Abstract
Despite improvements in healthcare delivery, mortality rates for high-output fistulae remain unchanged. The pathophysiology and causes of fistulae are reviewed in this article. An overview of the diagnostic procedures to delineate fistulae and underlying bowel disease together with their complications is included. Management of high-output fistulae consists of assessment and stabilization of patients, followed by conservative management by a multidisciplinary team until spontaneous or surgical closure of fistulae.
Keywords: High-output fistulae, pathophysiology, nutrition, somatostatin
High-output enterocutaneous fistulae (ECFs) continue to present a challenge in modern clinical practice. Forty years ago, such fistulae were characterized by mortality rates of up to 50%.1 Improved understanding of the pathophysiology combined with better surgical and nursing care led to a dramatic decline in mortality; the next three decades saw rates fall to between 5 and 20%.2,3,4,5 Thereafter mortality figures have remained stable despite a more integrated multidisciplinary care plan for patients with ECF. An analysis of 277 patients with ECF referred to St. Mark's Hospital over the past 11 years revealed an overall mortality rate of 10.8% (30 of 277) for fistula-related complications. All 6 of the patients who died within 30 days of surgery had high-output fistulae, and of the other 24, 15 patients had high-output fistulae. The total mortality for high-output fistula was therefore 7.6% (data not yet published). Unfortunately, many of the conditions that predispose to high-output fistulae are associated with short bowel syndrome, rendering treatment more difficult for the multidisciplinary team, and this may be a factor in the static mortality rates. It is also probable that patients with profound intestinal losses who were previously considered unsalvageable now undergo major resection and live to face the risk of subsequent fistulization.
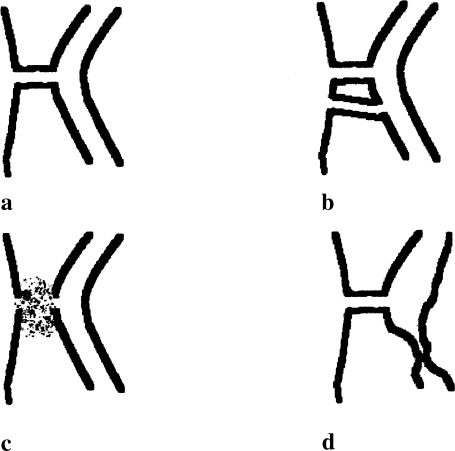
A high-output fistula may be defined pragmatically as one with an output of 500 mL/day or more. This may be further refined according to the anatomical site, and the present article focuses on the ECFs. A fistula may be simple, with a short track that communicates directly with the skin, or more complex, associated with abscess formation or involvement of multiple loops of bowel (Fig. 1).
Figure 1.
Types of fistulae. (A) Simple fistula; (B) complex fistula involving more than one bowel loop; (C) complex fistula with an abscess; (D) complex fistula with diseased/obstructed distal bowel.
Problems with high-output ECF arise because enteral secretions (including gastric, pancreatic, and biliary contributions) together with the oral intake bypass the absorptive process of the small intestine through the fistulous track, leading to malnutrition and fluid and electrolyte loss. The situation is worsened by sepsis, skin excoriation, or delayed wound closure in cases in which the external fistulous track is within or near an open wound. Understanding the pathophysiology of this process is a prerequisite to an integrated plan for the management of patients with high-output ECF.
PATHOPHYSIOLOGY
Causes of Fistulae
Surgery remains the commonest trigger for the development of ECFs, and recent surgery has occurred in at least 75 to 85% of cases.6 Fistulae occur predominantly on a background of surgery for cancer, inflammatory bowel disease, or lysis of adhesions. Surgery for peptic ulcer disease or pancreatitis or any emergency surgical procedure may also be complicated by fistula formation. Several perioperative factors are considered to increase the risk, including full-thickness bowel injury, damage to the mesenteric arteries, intestinal entrapment in fascial sutures, and excessively tight sutures with consequent ischemic necrosis. Inappropriate placement of surgical drains has also been thought to predispose to fistula formation. The postoperative failure of anastomotic healing, from any cause but worsened by malnutrition and sepsis, also initiates fistula formation. The presence of an open wound, laparostomy, or accompanying intestinal failure then militates against successful treatment. Spontaneous fistulae account for no more than 15 to 25% of ECFs, with Crohn's disease and intra-abdominal cancer as the commonest causes. ECFs may develop as a direct consequence of the primary malignancy but more often as a complication of radiotherapy. A retrospective analysis of cancer-associated fistulae showed that they were usually single and more often from the jejunum or ileum than from the colon.7 The commonest underlying malignancies were colorectal cancer in men and ovarian cancer in women. Metastasis was detected in the majority of cancer patients with ECF, indicating that this complication is most likely to occur in the setting of advanced disease.
A new analysis of ECFs from St Mark's Hospital shows that 85% were postoperative and 15% spontaneous; inflammatory bowel disease was present in 47% of postoperative fistulae and Crohn's disease specifically in no less than 83% of the spontaneous ones. Other causes of ECF are shown in Table 1.
Table 1.
Causes of Enterocutaneous Fistula
| Congenital |
| Patent vitelline duct |
| Postoperative |
| Inadvertent injury |
| Failure of anastomosis |
| Proximity of drain |
| Post-traumatic |
| Direct abdominal injury |
| Open abdominal wound |
| Inflammatory |
| Crohn's disease |
| Adjacent abscess |
| Postirradiation |
| Malignant |
| Adherence of tumor to adjacent bowel or abdominal wall with subsequent tumor necrosis |
Fistula Output
FLUID LOSSES
The effect of fistula output is best appreciated by an analysis of daily gastrointestinal secretions. The salivary and gastric glands each release up to 1.5 L of fluid per day. The pancreas produces about 1 L of fluid per day, and there is as much as 1 L of bile. About 200 mL/day originates from the duodenal Brunner's glands, with the rest of the small intestine secreting up to 1.8 L/day in health and a great deal more in the presence of obstruction or other small bowel disease. Therefore, 8 to 10 L of fluid flows through the jejunum each day, depending on the oral intake. In the intact functioning intestine 98% of this fluid is (re)absorbed; leaving only 100 to 200 mL of fluid to be excreted in the stool. Most (7–8 L) of this absorption occurs proximal to the ileocecal valve.
