Abstract
Ulcerative colitis is a chronic inflammatory disease of the colon with an increasing incidence worldwide. The medical management of this disease continues to expand as drugs to induce and maintain remission are sought to avoid the need for colectomy. This article will review the standard of care for the treatment of mild, moderate, and severe ulcerative colitis. The efficacy, optimal usage, and adverse events profile of agents such as 5-aminosalicylates, corticosteroids, azathioprine, and cyclosporine will be discussed and an algorithm for their use will be developed. Alternative and experimental therapies such as monoclonal antibodies, probiotics, and heparin will also be addressed.
Keywords: Ulcerative colitis, medical therapy, cyclosporine
Ulcerative colitis (UC) is a chronic inflammatory disease of the colon that affects up to 12 per 100,000 people in Western countries.1 The incidence may be increasing in developing nations but is more frequent in Caucasians and people of Jewish descent. Although there is an increase in families, the genetic trend is not as strong as in Crohn's disease.2 The peak age of incidence is in persons between 15 and 30 years old.1 Protective factors against the development of UC include cigarette smoking3 and appendectomy.4
The distribution of disease at diagnosis varies widely. Estimates from a Norwegian study report proctitis in 32% of patients, left-sided colitis in 33%, and more extensive colitis in 35%.5 Thirty-nine percent of patients with proctosigmoiditis can expect extension of their disease6 and 90% of all UC cases can expect a relapsing course.7 Approximately 4 to 9% will require colectomy in the year of diagnosis,5,6 with a subsequent risk of colectomy at 1% per year.7 The majority of UC patients will require medical therapy chronically throughout their lifetime; therefore, an understanding of the appropriate use of these agents is important for the physician caring for these patients.
Prior to initiating therapy, a patient must be evaluated for extent and severity of disease. Extent of disease is best assessed by colonoscopy with biopsy of grossly affected as well as unaffected areas. If disease is distal to the splenic flexure, topical therapy such as suppositories and enemas may be the first choice. For more extensive disease, oral agents or a combination of oral and topical agents are indicated. The severity of disease can be assessed by the Truelove and Witts score8 (Table 1). This article will focus on medications for induction and maintenance of remission in mild to moderate UC as well as colectomy-sparing therapy for severe colitis.
Table 1.
Truelove and Witts' Criteria for Evaluating the Severity of Ulcerative Colitis8
| Variable | Mild Disease | Severe Disease | Fulminant Disease |
|---|---|---|---|
| Moderate disease includes features of both mild and severe disease. | |||
| Stools (number/day) | < 4 | > 6 | > 10 |
| Blood in stool | Intermittent | Frequent | Continuous |
| Temperature (°C) | Normal | > 37.5° | > 37.5° |
| Pulse (beats/minute) | Normal | > 90 | > 90 |
| Hemoglobin | Normal | < 75% of normal value | Transfusion required |
| Erythrocyte sedimentation rate (mm/hr) | ≤30 | >30 | >30 |
| Colonic Features on x-ray | Air, edematous wall, thumbprinting | Dilatation | |
| Clinical signs | Abdominal tenderness | Abdominal distention and tenderness | |
AMINOSALICYLATES
Sulfasalazine and 5-aminosalicylate (5-ASA) drugs are the first line in drug therapy for the treatment of mild to moderate UC. Table 2 summarizes the available agents. Type and dose of therapy are determined by location and severity of disease. For distal disease, topical therapy is the most effective method of delivery. By metanalysis, rectally administered 5-ASA is superior to placebo and rectal corticosteroids for induction and maintenance of remission in distal UC.9 If the patient has proctitis, 5-ASA suppositories at a dose of 500 mg twice daily will induce and maintain remission.10,11 For disease up to the splenic flexure, 5-ASA enemas are effective for induction and maintenance of remission in doses of 2 to 4 g per enema.12,13
Table 2.
5-ASA Preparations
| Generic Name | Proprietary Names | 5-ASA Delivery Mechanism | Sites of Delivery | Daily Dose, Range |
|---|---|---|---|---|
| Mesalamine | Rowasa Salofalk | Enema suspension | Left colon and rectum | 1-4 g |
| Mesalamine | Canasa | Suppository | Rectum | 0.5-1.5 g |
| Mesalamine | Asacol | Eudragit-S coated tablets (release at pH > 7 | Terminal ileum, colon | 1.6-4.8 g |
| Mesalamine | Salofalk, Mesasal, Claversal | Mesalamine in sodium/glycerine buffer coated with Eudragit-L (release at pH > 6) | Distal jejunum, proximal ileum | 1.5-4 g |
| Mesalamine | Pentasa | Ethylcellulose coated microgranules (time and pH dependant release) | Entire small bowel, colon | 2-4 g |
| Sulfasalazine | Azulfidine | 5-ASA azo-bound to sulfapyridine | Colon | 1-4 g |
| Olsalazine | Dipentum | 5-ASA dimer linked by azo-bond | Colon | 1-3 g |
| Balsalazide | Colazaal | 5-ASA azo-bound to inert carrier | Colon | 2-6.75 g |
For more extensive disease, multiple oral 5-ASA preparations are available. Sulfasalazine was the first 5-ASA agent found to be effective in UC.14 Placebo-controlled trials have shown that sulfasalazine is effective in inducing15,16 and maintaining17,18 remission in mild to moderate UC. However, its use is limited by high rates of intolerance among patients. Side effects can include headache, abdominal pain, nausea, vomiting, skin rash, fever, hepatitis, hematologic abnormalities, folate deficiency, pancreatitis, systemic lupus erythematosus, and male infertility.19 Sulfasalazine should always be given with folate 1 mg daily and is contraindicated in men attempting conception.20
Sulfasalazine is a combination of 5-ASA azo-bound to the antibiotic sulfapyridine. It is the 5-ASA component that is the therapeutically active compound and the sulfapyridine moiety that is the cause of many of the side effects.21,22,23 This finding led to the development of alternative 5-ASA delivery systems for the treatment of UC and the discovery that it is the overall dose of mesalamine given, rather than the delivery system, that determines efficacy. Oral mesalamine agents with delayed- (Asacol) or timed-release (Pentasa) formulations at doses of 1.6 to 4.8 g/day are effective in inducing remission in mildly to moderately active UC.24,25,26 Doses of 0.8 to 4.8 g/day are effective in maintaining remission. Combination therapy, with oral mesalamine 2.4 g/day and rectal mesalamine 4 g/day, is more effective than either therapy alone.27 However, this may simply be a reflection of the overall dose of mesalamine received by the patient.