In the case of proximal fistulae there is a potential for serious compromise of fluid balance even when there is a reasonable extent of more distal intact small intestine, in that large fluid volumes may exit through the fistula rather than proceeding distally to allow absorption to occur. Enzymes and electrolytes (especially sodium and magnesium) inevitably accompany fluid, as do essential nutrients. The nutritional and fluid balance issues are less with distal fistulae, as small intestinal absorption leaves less fluid and fewer nutrients to be lost as long as the small bowel proximal to the fistula is in good functional condition. Reduction of gastrointestinal tract fluid secretion is an integral part of fistula treatment, as discussed in the following.8
METABOLIC DERANGEMENTS
It is not only the volume that is critical with high-output fistulae but also the composition of the secretions at various levels of the gastrointestinal tract.
Gastric juice contains hydrogen, chloride, sodium, and potassium ions, the concentrations differing significantly between the fasting and fed states. Feeding increases the hydrogen ion concentration from about 50 mM to up to 100 mM, increases chloride from 90 to 120 mM, but decreases sodium from 40 to about 25 mM.8 Duodenal fistulae consequently lead to loss of fluid rich in hydrogen and chloride ions, especially after eating, culminating in hypochloremic, hypokalemic metabolic alkalosis together with hypovolemia.
The combination of Brunner's gland secretions, bile, and pancreatic secretions serve to neutralize gastric acid secretion. The main electrolytes in bile are sodium and chloride, whereas bicarbonate and sodium make up most of the pancreatic juice. The sodium concentration of about 140 mM is independent of the activity state of the pancreas, but the bicarbonate concentration increases from 40 to up to 145 mM following stimulation.9 Fistulous discharge at the level of the upper jejunum is therefore associated with significant losses of sodium, chloride, and bicarbonate ions.
ENZYMES AND SKIN
By the time the gastric contents have reached the jejunum, an array of enzymes has been released and activated within the gut lumen. Salivary amylase and gastric pepsin, together with potent pancreatic proteolytic enzymes including trypsin, chymotrypsin, and carboxypeptidases, commence carbohydrate and protein digestion. Other pancreatic enzymes including lipase, amylase, phospholipase, and cholesterol esterase are also activated and are found in the effluent from high-output fistulae. These enzymes contribute to delay in wound closure and can lead to major excoriation of previously normal skin.
THE ROLE OF GUT HORMONES
Gastric acid secretion is accompanied by the release of gastrin, histamine, somatostatin, and cholecystokinin (CCK).10 Gastrin and histamine exert a paracrine and endocrine effect to activate the acid-secreting parietal cell and the somatostatin-secreting D cell. CCK is released from duodenal and upper jejunal mucosa when food enters the small bowel. In addition to stimulating pancreatic enzyme secretion, CCK is a potent gastrin stimulus and serves to perpetuate gastric acid secretion. It also stimulates the release of gastric somatostatin. Both of these actions are mediated in an endocrine manner. Somatostatin, on the other hand, is a potent inhibitory hormone and switches off gastric acid secretion by direct action on the parietal and enterochromaffin-like cells and indirectly by inhibiting gastrin secretion. Somatostatin thus mediates its actions in endocrine, paracrine, and neurocrine manners to regulate gastrointestinal motility and secretion. Its potential therapeutic role in the management of high-output fistulae is discussed later.
Glucagon-like peptide 2 (GLP2) has attracted considerable attention as a therapeutic agent for intestinal injury since its identification as a potent stimulator of mucosal epithelial proliferation.11 Preliminary trials in patients with short bowel syndrome have yielded improvements in intestinal absorption of both fluids and nutrients.12 A GLP2-induced reduction of gastric acid secretion and gastric motility is perhaps particularly relevant in terms of its potential as a therapeutic agent for patients with ECF. As yet there are no studies specifically examining the effects of GLP2 on high-output fistulae.
ASSOCIATED SHORT BOWEL FEATURES
Fistulae related to intestinal surgery, inflammatory bowel disease, and radiation enteritis are often accompanied by short bowel syndrome, which may occur because the residual bowel is actually short, because it is functionally impaired, or because the dominance of effluent from a proximal fistula precludes reabsorption from distal (otherwise intact) intestine. Problems with fluid balance and reduced absorptive capacity for nutrients are then to be expected. Early recognition of these complicating factors is crucial to a good outcome.
DIAGNOSIS OF FISTULA
A fistula constitutes an abnormal communication between two epithelial surfaces, and clinical examination usually identifies the external site of an ECF but rarely identifies its source or internal opening. Moreover, complex fistulous tracks may communicate with organs other than the gastrointestinal tract, especially the bladder and the gynecological organs. A range of radiological examinations is available for identification and classification of many of the more complex fistulae.
Fistulogram
Injecting contrast material through the external opening is useful to identify the fistula track and its internal opening. Despite the advent of more modern modalities, this is still often the best means to identify the fistula tract when an external opening is present.13 In cases in which there is no flow of contrast material (even using an occlusion balloon) and the internal anatomy remains obscure, further imaging is required.
Ultrasonography
The sensitivity and specificity of ultrasonography (US) in assessing fistulae have been examined mainly for fistulae associated with Crohn's disease. In a series of 98 consecutive inpatients with Crohn's disease, 8 of 12 fistulas were detected by US.14 Another study of 213 patients revealed a sensitivity of 87% and specificity of 90%.15 In both studies US was better at detecting abscesses and stenosis, and it might be thought that US has a limited role in fistula practice. However, modifying the technique by the instillation of hydrogen peroxide—enhanced ultrasound-fistulography—has rendered US as accurate, safe, and simple as radiographic fistulography while retaining the additional benefit of detecting coexisting abdominal complications and diseased bowel segments.16
Barium Follow-Through
Barium studies are no longer considered the method of choice for the detection of internal fistulae. Barium follow-through (BaFT) can, nonetheless, be very helpful in determining the fistula site in relation to the rest of the bowel. The extent of mucosal damage from any underlying intestinal disease and the length of remaining bowel can also be assessed. Barium studies can certainly be used to complement other imaging modalities,17 but they give relatively little information on the structures around the bowel and, because they are essentially low-pressure contrast studies, it is common that tracks are filled incompletely or not at all.
BARIUM FOLLOW-THROUGH VERSUS ULTRASOUND SCANNING
The diagnostic accuracy of US and barium studies is similar for detecting fistulae, but US is superior in the detection of intra-abdominal abscesses. A combination of the two modalities further improves the diagnostic accuracy.17
BARIUM FOLLOW-THROUGH VERSUS COMPUTED TOMOGRAPHY
Compared with computed tomography (CT), barium studies are probably still superior in revealing enteroenteric fistulae, sinus tracts, strictures, and postsurgical anatomy. However, these modest advantages are gradually being eroded with each new generation of scanner. CT is undoubtedly better in detecting abscesses and in the assessment of multiple or complex fistulae, especially those that involve nongastrointestinal organs, such as bladder, and when fistulae involve the psoas muscle or sacrum. The two modalities remain complementary for the more complex cases.18
BARIUM FOLLOW-THROUGH VERSUS WATER-SOLUBLE CONTRAST AGENTS
Water-soluble contrast agents are preferable to barium when an anastomotic leak is suspected or there is other reason to fear direct communication with the peritoneal space, as barium peritonitis is dangerous in the short and long term. Diagnosis is almost as accurate as with barium studies but there is less definition, especially with more distal lesions.19 Water-soluble contrast examinations through the rectum or in retrograde fashion from stomas can also be highly contributory.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is now unequivocally superior to US in localization of inflamed bowel and in recognizing fistulae, stenosis, and abscesses, but it is costly and not always readily available.20 Administration of contrast medium has improved visualization of the intestinal wall as in the circumscribed thickening of Crohn's disease, and extraintestinal intra-abdominal processes are unlikely to be missed.
A recent study suggests that MRI has a diagnostic efficacy equal to that of barium studies in the primary diagnosis of Crohn's disease and that it is now clearly more sensitive in the detection of extraintestinal manifestations such as fistulae or abscesses.21 The superiority of MRI in disease detection and the advantages of avoiding radiation exposure, together with the introduction of faster imaging techniques (e.g., breath-hold and respiration-triggered protocols), make for a realistic early prospect of MRI replacing barium studies. Irrespective of the cost, MRI is indicated for clinically complex fistulae, recurrent fistulae, and fistulae complicating Crohn's disease; for some years now it has been recognized to be invaluable for the assessment of pelvic or perineal fistulating disease.20,22 In the therapeutic setting, MRI has allowed detection of residual fistulae where clinical impressions and surgical assessment under anesthetic have been misleading, but its ultimate role in this situation has yet to be defined.23
MANAGEMENT
The management of gastrointestinal fistulae may wisely be considered as a three-phase process: assessment, stabilization, and interval surgery.
Assessment
The first aim of assessment is to achieve a thorough understanding of the fistula and its impact on the patient. Imaging to classify the anatomy and etiology of the fistulae can be performed while the physiological impact is investigated with strict charting of fluid intake and losses in urine and stool and from each fistula, stoma, and wound. Attentive and systematic nursing also permits the assessment of the psychological state of the patient, which may be parlous indeed.
Stabilization
FLUID LOSSES
Renal function may be compromised by secondary hyperaldosteronism provoked by sodium loss through the fistula. A urinary sodium concentration below 20 mM suggests inadequate fluid replacement, and this is certain when the level falls below 10 mM. These need not be timed collections but can be simple random urine samples, as it is the concentration of sodium that provides the necessary information. Fluid volumes can be replaced intravenously or orally. As a rule to guide fluid replacement, each liter of fluid lost through an ECF or stoma contains 100 mmol of sodium. With oral replacement treatment it is important to add sodium, as a jejunal luminal concentration less than 90 mM is associated with a net sodium loss. Loperamide and codeine phosphate help to slow intestinal transit and may reduce fistula output by this mechanism. Gastric volume secretion is minimized by gastric acid inhibition with proton pump inhibitors, which also has a small effect in reducing electrolyte loss.24 Both lansoprazole and omeprazole significantly decrease basal and pentagastrin-induced acid secretion25 with a dose-dependent response26; twice-daily regimes are more effective than once-daily ones.27
ELECTROLYTE LOSSES
Electrolyte losses are replaced whenever possible by oral electrolyte therapy. The typical patient is encouraged to limit ordinary (hypotonic, sodium-free) fluid intake severely—perhaps to no more than 0.5 L in 24 hours. This is balanced by the addition of electrolyte or oral rehydration solution. Each liter of electrolyte solution should contain 90 mmol of sodium and 111 mmol of glucose, as in the World Health Organization solution. Although ideally it should replace in equal volume the fluid collected from fistulae and stomas, it is unrealistic to expect patients to manage more than 1.5 to 2.0 L in 24 hours. Where greater volumes are required, parenteral support is needed.
NUTRITION
Malnutrition is an adverse prognostic factor known to predispose to sepsis and mortality in patients with high-output fistulae. It is not surprising therefore to find several studies attempting to improve outcomes by treating malnutrition. Their aims have been to reduce mortality and to promote fistula closure by preventing sepsis or allowing its control. The key questions are therefore whether nutrition promotes fistula closure and reduces mortality and, if so, by which route, parenteral or enteral, it should be given.