Olsalazine and balsalazide are 5-ASA agents that have diazo bonds, which are released by colonic bacteria. Olsalazine is effective for induction28,29 and maintenance30,31 of remission in UC, but its use is limited by worsened diarrhea.32 Balsalazide is composed of a 5-ASA linked to an inert carrier molecule. Although one study did show significant efficacy of balsalazide over an equivalent dose of mesalamine,33 other studies have shown an efficacy equal to sulfasalazine34 and mesalamine35,36 for induction of remission in mild to moderate UC.
Though 5-ASA agents are considered safe, some toxicity can be seen. Aside from the complications attributed to sulfasalazine above, the most frequently reported side effects of 5-ASA agents include dizziness, fever, headache, abdominal pain, nausea, and rash.25,26 Rare but serious adverse events include pulmonary toxicity, pericarditis, hepatitis, pancreatitis, aplastic anemia, leukopenia, and thrombocytopenia.37,38,39,40 Though interstitial nephritis has been reported,41 the frequency of renal insufficiency was low in large safety and pharmacovigilance databases for Asacol and Pentasa.42,43 Finally, a minority of patients will experience worsening diarrhea and abdominal pain due to a hypersensitivity reaction to 5-aminosalicylate.43a
In summary, 5-ASA agents are safe and effective for the induction and maintenance of remission in mild to moderate UC. Its use in severe colitis is not well studied. Data support the concept that the optimum use of aminosalicylates in active UC demands the highest tolerated dose, whether administered orally, rectally, or in combination. In quiescent disease, lower doses may be more tolerable to the patient and are less costly, although again, there is the general theme of dose response.
CORTICOSTEROIDS
The discovery that corticosteroids were effective in UC had a significant positive impact on a disease with a previous high mortality. Mortality rates dropped from a high of 61%44 to 4 to 7%.45 However, today the side effects of corticosteroids make it a less desirable though sometimes unavoidable agent in the therapy of UC.
In a population-based study in Olmstead County, Minnesota, 34% of UC patients required corticosteroids at some point in their disease course.46 Therapy with corticosteroids resulted in complete remission in 54%, partial remission in 30%, and no response in 16%. At 1 year from initiation of corticosteroid therapy, prolonged response without steroids or surgery was seen in 49%, corticosteroid dependence in 22%, and surgery in 29%.
Resistance to corticosteroids is seen in 16 to 20% of patients.46,47 Several potential mechanisms for resistance to corticosteroid therapy in patients with inflammatory bowel disease (IBD) have been described, including an increase in the expression of glucocorticoid receptor β48 and increased expression of the multidrug resistance-1 gene (MDR-1). The latter results in an increased expression of the membrane-based drug efflux pump P-glycoprotein 170 that pumps corticosteroids out of cells, thus lowering the intracellular concentration.49
Corticosteroids can be administered as oral (cortisone, prednisone, prednisolone, budesonide), intravenous (prednisolone, methylprednisolone, corticotropin), or rectal (beclomethasone, tixicortol, budesonide, prednisolone metasulfobenzoate) formulations. Truelove and Witts reported the efficacy of cortisone 100 mg per day in UC in 1955.8 Baron and colleagues then reported that 40 mg of prednisone was more effective than 20 mg and equally effective as 60 mg, but with fewer side effects.50 Finally, single daily dosing of prednisone 40 mg daily was equally effective as 10 mg four times a day.51 It is from these early studies that the current maxim of prednisone 40 mg per day for moderate to severe UC originated. No maintenance benefit of corticosteroids in UC has been found.52
Rectal corticosteroids are effective in left-sided UC. They provide quick relief for patients with tenesmus and bleeding. Rectal hydrocortisone 100 mg53 and prednisolone 10 mg54 have been proven effective by controlled trials for induction of remission, but not for maintenance.55 Budesonide enemas, which have minimal systemic absorption, are also effective for induction but not for maintenance of remission in left-sided UC.56 However, by metanalysis, topical corticosteroids were not as effective as topical mesalamine therapies for ulcerative proctitis and left-sided UC.57 Also, rectal corticosteroids are well absorbed and can result in suppression of the adrenal axis.58
Prednisone and prednisolone are well absorbed after oral administration and bioavailability is high, averaging over 70%. However, the absorption may be decreased in patients with severe UC in whom oral administration of prednisolone resulted in a lower peak plasma concentration and a slower rate of decrease in the plasma concentration compared with healthy volunteers.59 Patients with severe UC who receive 60 mg/day of intravenous prednisolone have a 73% response rate in 5 days.60 Some patients are slower responders and will require 7 to 10 days to respond. No controlled trials have addressed the effectiveness of single, multiple, or continuous infusion of corticosteroids in severe UC.61 Intramuscular corticotropin (adrenal corticotropin hormone, ACTH) at 80 U/day showed a similar benefit to cortisone 200 mg/day in patients with active UC.55 In severe UC, corticotropin 80 to 120 U/day was similar to hydrocortisone 300 to 400 mg/day.62,63,64 However, some deaths were reported in IV ACTH due to adrenocortical necrosis.64
Corticosteroid toxicity is frequent and often results in resistance on the part of patients to reinitiate therapy if they have used it before. Short-term toxicities observed include moon face (47%), acne (30%), infection (27%), ecchymoses (17%), hypertension (15%), hirsutism (7%), petechial bleeding (6%), and striae (6%). Prolonged corticosteroid therapy can result in multiple serious side effects including hypertension, new onset diabetes mellitus, infection, osteonecrosis, steroid associated osteoporosis, myopathy, psychosis, cataracts, and glaucoma.65,66,67 Table 3 lists potential side effects of corticosteroid therapy.