The closure of 81% of ECFs was reported by Deitel in a study involving 86 patients with 100 ECFs.28 One or more of three nutritional modalities was used according to clinical criteria: central parenteral nutrition, peripheral parenteral nutrition, or elemental enteral nutrition. A decade later, a retrospective analysis of 108 patients with 114 fistulae who had received parenteral nutrition showed that 61% of the fistulae closed spontaneously.29 The mortality rates were 9.5% and 10%, respectively—a vast improvement on the 40% seen in earlier trials but not different from the mortality rate of 10.8% at St Mark's Hospital, where parenteral nutrition is avoided whenever possible.
Use of an elemental diet was claimed to result in closure of ECFs in 64.8% of cases.30 However, only 11 of the 37 patients were given an exclusive elemental diet; the other 26 were initially treated with intravenous nutrition and subsequently maintained with an elemental diet; the authors did not analyze the response to an elemental diet alone. Mortality was 16.2% and operative closure was needed in 16.2%.
A study with 234 ECF patients looked at the effect of continuous enteral nutrition.31 The spontaneous closure rate was lower at 38% and the mortality was 11%. This mortality rate was similar to that in the large parenteral nutrition studies32,33 but greater than the 5.3% mortality reported by McIntyre et al.34 The wide differences in closure and mortality rates probably reflect the highly heterogeneous group of patients with ECFs, including those who had postoperative complications alone and those in whom the fistula was in conjunction with malignancy, radiation damage, or inflammatory bowel disease.
In an attempt to remove some of these confounding factors, Sitges-Serra et al assessed the outcomes for postoperative fistulae only. Seventy-five patients with 87 postoperative fistulae were kept with nothing by mouth and commenced on intravenous nutrition. Fluid and electrolyte imbalance and sepsis were aggressively treated in all patients. The authors reported a 71% spontaneous closure rate, but subgroup analysis for high-output fistulae revealed a lower closure rate of 54%.4 Despite the efforts to create a homogeneous study group, methodological flaws remain, including the still wide spectrum of underlying diseases leading to surgery and the lack of a control group managed without intravenous nutrition. A further study, but with smaller numbers of patients, showed that exclusive intravenous nutrition was associated with closure of 50% of fistulae, with 11 of 28 patients responding (39%); some patients had more than one fistula. Overall mortality was 22% despite optimizing nutrition with exclusive parenteral nutrition for 4 weeks and proceeding to surgery only if there was not a response to this.33
Although many studies have investigated the value of parenteral nutrition or enteral nutrition in promoting the closure of intestinal fistulae, none has directly compared their outcomes. An animal study has, however, found an 81% reduction in fistula output with an elemental diet compared with a regular diet and showed that intravenous nutrition reduced the output by 93%.35 Concomitantly, there were 71% fewer calories lost with enteral nutrition and 93% fewer with intravenous nutrition. These results have not been reproduced in humans but lend support to the concept of energetic attention to better nutrition in fistula patients, even if spontaneous closure does not occur.
The preceding studies not only suggest that nutrition promotes healing but also identify subgroups of fistula patients who are unlikely to respond to conservative measures and will eventually need surgery for closure. Cancer patients were highlighted as a poor prognostic group; a retrospective analysis of 25 cancer patients with ECFs showed that malnutrition and sepsis developed in as many as 60% of patients. Additional exacerbating factors with an impact on the reported 30-day mortality of 16% included previous radiotherapy, location and output from the fistulae, and hypoalbuminemia.36 Furthermore, the presence of a fistula had a strong negative impact on the provision of further anticancer therapy.
SKIN CARE
The multidisciplinary team involved in caring for patients with high-output fistula is incomplete without the specialist stoma nurse. The contributions of the stoma nurse are indispensable, but it must be acknowledged that many of these are empirical rather than research based.
Even with simple high-output fistulae, skin care may be severely compromised; the enzyme-rich output can lead to skin excoriation within 3 to 4 hours. Excoriation is unpleasant for patients and reduces adhesion of stoma appliances. The risk of excoriation can be reduced with the use of barrier creams, sprays, or wipes.37 If the skin has already become wet or excoriated, a drying method should be instituted. There is no proven drying method; instead, the decision is made on an individual basis. The options include suction of predicted high outputs after meals, cloth wipes, and the use of hair dryers (which become mandatory for some barrier materials).38 Complex fistulae draining into open wounds delay healing by damaging the wound edges; selection of a well-fitting wound drainage bag system minimizes persistent irritation. Simple measures such as restricting oral intake when the fistula appliance is due to be changed and appropriate positioning of the patient to gain assistance from gravity reduce the need for suction during changes and shorten the time taken.
PRIMARY UNDERLYING DISEASE
Fistulae related to inflammatory bowel disease are amenable to therapy with one or more of the growing number of immunosuppressant and biological therapies39; control rather than resolution should be the expectation. Treatment of underlying abscesses causing fistulation (e.g., in diverticulitis) with aggressive antibiotic therapy and drainage also assists healing. There is no established treatment for radiation-induced fistula, but the fistula directly related to an area of malignancy may respond to chemotherapy.
SOMATOSTATIN
Because somatostatin decreases acid and pancreatic secretion and gut motility, it would seem a good candidate for successful reduction in fistula output; its very short duration of action would favor one of its longer acting analogues. These potentially beneficial effects would have to override the likely detrimental effects from reduction in splanchnic blood flow and reduction of intestinal nutrient absorption.40 Caution must also be exercised to avoid misinterpretation in the light of the evidence for down-regulation of somatostatin receptors, their differential affinity for the several biologically active somatostatin fragments, and the effect of the feeding or starving state.
Clinical trials of somatostatin have, in fact, been disappointing, perhaps partly because of these divergent actions but also because of the paucity of controlled trials, the small numbers of patients in each trial, and the heterogeneity of patients with high-output fistulae. Furthermore, there have been wide variations in the timing of the treatment with respect to the onset of the fistulae, a range of different outcome measures, and variations of the doses and durations of treatment before those outcomes have been recorded.
COMPARATIVE STUDIES
Six published trials have compared conservative treatment versus conservative treatment with somatostatin or its analogues. The outcomes measured included fistula output, closure rates, and time to closure.
Somatostatin-14
There have been three trials with somatostatin-14 (Table 2). One study included only pancreatic fistulae and is not considered further here.41 The other two studied postoperative ECFs42,43 and showed significantly reduced outputs compared with conservative treatment with intravenous nutrition alone. Although the first of these studies compared equal numbers of patients,44 the sites of fistulae varied, including origins from pancreas, duodenum, jejunum, ileum, and colon. The second study selected 61 patients in an intensive care setting, all of whom had small bowel fistulae, but the two arms of the study were unbalanced (e.g., 70% were in the conservative group). Significantly reduced fistula output and increased closure rates were reported but may have reflected other influences in this unblinded and open-treatment study.43 The time to fistula closure (where this occurred) was significantly shorter for the groups receiving somatostatin in both studies.
Table 2.
Somatostatin-14 Studies
| Study | n | Treatment | Patient Characteristics | Effect on Output | Closure Rate | Time to Closure |
|---|---|---|---|---|---|---|
| ECF, enterocutaneous fistula; PO, postoperative; S, somatostatin-14; TPN, total parenteral nutrition. | ||||||
| Torres et al44 | 2 | TPN | PO, mixed fistula sites | p < .05 | No effect | Shorter |
| 0 | TPN + S | |||||
| 2 | ||||||
| 0 | ||||||
| Planas et al43 | 4 | TPN | PO, ECF, admitted to ITU | p < .05 | Effect | Shorter |
| 6 | TPN + S | |||||
| 1 | ||||||
| 5 | ||||||
Octreotide
Three trials with octreotide have investigated its effects on fistula output and closure rates (Table 3).45,46,47 Of these, only one, an unusually designed double-blind crossover study with 14 patients, showed diminished fistula output.45 In this study, after an initial 7-day stabilization period, patients were enrolled into one of two groups. Group 1 received octreotide for 2 days, placebo for 2 days, and then regular octreotide until closure. Group 2 received the placebo first and then octreotide continuing until closure. The investigators suggested that interrupting the octreotide treatment with placebo led to an increased output. The two other studies showed no difference from placebo46 or placebo combined with intravenous nutrition.47 In no trial has octreotide affected the proportion of patients with fistula closure. Furthermore, the study by Sancho et al47 failed to show any advantage of octreotide in terms of the time to closure.
Table 3.
Octreotide Studies
| Study | n | Treatment | Patient Characteristics | Effect on Output | Closure Rate | Time to Closure |
|---|---|---|---|---|---|---|
| ECF, enterocutaneous fistula; NA, not applicable; NS, not significant; O, octreotide; Pl, placebo; PO, postoperative; S, somatostatin-14; TPN, total parenteral nutrition. | ||||||
| Nubiola-Calonge et al45 | 6 | Pl, O, O | PO, ECF | p < .01 | No effect | NA |
| 8 | O, Pl, O | |||||
| Scott et al46 | 8 | Pl | PO, ECF | NS | No effect | NA |
| 1 | O | |||||
| 1 | ||||||
| Sancho et al47 | 1 | Pl + TPN | PO, mixed | NS | No effect | No change |
| 7 | O + TPN | |||||
| 1 | ||||||
| 4 | ||||||
| Hernandez-Aranda et al48 | 4 | TPN | PO, ECF | NA | No effect | Shorter |
| 5 | O + TPN | |||||
| 4 | ||||||
| 0 | ||||||
Fistula closure time was the main outcome for a study by Hernández-Aranda and colleagues.48 Forty-five patients were given conservative treatment with intravenous nutrition alone, and 40 had additional octreotide. In the group that received octreotide, fistula closure time was significantly reduced and duration of nutritional support requirement was also reduced, but duration of hospital stay was not affected significantly. Of note is that the fistula closure rate in the control group was very low compared with other trials (56%), which may have been the main factor resulting in statistical significance.
NONCOMPARATIVE STUDIES
There are many more noncomparative than controlled studies in the literature, and a comprehensive review of these was conducted by Hesse et al.49 Methodological limitations render their conclusions highly uncertain. Of the three larger studies that investigated somatostatin-14, two showed a reduction in fistula output (of 70% at day 1)50,51 and the third showed no change.52 The octreotide studies also reported divergently. One study with 27 patients showed no significant reduction in output at day 1.53 This study is of particular importance as it included patients with both high-output and low-output ECF, and the authors were able to demonstrate that the outcome was independent of the initial fistula output. On the other hand, a smaller study of only 12 patients suggested that octreotide significantly reduced fistula output; this may reflect selection of patients limited to those with pancreatic fistulae.54
There is therefore insufficient evidence to recommend the use of somatostatin or its analogues in the management of high-output ECF. Until larger and better-designed trials have been conducted, their value should be considered to remain unproved and their use strictly limited because they may not be without risk.55
Surgery
A careful search for features predictive of the failure of spontaneous ECF closure is mandatory to identify patients who are unlikely to respond to conservative measures and for whom surgery should be planned. These poor prognostic factors include complete intestinal disruption; mucocutaneous continuity; the coexistence of large abscesses, especially if there is severe intraperitoneal infection; and adjacent active bowel disease (usually Crohn's or radiation damage).6,48 The pressure differential that results from a wide (> 1 cm), short (< 2 cm) fistula tract reduces the chance of resolution even in the absence of distal obstruction. Fistulae involving stomach, lateral duodenum, ligament of Treitz, or bladder also have a worse prognosis.56 The presence of malignancy, foreign bodies, age older than 65 years, and a fistula output that cannot be brought below 500 mL/day all appear associated with an unfavorable prognosis.57
However, the only patients for whom early surgery should be considered are those with undrained sepsis. The most frequent error in ECF management is one of commission, with premature “restorative” surgery followed by wound breakdown, anastomosis, or newly compromised intestine.