Table 3.
Adverse Effects Associated with Systemic Corticosteroid Therapy
| Adapted from Yang and Lichtenstein163 with permission. | |
| Cutaneous | Atrophy, striae, vascular effects, purpura, alopecia, pigmentation, acne, easy bruising |
| Cardiovascular | Hypertension, edema, atherosclerosis |
| Gastrointestinal | Nausea, vomiting, intestinal perforation, pancreatitis, esophagitis |
| Gynecological/obstetrical | Amenorrhea, gestational diabetes, adrenal suppression of infant |
| Neuropsychiatric | Psychosis, peripheral neuropathy, pseudotumor cerebri, depression/mood disorders, impaired cognitive function, seizures, insomnia, irritability |
| Metabolic | Hyperglycemia, hyperlipidemia, obesity, hypocalcemia, hypokalemia, buffalo hump |
| Musculoskeletal | Osteoporosis/osteopenia, aseptic necrosis, growth retardation, muscle atrophy, myopathy |
| Hematological | Leukocytosis, lymphopenia, eosinophenia, infection, immunosuppression, impaired fibroplasia, decreased mitotic rate |
| Ophthalmalogical | Cataracts, glaucoma, infection, exophthalmoses, hemorrhage |
| Endocrine | Hypothalamic-pituitary-adrenal axis suppression, hirsutism, moon facies |
| Pediatric | Growth retardation |
For moderate to severe UC, the preferred initial prednisone dose is 40 mg/day administered as a single dose. The optimal tapering strategy has not been determined, but experienced clinicians will typically treat the patient with prednisone 40 mg/day for 2 to 4 weeks, then taper by 5 mg/week to a daily dose of 20 mg/day, then slow the taper to 2.5 mg/week until prednisone is discontinued. For severe UC, requiring hospitalization, hydrocortisone 300 to 400 mg/day or methylprednisolone 40 to 60 mg/day is used. Five to seven days are required prior to determining whether the patient has failed steroids.
ANTIBIOTICS
The lack of efficacy of antibiotics in the treatment of UC and Crohn's disease is somewhat surprising given the presumed role of bacteria in the etiology of IBD. One placebo-controlled trial of ciprofloxacin in moderately active UC showed benefit68 while another was negative.69 The addition of intravenous ciprofloxacin to steroids in severe UC was also not of benefit.70 Oral tobramycin had short-term efficacy71 in UC but could not maintain remission.72 In acute severe UC, the combination of tobramycin and metronidazole,73 oral vancomycin alone,74 or intravenous metronidazole alone75 were not of added benefit to corticosteroids. Finally, in a small placebo-controlled trial, rifaximin, a nonabsorbed, broad-spectrum antibiotic, was not statistically better than placebo in overall clinical outcome in patients with steroid-refractory severe UC, but did have a significant reduction in stool frequency, rectal bleeding, and sigmoidoscopic score compared with placebo.76 Larger trials are underway.
Antibiotics should not be used without evidence of infection in patients with mild to moderate UC. Although evidence does not support their use in severe UC, in clinical practice the hospitalized patient may receive antibiotics as prophylaxis against bacterial translocation in the severely inflamed colon.
PROBIOTICS
Probiotics are live nonpathogenic organisms that confer health benefits by improving the microbial balance. While the formulation VSL3 has shown clear benefit for prevention of pouchitis after ileal-pouch surgery77 and maintenance of remission in chronic pouchitis,78 their benefit and that of other probiotics formulations in UC are still to be proven. Two small controlled studies have shown that E. coli Nissle is effective for maintenance of remission in UC.79,80 An open label trial of VSL3 in mildly to moderately active UC demonstrated a remission rate of 63%. However, patients were on other agents such as mesalamine and steroids.81 Larger controlled trials are needed to prove efficacy in both induction and maintenance of remission in UC.
NICOTINE
Nonsmokers and former smokers have higher rates of UC than current smokers.3 Also, smokers with UC who stop smoking experience increased severity of disease.82 The mechanism of this effect is thought to be due to nicotine, but is not completely elucidated.83,84 Placebo-controlled trials of transdermal nicotine patches demonstrated efficacy in achieving clinical remission or improvement at doses of 25 mg/24 hours85 and 22 mg/24 hours.86 However, it was not effective for maintenance,87 although an uncontrolled study suggested that patients who are treated with transdermal nicotine maintain their response longer than those treated with corticosteroids.88 Nicotine enemas also demonstrated benefit in uncontrolled trials.89,90 The major drawback of nicotine use is the high percentage of side effects, especially in patients who have never smoked before. These side effects include skin irritation, lightheadedness, nausea, vomiting, diaphoresis, central nervous system disturbances, and insomnia.84
IMMUNOSUPPRESSANTS
While 5-ASA agents are the first line for induction and maintenance of remission in mild to moderate UC and steroids are used for induction of remission in moderate to severe UC, immune modifier drugs are used to induce remission in steroid-dependent or steroid-refractory disease, maintain remission in those patients for whom 5-ASA agents are inadequate, and as salvage therapy in severe disease refractory to steroid therapy.