Surgical intervention for poor-prognosis ECF should be delayed for at least 6 weeks and preferably for more than 3 months from the time of the most recent prior surgery. Patients with radiation damage and active malignancy present special problems that are necessarily dealt with on a case-by-case basis.
Elective surgical intervention should also be considered for the group of patients without poor prognostic factors but in whom spontaneous closure has not occurred by 4 to 6 months.
This is not the place for a critique of operative technique, but it may be useful to summarize recent procedures for ECF at St. Mark's Hospital. The commonest major procedure was interval resection of fistula together with reanastomosis. Less common procedures included resection, reanastomosis with a defunctioning stoma, and creation of a defunctioning stoma alone; only rarely were fistulae oversewn or bypassed.5
Endoscopic closure of resistant fistulae by the injection of fibrin glue was reported to lead to closure in 14 of the 15 patients so treated but with mortality “unrelated to fistulae or its treatment” in 2 patients.58 Our own experience has been much less favorable, and widespread adoption of the technique should not precede clearly positive controlled studies.
REFERENCES
- 1.Edmunds L H, Jr, Williams G M, Welch C E. External fistulas arising from the gastro-intestinal tract. Ann Surg. 1960;152:445–471. doi: 10.1097/00000658-196009000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rocchio M A, Cha C J, Haas K F, Randall H T. Use of chemically defined diets in the management of patients with high output gastrointestinal cutaneous fistulas. Am J Surg. 1974;127:148–156. doi: 10.1016/0002-9610(74)90151-2. [DOI] [PubMed] [Google Scholar]
- 3.Deitel M. Nutritional management of external gastrointestinal fistulas. Can J Surg. 1976;19:505–509. [PubMed] [Google Scholar]
- 4.Sitges-Serra A, Jaurrieta E, Sitges-Creus A. Management of postoperative enterocutaneous fistulas: the roles of parenteral nutrition and surgery. Br J Surg. 1982;69:147–150. doi: 10.1002/bjs.1800690310. [DOI] [PubMed] [Google Scholar]
- 5.McIntyre P B, Ritchie J K, Hawley P R. Management of enterocutaneous fistulas: a review of 132 cases. Br J Surg. 1984;71:293–296. doi: 10.1002/bjs.1800710416. [DOI] [PubMed] [Google Scholar]
- 6.Athanassiades S, Notis P, Tountas C. Fistulas of the gastrointestinal tract. Experience with eighty-one cases. Am J Surg. 1975;130:26–28. doi: 10.1016/0002-9610(75)90450-x. [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain R S, Kaufman H L, Danforth D N. Enterocutaneous fistula in cancer patients: etiology, management, outcome, and impact on further treatment. Am Surg. 1998;64:1204–1211. [PubMed] [Google Scholar]
- 8.Feldman M, Goldschmiedt M. Gastric HCO3-secretion: relationship with Na+ secretion and effect of acetazolamide in humans. Am J Physiol. 1991;261(2 Pt 1):G320–G326. doi: 10.1152/ajpgi.1991.261.2.G320. [DOI] [PubMed] [Google Scholar]
- 9.Read N W, Cooper K, Fordtran J S. Effect of modified sham feeding on jejunal transport and pancreatic and biliary secretion in man. Am J Physiol. 1978;234:E417–E420. doi: 10.1152/ajpendo.1978.234.4.E417. [DOI] [PubMed] [Google Scholar]
- 10.Kidd M, Modlin I M, Tang L H. Gastrin and the enterochromaffin-like cell: an acid update. Dig Surg. 1998;15:209–217. doi: 10.1159/000018616. [DOI] [PubMed] [Google Scholar]
- 11.Drucker D J, Erlich P, Asa S L, Brubaker P L. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci USA. 1996;93:7911–7916. doi: 10.1073/pnas.93.15.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeppesen P B, Hartmann B, Thulesen J, et al. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology. 2001;120:806–815. doi: 10.1053/gast.2001.22555. [DOI] [PubMed] [Google Scholar]
- 13.Alexander E S, Weinberg S, Clark R A, Belkin R D. Fistulas and sinus tracts: radiographic evaluation, management, and outcome. Gastrointest Radiol. 1982;7:135–140. doi: 10.1007/BF01887627. [DOI] [PubMed] [Google Scholar]
- 14.Cresci G A, Martindale R G. Metabolic and nutritional management of a patient with multiple enterocutaneous fistulas. Nutrition. 1997;13:446–448. doi: 10.1016/s0899-9007(97)00094-4. [DOI] [PubMed] [Google Scholar]
- 15.Gasche C, Moser G, Turetschek K, Schober E, Moeschl P, Oberhuber G. Transabdominal bowel sonography for the detection of intestinal complications in Crohn's disease. Gut. 1999;44:112–117. doi: 10.1136/gut.44.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maconi G, Parente F, Porro G B. Hydrogen peroxide enhanced ultrasound-fistulography in the assessment of enterocutaneous fistulas complicating Crohn's disease. Gut. 1999;45:874–878. doi: 10.1136/gut.45.6.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maconi G, Sampietro G M, Parente F, et al. Contrast radiology, computed tomography and ultrasonography in detecting internal fistulas and intra-abdominal abscesses in Crohn's disease: a prospective comparative study. Am J Gastroenterol. 2003;98:1545–1555. doi: 10.1111/j.1572-0241.2003.07521.x. [DOI] [PubMed] [Google Scholar]
- 18.Orel S G, Rubesin S E, Jones B, Fishman E K, Bayless T M, Siegelman S S. Computed tomography vs barium studies in the acutely symptomatic patient with Crohn disease. J Comput Assist Tomogr. 1987;11:1009–1016. doi: 10.1097/00004728-198711000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Jobling C, Halligan S, Bartram C. The use of non-ionic water-soluble contrast agents for small bowel follow-through examination. Eur Radiol. 1999;9:706–710. doi: 10.1007/s003300050737. [DOI] [PubMed] [Google Scholar]
- 20.Potthast S, Rieber A, Von Tirpitz C, Wruk D, Adler G, Brambs H J. Ultrasound and magnetic resonance imaging in Crohn's disease: a comparison. Eur Radiol. 2002;12:1416–1422. doi: 10.1007/s00330-001-1191-3. [DOI] [PubMed] [Google Scholar]
- 21.Rieber A, Nussle K, Reinshagen M, Brambs H J, Gabelmann A. MRI of the abdomen with positive oral contrast agents for the diagnosis of inflammatory small bowel disease. Abdom Imaging. 2002;27:394–399. doi: 10.1007/s00261-001-0120-x. [DOI] [PubMed] [Google Scholar]
- 22.Koelbel G, Schmiedl U, Majer M C, et al. Diagnosis of fistulae and sinus tracts in patients with Crohn disease: value of MR imaging. AJR Am J Roentgenol. 1989;152:999–1003. doi: 10.2214/ajr.152.5.999. [DOI] [PubMed] [Google Scholar]
- 23.Bell S J, Halligan S, Windsor A C, Williams A B, Wiesel P, Kamm M A. Response of fistulating Crohn's disease to infliximab treatment assessed by magnetic resonance imaging. Aliment Pharmacol Ther. 2003;17:387–393. doi: 10.1046/j.1365-2036.2003.01427.x. [DOI] [PubMed] [Google Scholar]
- 24.Dombrowski S R, Mirtallo J M. Drug therapy and nutritional management of patients with gastrointestinal fistulas. Clin Pharm. 1984;3:264–272. [PubMed] [Google Scholar]
- 25.Bruley des Varennes S, Levy P, Lartigue S, Dellatolas F, Lemaire M, Galmiche J P. Comparison of lansoprazole with omeprazole on 24-hour intragastric pH, acid secretion and serum gastrin in healthy volunteers. Aliment Pharmacol Ther. 1994;8:309–314. doi: 10.1111/j.1365-2036.1994.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 26.Londong W, Londong V, Cederberg C, Steffen H. Dose-response study of omeprazole on meal-stimulated gastric acid secretion and gastrin release. Gastroenterology. 1983;85:1373–1378. [PubMed] [Google Scholar]
- 27.Katz P O, Hatlebakk J G, Castell D O. Gastric acidity and acid breakthrough with twice-daily omeprazole or lansoprazole. Aliment Pharmacol Ther. 2000;14:709–714. doi: 10.1046/j.1365-2036.2000.00775.x. [DOI] [PubMed] [Google Scholar]
- 28.Deitel M. Nutritional management of external gastrointestinal fistulas. Can J Surg. 1976;19:505–509. [PubMed] [Google Scholar]
- 29.Rose D, Yarborough M F, Canizaro P C, Lowry S F. One hundred and fourteen fistulas of the gastrointestinal tract treated with total parenteral nutrition. Surg Gynecol Obstet. 1986;163:345–350. [PubMed] [Google Scholar]
- 30.Rocchio M A, Cha C J, Haas K F, Randall H T. Use of chemically defined diets in the management of patients with high output gastrointestinal cutaneous fistulas. Am J Surg. 1974;127:148–156. doi: 10.1016/0002-9610(74)90151-2. [DOI] [PubMed] [Google Scholar]
- 31.Levy E, Frileux P, Cugnenc P H, Honiger J, Ollivier J M, Parc R. High-output external fistulae of the small bowel: management with continuous enteral nutrition. Br J Surg. 1989;76:676–679. doi: 10.1002/bjs.1800760708. [DOI] [PubMed] [Google Scholar]
- 32.Sitges-Serra A, Jaurrieta E, Sitges-Creus A. Management of postoperative enterocutaneous fistulas: the roles of parenteral nutrition and surgery. Br J Surg. 1982;69:147–150. doi: 10.1002/bjs.1800690310. [DOI] [PubMed] [Google Scholar]
- 33.Zera R T, Bubrick M P, Sternquist J C, Hitchcock C R. Enterocutaneous fistulas. Effects of total parenteral nutrition and surgery. Dis Colon Rectum. 1983;26:109–112. doi: 10.1007/BF02562587. [DOI] [PubMed] [Google Scholar]
- 34.McIntyre P B, Ritchie J K, Hawley P R. Management of enterocutaneous fistulas: a review of 132 cases. Br J Surg. 1984;71:293–296. doi: 10.1002/bjs.1800710416. [DOI] [PubMed] [Google Scholar]
- 35.Wolfe B M, Keltner R M, Willman V L. Intestinal fistula output in regular, elemental, and intravenous alimentation. Am J Surg. 1972;124:803–806. doi: 10.1016/0002-9610(72)90144-4. [DOI] [PubMed] [Google Scholar]
- 36.Chamberlain R S, Kaufman H L, Danforth D N. Enterocutaneous fistula in cancer patients: etiology, management, outcome, and impact on further treatment. Am Surg. 1998;64:1204–1211. [PubMed] [Google Scholar]
- 37.Frost S. Stoma care: managing high-output fistulas. Nurs Stand. 1991;5:25–27. [PubMed] [Google Scholar]
- 38.Burch J. The nursing care of a patient with enterocutaneous faecal fistulae. Br J Nurs. 2003;12:736–740. doi: 10.12968/bjon.2003.12.12.11336. [DOI] [PubMed] [Google Scholar]
- 39.Lichtenstein G R. Treatment of fistulizing Crohn's disease. Gastroenterology. 2000;119:1132–1147. doi: 10.1053/gast.2000.18165. [DOI] [PubMed] [Google Scholar]
- 40.Walsh J H. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994. Gastrointestinal hormones. pp. 1–128.