Azathioprine/6-Mercaptopurine
6-mercaptopurine (6-MP) and its prodrug azathioprine (AZA) are purine antimetabolite drugs demonstrated to be effective for the induction and maintenance of remission in UC and have proven steroid-sparing effects. The efficacy of 6-MP was recognized as early as 1962 in a case report by Bean.91 Though some controlled studies of AZA versus placebo and AZA versus sulfasalazine in the treatment of acute attacks of colitis found no significant benefit,89,92 others found that AZA use resulted in improved disease activity, a decreased need for steroids,93 and prolonged rates of remission.94 Multiple uncontrolled studies confirmed the benefits of AZA/6-MP.95,96,97,98,99
Effective doses of AZA are 2.0 to 3.0 mg/kg/day and of 6-MP are 1.0 to 1.5 mg/kg/day and may take up to 17 weeks to take complete effect.100 Though some physicians begin at low doses and titrate upwards, our practice is to begin at full dose with careful monitoring of the compete blood count. There is no role for intravenous loading of AZA in severe UC.101 Thiopurine S-methyltransferase (TPMT) phenotype or genotype can aid in determining safety and optimal dosage of AZA/6-MP. Low to intermediate levels of TPMT are associated with leukopenia in rheumatoid arthritis102 and with Crohn's disease.103 Based on these observations, it is recommended that patients with normal TPMT activity receive standard doses of AZA or 6-MP. Patients with intermediate activity should receive 50% of the standard dose and those who have no TPMT activity should not be treated with the drug.104 The use of metabolite levels (6-TGN [thioguanine nucleotides] and 6-MMP [6-methylmercaptopurine]) to gauge optimal dosing of AZA/6-MP is controversial. Though two studies supported its use,105,106 three others failed to demonstrate a consistent relationship between clinical efficacy and erythrocyte 6-TGN concentrations.107,108,109
Allergic reactions occur in 5% of patients taking AZA or 6-MP and include pancreatitis, fever, rash, malaise, nausea, diarrhea, and some cases of hepatitis.110 Nonallergic reactions include bone marrow suppression leading to leukopenia, anemia or thrombocytopenia, opportunistic infection, and hepatitis. Lymphoma does not appear to be increased above what is expected in IBD,110,111,112 though there may be an increase in Epstein-Barr virus-associated lymphomas in patients treated with AZA/6-MP.113
Methotrexate
Methotrexate (MTX) has demonstrated benefit for the induction and maintenance of remission in Crohn's disease114,115; however, its benefit in UC is not well established. Uncontrolled data have shown response in small series of patients with UC.116,117,118 The only controlled trial in UC was by Oren and associates,119 which compared oral MTX 12.5 mg/week with placebo in 67 patients with chronic active UC. No difference was found between the MTX and placebo group in remission and relapse rates. A recent study reported on patients with steroid-dependent or steroid-resistant active UC.120 Ten patients were intolerant or resistant to AZA and were switched to MTX 12.5 mg IM/ week. Six of 10 (60%) achieved clinical remission, 40% achieved clinical response, and 20% subsequently relapsed. Available data suggest that AZA/6-MP should be the first choice for maintenance and steroid sparing in UC, but MTX can be tried in those who are intolerant or resistant to AZA/6-MP. Our usual starting dose is 25 mg SQ/week, though once remission is achieved, 15 mg SQ/week can be used for maintenance.
MTX should always be given with folic acid 1 mg per day. Use in patients who are diabetic, obese, use excessive alcohol, or have known liver abnormalities is contraindicated. MTX is teratogenic and should not be used in men or women attempting conception. Increased serum transaminases and hypersensitivity reactions such as rash and pneumonitis can sometimes be seen.
Cyclosporine
Cyclosporine (CSA) is a calcineurin inhibitor that is used as salvage therapy for induction of remission in severe, steroid-refractory UC that would otherwise require colectomy. There are four randomized trials that have demonstrated the efficacy of CSA in severe UC. The first, by Lichtiger et al, found that 9/11 (82%) steroid-refractory UC patients had clinical response with 4 mg/kg/day of CSA in combination with intravenous steroids, versus none of placebo-treated patients on intravenous steroids alone.121 Two other controlled studies suggested that CSA alone at a dose of 4 mg/kg/day without steroids is effective in inducing remission in severe UC.122,123 Finally, a study by Van Assche and colleagues found that 2 mg/kg/day of CSA is equivalent to 4 mg/kg/day in achieving response in severe UC.124
Patients who respond to 4 mg/kg/day of IV CSA are continued on the drug for 7 to 10 days. The target whole blood CSA level is 300 to 350 ng/ml for the 4 mg/kg/day dose or 150 to 250 ng/ml for the 2 mg/kg/day dose. They are then converted to oral CSA at a dose of 8 mg/kg/day or twice the IV dose in hospital.125 The desired CSA level on oral dose is 150 to 300 ng/ml. Unfortunately, 45% of patients on oral CSA alone will require colectomy at 6 months.126 This can be decreased to 20% by the addition of 6-MP/AZA at discharge from the hospital.126 Patients are also continued on prednisone, which is tapered during outpatient follow-up. This regimen of triple immunosuppressive therapy with CSA, 6-MP/AZA, and prednisone can lead to significant infectious complications. For this reason, trimethoprim/sulfamethoxazole is added for prophylaxis. One uncontrolled study suggested that patients responding to IV CSA can be started on oral AZA without oral CSA, and prednisone can be tapered accordingly127; however, the colectomy rate was 41%.
An oral, microemulsion form of CSA (Neoral) has been developed which has increased oral bioavailability and improved absorption from the small bowel.128 The pharmokinetic parameters of CSA microemulsion in patients with IBD appear to be similar to those of healthy volunteers.129 Three small series have described efficacy of oral microemulsion CSA in severe UC,130,131,132 though larger controlled trials are needed.
In a report from Mount Sinai Hospital on 111 IBD patients treated with CSA, the most frequent adverse events were paresthesias (51%), hypertension (43%), hypertrichosis (27%), renal insufficiency (23%), infections (20%), gingival hyperplasia (4%), seizures (3%), death (2%), and anaphylaxis (1%).133 In a similar report from the University of Chicago on 74 patients with IBD treated with CSA, 54% experienced adverse events including severe events such as Pneumocystis carinii pneumonia in two patients, abdominal abscess, grand mal seizure, mycotic aneurysm, and renal insufficiency.126 Table 4 lists drug interactions of CSA and tacrolimus.
Table 4.
Potential Drug Interactions of Cyclosporine and Tacrolimus
| Adapted from Kornbluth, et al125 with permission. | |
| Inhibition of cytochrome P450 | Calcium channel blockers |
| Increased CSA levels | Bromocriptine |
| Metoclopramide | |
| Imidazoles | |
| Macrolide antibiotics | |
| Methylprednisolone | |
| Protease inhibitors | |
| Grapefruit juice | |
| Induction of cytochrome P450 | Rifampin |
| Decrease cyclosporine levels | Phenobarbital |
| Phenytoin | |
| Carbamazepine | |
| Reverse transcriptase inhibitors | |
| St. John's wort | |
CSA in severe UC is definitely effective, but its side-effect profile and tangible long-term failure rate must be discussed in depth with the patient debating colectomy versus salvage medical therapy. Also, patients who are not tolerant of AZA/6-MP are not good candidates for CSA therapy, as CSA alone has a high colectomy rate over time. Side effects may be decreased by using lower doses of IV CSA at 2 mg/kg/day, using antibiotic prophylaxis, or avoiding triple therapy with oral CSA, AZA and prednisone.
Tacrolimus
Tacrolimus is a calcineurin inhibitor like CSA. Controlled trials in severe UC have not been conducted to date, but multiple case series suggest efficacy. The first study was by Bousvaros et al134 and described a 69% clinical response rate in 13 patients with steroid-refractory UC. However, at 1 year, only 38% of patients avoided colectomy. Three case series note salvage therapy with tacrolimus in steroid-resistant or steroid-dependent UC135,136 as well as in a patient with toxic megacolon.137 One trial compared intravenous to oral tacrolimus in 38 patients with refractory UC.138 Oral and IV dosing was equivalent. Eighteen of 38 patients (47%) improved within 14 days. Thirty-five of 38 patients (92%) avoided colectomy at 28 days, but at 2 years, the colectomy rate was 50%.
The dose of oral tacrolimus is 0.1 to 0.2 mg/kg/day given in divided doses twice daily. The serum trough levels are 4 to 6 ng/ml. Patients should be monitored closely for evidence of infections and trimethoprim/sulfamethoxazole prophylaxis should be used. Side effects include transient renal insufficiency, tremor, paresthesias, hyperkalemia, and hypertension.135 These often resolve with lowering of the dose.
Infliximab
Infliximab is a chimeric monoclonal antibody to tumor necrosis factor-α (TNF), a key inflammatory cytokine. While infliximab has made a dramatic impact on the treatment of Crohn's disease,139 its role in UC is not clear. Sands and associates reported 11 patients in a controlled trial of infliximab in severe, steroid-refractory UC.140 Four of eight patients (50%) who received infliximab had a clinical response, although one subsequently required colectomy. Two small case series report response in severe UC,141,142 but a larger randomized controlled trial was negative.143 In this latter trial, patients with severe, steroid-refractory UC were randomized to infliximab 5 mg/kg or placebo at weeks 0 and 2. After 6 weeks, remission was achieved in 8/22 infliximab patients and 6/20 placebo (not significant). A controlled trial of infliximab in nonsteroid-refractory patients144 randomized patients to either infliximab 5 mg/kg at 0, 2, and 6 weeks or intravenous prednisolone at 1.5 mg/kg daily for 2 weeks followed by a taper. Five of 6 patients receiving infliximab and 6/7 patients receiving steroids had a response. The two agents appear to be equivalent in this small study.
Clinical experience and a placebo-controlled trial143 suggest that infliximab is not effective in steroid-refractory UC. This is further supported by a retrospective analysis of 27 patients with active UC who received infliximab.145 While 44% of all UC patients achieved remission and 22% had a partial response, steroid-refractory patients were less likely to respond when compared with steroid-responsive patients (33% vs 83%; p = 0.026). Infliximab should not be used in severe, steroid-refractory UC. Evidence for its use in steroid-responsive disease is anecdotal at best and controlled studies are needed.
EXPERIMENTAL AGENTS
Heparin
Thrombotic events associated with UC and histologic evidence of microvascular thrombosis on colon biopsy suggested that anticoagulation may be an effective therapy for UC.146 Uncontrolled studies did find unfractionated heparin to be of benefit,147,148 but small controlled studies comparing unfractionated heparin to corticosteroids had mixed results.149,150 Larger, placebo-controlled trials of low-molecular-weight heparin in studies of 100 patients and 138 patients, respectively, found no significant benefit over placebo.151,152 Heparin is not effective for the treatment of UC but does appear to be safe with no increased risk of gastrointestinal bleeding, should the need for anticoagulation for other reasons arise in these patients.
BIOLOGICS: CYTOKINES AND ANTIBODIES
Investigational agents with preliminary reports of efficacy include vepolimomab (monoclonal antibody [mAb] to vascular adhesion protein-1)153; interleukin-2 (IL-2) antagonists such as basiliximab, which may increase response to steroids in steroid-resistant UC154; anti-CD3 antibodies, such as visilizumab, which has shown preliminary efficacy in steroid-resistant UC155; antibody to α4β7, such as MLN-02, which mediates recruitment of lymphocytes to the gut and demonstrated safety and efficacy in a controlled trial in patients with active UC156; and interferon-β, which showed some clinical response in patients with steroid-refractory UC in a phase II, placebo-controlled trial.157 Multiple other agents exist, but for all new agents, while preliminary results are exciting, randomized controlled trials are needed before widespread use is initiated.