- 41.Pederzoli P, Bassi C, Falconi M, Albrigo R, Vantini I, Micciolo R. Conservative treatment of external pancreatic fistulas with parenteral nutrition alone or in combination with continuous intravenous infusion of somatostatin, glucagon or calcitonin. Surg Gynecol Obstet. 1986;163:428–432. [PubMed] [Google Scholar]
- 42.Torres A J, Landa J I, Moreno-Azcoita M, et al. Somatostatin in the management of gastrointestinal fistulas. A multicenter trial. Arch Surg. 1992;127:97–99. doi: 10.1001/archsurg.1992.01420010115018. [DOI] [PubMed] [Google Scholar]
- 43.Planas M, Porta I, Angles R, Baena J A, Serra J, Padro J B. [Somatostatin and/or total parenteral nutrition for the treatment of intestinal fistulas] Rev Esp Enferm Dig. 1990;78:345–347. [PubMed] [Google Scholar]
- 44.Torres A J, Landa J I, Moreno-Azcoita M, et al. Somatostatin in the management of gastrointestinal fistulas. A multicenter trial. Arch Surg. 1992;127(1):97–99. doi: 10.1001/archsurg.1992.01420010115018. [DOI] [PubMed] [Google Scholar]
- 45.Nubiola-Calonge P, Badia J M, Sancho J, Gil M J, Segura M, Sitges-Serra A. Blind evaluation of the effect of octreotide (SMS 201–995), a somatostatin analogue, on small-bowel fistula output. Lancet. 1987;2:672–674. doi: 10.1016/s0140-6736(87)92452-4. [DOI] [PubMed] [Google Scholar]
- 46.Scott N A, Finnegan S, Irving M H. Octreotide and gastrointestinal fistulae. Digestion. 1990;45(Suppl 1):XS66–70. discussion 70–71. doi: 10.1159/000200265. [DOI] [PubMed] [Google Scholar]
- 47.Sancho J J, di Costanzo J, Nubiola P, et al. Randomized double-blind placebo-controlled trial of early octreotide in patients with postoperative enterocutaneous fistula. Br J Surg. 1995;82:638–641. doi: 10.1002/bjs.1800820521. [DOI] [PubMed] [Google Scholar]
- 48.Hernandez-Aranda J C, Gallo-Chico B, Flores-Ramirez L A, Avalos-Huante R, Magos-Vazquez F J, Ramirez-Barba E J. [Treatment of enterocutaneous fistula with or without octreotide and parenteral nutrition] Nutr Hosp. 1996;11:226–229. [PubMed] [Google Scholar]
- 49.Hesse U, Ysebaert D, de Hemptinne B. Role of somatostatin-14 and its analogues in the management of gastrointestinal fistulae: clinical data. Gut. 2001;49(Suppl 4):iv11–iv21. doi: 10.1136/gut.49.suppl_4.iv11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.di Costanzo J, Cano N, Martin J, et al. Treatment of external gastrointestinal fistulas by a combination of total parenteral nutrition and somatostatin. JPEN J Parenter Enteral Nutr. 1987;11:465–470. doi: 10.1177/0148607187011005465. [DOI] [PubMed] [Google Scholar]
- 51.Ysebaert D, Hee R Van, Hubens G, Vaneerdeweg W, Eyskens E. Management of digestive fistulas. Scand J Gastroenterol Suppl. 1994;207:42–44. doi: 10.3109/00365529409104194. [DOI] [PubMed] [Google Scholar]
- 52.Hild P, Dobroschke J, Henneking K, Rieck B. Treatment of enterocutaneous fistulas with somatostatin. Lancet. 1986;2:626. doi: 10.1016/s0140-6736(86)92445-1. [DOI] [PubMed] [Google Scholar]
- 53.Nubiola P, Badia J M, Martinez-Rodenas F, et al. Treatment of 27 postoperative enterocutaneous fistulas with the long half-life somatostatin analogue SMS 201–995. Ann Surg. 1989;210:56–58. doi: 10.1097/00000658-198907000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barnes S M, Kontny B G, Prinz R A. Somatostatin analog treatment of pancreatic fistulas. Int J Pancreatol. 1993;14:181–188. doi: 10.1007/BF02786125. [DOI] [PubMed] [Google Scholar]
- 55.O'Keefe S J, Haymond M W, Bennet W M, Oswald B, Nelson D K, Shorter R G. Long-acting somatostatin analogue therapy and protein metabolism in patients with jejunostomies. Gastroenterology. 1994;107:379–388. doi: 10.1016/0016-5085(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 56.Berry S M, Fischer J E. Classification and pathophysiology of enterocutaneous fistulas. Surg Clin North Am. 1996;76:1009–1018. doi: 10.1016/s0039-6109(05)70495-3. [DOI] [PubMed] [Google Scholar]
- 57.Athanassiades S, Notis P, Tountas C. Fistulas of the gastrointestinal tract. Experience with eighty-one cases. Am J Surg. 1975;130:26–28. doi: 10.1016/0002-9610(75)90450-x. [DOI] [PubMed] [Google Scholar]
- 58.Rabago L R, Ventosa N, Castro J L, Marco J, Herrera N, Gea F. Endoscopic treatment of postoperative fistulas resistant to conservative management using biological fibrin glue. Endoscopy. 2002;34:632–638. doi: 10.1055/s-2002-33237. [DOI] [PubMed] [Google Scholar]