LEUKOCYTAPHERESIS
One novel technique for the treatment of severe UC is leuckocytapheresis. Based on the theory that inflammation and damage to the colonic mucosa are caused by products of activated granulocytes, monocytes, and macrophages, an extracorporeal leukocytapheresis column (LCAP) was developed to remove these cells from the peripheral circulation, with reported efficacy in UC.158 A randomized controlled trial in 76 active UC patients reported that addition of LCAP to corticosteroids improved clinical response with a reduction in steroid dosage.159 These results were corroborated by a recent randomized trial from the same group showing that LCAP was more effective than sham perfusion (80% vs 33%; p < 0.05) in eliciting clinical response in patients with active UC.160
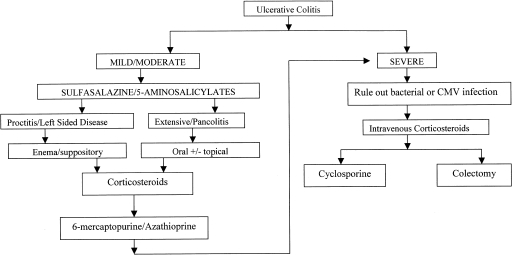
ALGORITHM FOR THE TREATMENT OF ULCERATIVE COLITIS
Figure 1 outlines the algorithm for the treatment of a flare of UC. When a patient presents with a flare, the diagnosis should be confirmed and the severity of the disease established. Colonoscopy with biopsy to confirm the diagnosis of UC and establish the extent of the disease should be performed in all new and established cases. A small bowel follow-through should be performed once at some point in the disease course to rule out the diagnosis of Crohn's disease. Stool studies should be sent in all acute flares to rule out superinfection with Clostridium difficile, bacteria, or ova and parasites. In a patient with severe UC, an unprepped flexible sigmoidoscopy rather than full colonoscopy should be performed with biopsies of the rectum for histology and viral culture. This will confirm the severity of the disease and will also rule out cytomegalovirus (CMV). One study reported that 36% of patients with steroid-refractory colitis had CMV on rectal specimens.161 The majority of these patients responded to antiviral therapy with foscarnet or ganciclovir. The severity of the disease can be determined by the Truelove and Witts score (Table 1).
Figure 1.
An algorithm for the medial management of mild, moderate, and severe ulcerative colitis. Progression along arrows is indicated if prior therapies fail.
For mild to moderate disease the first-line therapy is 5-ASA agents. If this is ineffective, steroids can be used to induce remission. If the patient cannot be tapered off the steroids or relapses after steroid withdrawal, immunosuppression with AZA/6-MP should be initiated for steroid-sparing and maintenance effects. In the patient with severe disease not responding to oral steroids, intravenous steroids are indicated in an inpatient setting. If there is no response after 5 to 7 days, CSA should be offered if the patient is an appropriate candidate. Patients who are intolerant of AZA/6-MP are not good candidates as colectomy rates are high in patients on CSA alone. Theoretically, MTX can be used instead, though its use in UC has less supportive evidence. Patient reluctance to use CSA or failure to respond to CSA would then lead to colectomy.
Aggressive medical therapy with immunosuppressants does not increase the risk of postoperative complications after colectomy and ileal pouch-anal anastomosis.162 Factors that predicted an increase in short-term complications were high-dose steroids and severe disease. Variations on this algorithm with experimental or anecdotal agents can be tried as long as the patient is fully informed and the physician is comfortable with these drugs. However, larger controlled trials are needed before widespread use can be adopted.
REFERENCES
- 1.Andres P G, Friedman L S. Epidemiology and the natural course of inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:255–281. doi: 10.1016/s0889-8553(05)70056-x. [DOI] [PubMed] [Google Scholar]
- 2.Halfvarson J, Bodin L, Tysk C, Lindberg E, Jarnerot G. Inflammatory bowel disease in a Swedish twin cohort: a long-term follow-up of concordance and clinical characteristics. Gastroenterology. 2003;124:1767–1773. doi: 10.1016/s0016-5085(03)00385-8. [DOI] [PubMed] [Google Scholar]
- 3.Harries A D, Baird A, Rhodes J. Non-smoking: a feature of ulcerative colitis. Br Med J (Clin Res Ed) 1982;284:706. doi: 10.1136/bmj.284.6317.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson R E, Olaison G, Tysk C, Ekbom A. Appendectomy and protection against ulcerative colitis. N Engl J Med. 2001;344:808–814. doi: 10.1056/NEJM200103153441104. [DOI] [PubMed] [Google Scholar]
- 5.Moum B, Vatn M H, Ekbom A, et al. Incidence of inflammatory bowel disease in southeastern Norway: evaluation of methods after 1 year of registration. Southeastern Norway IBD Study Group of Gastroenterologists. Digestion. 1995;56:377–381. doi: 10.1159/000201262. [DOI] [PubMed] [Google Scholar]
- 6.Langholz E, Munkholm P, Davidsen M, Binder V. Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology. 1994;107:3–11. doi: 10.1016/0016-5085(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 7.Langholz E, Munkholm P, Davidsen M, Nielsen O H, Binder V. Changes in extent of ulcerative colitis: a study on the course and prognostic factors. Scand J Gastroenterol. 1996;31:260–266. doi: 10.3109/00365529609004876. [DOI] [PubMed] [Google Scholar]
- 8.Truelove S C, Witts L. Cortisone in ulcerative colitis: final report on a therapeutic trial. BMJ. 1955;2:1041–1048. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall J K, Irvine E J. Rectal aminosalicylate therapy for distal ulcerative colitis: a meta-analysis. Aliment Pharmacol Ther. 1995;9:293–300. doi: 10.1111/j.1365-2036.1995.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 10.D'Arienzo A, Panarese A, D'Armiento F P, et al. 5-aminosalicylic acid suppositories in the maintenance of remission in idiopathic proctitis or proctosigmoiditis: a double-blind placebo-controlled clinical trial. Am J Gastroenterol. 1990;85:1079–1082. [PubMed] [Google Scholar]
- 11.Campieri M, De Franchis R, Bianchi Porro G, Ranzi T, Brunetti G, Barbara L. Mesalazine (5-aminosalicylic acid) suppositories in the treatment of ulcerative proctitis or distal proctosigmoiditis. A randomized controlled trial. Scand J Gastroenterol. 1990;25:663–668. doi: 10.3109/00365529008997590. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland L R, Martin F, Greer S, et al. 5-aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology. 1987;92:1894–1898. doi: 10.1016/0016-5085(87)90621-4. [DOI] [PubMed] [Google Scholar]
- 13.d'Albasio G, Trallori G, Ghetti A, et al. Intermittent therapy with high-dose 5-aminosalicylic acid enemas for maintaining remission in ulcerative proctosigmoiditis. Dis Colon Rectum. 1990;33:394–397. doi: 10.1007/BF02156265. [DOI] [PubMed] [Google Scholar]
- 14.Svartz N. Salazopyrine, a new sulfanilamide preparation. Acta Med Scand. 1942;110:557. [Google Scholar]
- 15.Dick A P, Grayson M, Carpenter R G, Petrie A. Controlled trial of sulphasalazine in the treatment of ulcerative colitis. Gut. 1964;50:437–442. doi: 10.1136/gut.5.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baron J H, Connell A, Lennard-Jones J E, Jones F A. Sulphasalzine and salicylazosulphadimidine in ulcerative colitis. Lancet. 1962;1:1094–1096. doi: 10.1016/s0140-6736(62)92080-9. [DOI] [PubMed] [Google Scholar]
- 17.Misiewicz J J, Lennard-Jones J E, Connell A M, Baron J H, Avery Jones F. Controlled trial of sulphasalazine in maintenance therapy for ulcerative colitis. Lancet. 1965;1:185–188. doi: 10.1016/s0140-6736(65)90973-6. [DOI] [PubMed] [Google Scholar]
- 18.Dissanayake A S, Truelove S C. A controlled therapeutic trial of long-term maintenance treatment of ulcerative colitis with sulphazalazine (Salazopyrin) Gut. 1973;14:923–926. doi: 10.1136/gut.14.12.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taffet S L, Das K M. Sulfasalazine. Adverse effects and desensitization. Dig Dis Sci. 1983;28:833–842. doi: 10.1007/BF01296907. [DOI] [PubMed] [Google Scholar]
- 20.O'Morain C, Smethurst P, Dore C J, Levi A J. Reversible male infertility due to sulphasalazine: studies in man and rat. Gut. 1984;25:1078–1084. doi: 10.1136/gut.25.10.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das K M, Eastwood M A, McManus J P, Sircus W. The metabolism of salicylazosulphapyridine in ulcerative colitis. I. The relationship between metabolites and the response to treatment in inpatients. Gut. 1973;14:631–641. doi: 10.1136/gut.14.8.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klotz U, Maier K, Fischer C, Heinkel K. Therapeutic efficacy of sulfasalazine and its metabolites in patients with ulcerative colitis and Crohn's disease. N Engl J Med. 1980;303:1499–1502. doi: 10.1056/NEJM198012253032602. [DOI] [PubMed] [Google Scholar]
- 23.Khan A K, Piris J, Truelove S C. An experiment to determine the active therapeutic moiety of sulphasalazine. Lancet. 1977;2:892–895. doi: 10.1016/s0140-6736(77)90831-5. [DOI] [PubMed] [Google Scholar]
- 24.Hanauer S, Schwartz J, Robinson M, et al. Mesalamine capsules for treatment of active ulcerative colitis: results of a controlled trial Pentasa Study Group. Am J Gastroenterol. 1993;88:1188–1197. [PubMed] [Google Scholar]
- 25.Sninsky C A, Cort D H, Shanahan F, et al. Oral mesalamine (Asacol) for mildly to moderately active ulcerative colitis. A multicenter study. Ann Intern Med. 1991;115:350–355. doi: 10.7326/0003-4819-115-5-350. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder K W, Tremaine W J, Ilstrup D M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 27.Safdi M, DeMicco M, Sninsky C, et al. A double-blind comparison of oral versus rectal mesalamine versus combination therapy in the treatment of distal ulcerative colitis. Am J Gastroenterol. 1997;92:1867–1871. [PubMed] [Google Scholar]
- 28.Rao S S, Dundas S A, Holdsworth C D, Cann P A, Palmer K R, Corbett C L. Olsalazine or sulphasalazine in first attacks of ulcerative colitis? A double-blind study. Gut. 1989;30:675–679. doi: 10.1136/gut.30.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willoughby C P, Cowan R E, Gould S R, Machell R J, Stewart J B. Double-blind comparison of olsalazine and sulphasalazine in active ulcerative colitis. Scand J Gastroenterol Suppl. 1988;148:40–44. doi: 10.3109/00365528809101546. [DOI] [PubMed] [Google Scholar]
- 30.Courtney M G, Nunes D P, Bergin C F, et al. Randomised comparison of olsalazine and mesalazine in prevention of relapses in ulcerative colitis. Lancet. 1992;339:1279–1281. doi: 10.1016/0140-6736(92)91601-4. [DOI] [PubMed] [Google Scholar]
- 31.Sandberg-Gertzen H, Jarnerot G, Kraaz W. Azodisal sodium in the treatment of ulcerative colitis. A study of tolerance and relapse-prevention properties. Gastroenterology. 1986;90:1024–1030. doi: 10.1016/0016-5085(86)90882-6. [DOI] [PubMed] [Google Scholar]
- 32.Meyers S, Sachar D B, Present D H, Janowitz H D. Olsalazine sodium in the treatment of ulcerative colitis among patients intolerant of sulfasalazine. A prospective, randomized, placebo-controlled, double-blind, dose-ranging clinical trial. Gastroenterology. 1987;93:1255–1262. doi: 10.1016/0016-5085(87)90253-8. [DOI] [PubMed] [Google Scholar]
- 33.Green J R, Lobo A J, Holdsworth C D, et al. Balsalazide is more effective and better tolerated than mesalamine in the treatment of acute ulcerative colitis. The Abacus Investigator Group. Gastroenterology. 1998;114:15–22. doi: 10.1016/s0016-5085(98)70627-4. [DOI] [PubMed] [Google Scholar]
- 34.Green J R, Mansfield J C, Gibson J A, Kerr G D, Thornton P C. A double-blind comparison of balsalazide, 6.75 g daily, and sulfasalazine, 3 g daily, in patients with newly diagnosed or relapsed active ulcerative colitis. Aliment Pharmacol Ther. 2002;16:61–68. doi: 10.1046/j.1365-2036.2002.01150.x. [DOI] [PubMed] [Google Scholar]
- 35.Levine D S, Riff D S, Pruitt R, et al. A randomized, double blind, dose-response comparison of balsalazide (6.75 g), balsalazide (2.25 g), and mesalamine (2.4 g) in the treatment of active, mild-to-moderate ulcerative colitis. Am J Gastroenterol. 2002;97:1398–1407. doi: 10.1111/j.1572-0241.2002.05781.x. [DOI] [PubMed] [Google Scholar]
- 36.Pruitt R, Hanson J, Safdi M, et al. Balsalazide is superior to mesalamine in the time to improvement of signs and symptoms of acute mild-to-moderate ulcerative colitis. Am J Gastroenterol. 2002;97:3078–3086. doi: 10.1111/j.1572-0241.2002.07103.x. [DOI] [PubMed] [Google Scholar]
- 37.Bitton A, Peppercorn M A, Hanrahan J P, Upton M P. Mesalamine-induced lung toxicity. Am J Gastroenterol. 1996;91:1039–1040. [PubMed] [Google Scholar]
- 38.Iaquinto G, Sorrentini I, Petillo F E, Berardesca G. Pleuropericarditis in a patient with ulcerative colitis in longstanding 5-aminosalicylic acid therapy. Ital J Gastroenterol. 1994;26:145–147. [PubMed] [Google Scholar]
- 39.Deltenre P, Berson A, Marcellin P, Degott C, Biour M, Pessayre D. Mesalazine (5-aminosalicylic acid) induced chronic hepatitis. Gut. 1999;44:886–888. doi: 10.1136/gut.44.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandez J, Sala M, Panes J, Feu F, Navarro S, Teres J. Acute pancreatitis after long-term 5-aminosalicylic acid therapy. Am J Gastroenterol. 1997;92:2302–2303. [PubMed] [Google Scholar]
- 41.Brouillard M, Gheerbrant J D, Gheysens Y, et al. Chronic interstitial nephritis and mesalazine: 3 new cases? [in French] Gastroenterol Clin Biol. 1998;22:724–726. [PubMed] [Google Scholar]
- 42.Hanauer S B, Verst-Brasch C, Regalli G. Renal safety of long-term mesalamine therapy in inflammatory bowel disease (IBD) Gastroenterology. 1997;112:A991. [Google Scholar]
- 43.Marteau P, Nelet F, Le Lu M, Devaux C. Adverse events in patients treated with 5-aminosalicyclic acid: 1993-1994 pharmacovigilance report for Pentasa in France. Aliment Pharmacol Ther. 1996;10:949–956. doi: 10.1046/j.1365-2036.1996.92264000.x. [DOI] [PubMed] [Google Scholar]
- 43a.Sturgeon J B, Bhatia P, Hermens D, Miner P B., Jr Exacerbation of chronic ulcerative colitis with mesalamine. Gastroenterology. 1995;108:1889–1893. doi: 10.1016/0016-5085(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 44.Edwards F, Truelove S C. The course and prognosis of ulcerative colitis. Gut. 1963;41:299–315. doi: 10.1136/gut.4.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ekbom A, Helmick C G, Zack M, Holmberg L, Adami H O. Survival and causes of death in patients with inflammatory bowel disease: a population-based study. Gastroenterology. 1992;103:954–960. doi: 10.1016/0016-5085(92)90029-x. [DOI] [PubMed] [Google Scholar]
- 46.Faubion W A, Jr, Loftus E V, Jr, Harmsen W S, Zinsmeister A R, Sandborn W J. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–260. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- 47.Munkholm P, Langholz E, Davidsen M, Binder V. Frequency of glucocorticoid resistance and dependency in Crohn's disease. Gut. 1994;35:360–362. doi: 10.1136/gut.35.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Honda M, Orii F, Ayabe T, et al. Expression of glucocorticoid receptor beta in lymphocytes of patients with glucocorticoid-resistant ulcerative colitis. Gastroenterology. 2000;118:859–866. doi: 10.1016/s0016-5085(00)70172-7. [DOI] [PubMed] [Google Scholar]
- 49.Farrell R J, Murphy A, Long A, et al. High multidrug resistance (P-glycoprotein 170) expression in inflammatory bowel disease patients who fail medical therapy. Gastroenterology. 2000;118:279–288. doi: 10.1016/s0016-5085(00)70210-1. [DOI] [PubMed] [Google Scholar]
- 50.Baron J H, Connell A M, Kanaghinis T G, Lennard-Jones J E, Jones A F. Out-patient treatment of ulcerative colitis. Comparison between three doses of oral prednisone. BMJ. 1962;5302:441–443. doi: 10.1136/bmj.2.5302.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Powell-Tuck J, Bown R L, Lennard-Jones J E. A comparison of oral prednisolone given as single or multiple daily doses for active proctocolitis. Scand J Gastroenterol. 1978;13:833–837. doi: 10.3109/00365527809182199. [DOI] [PubMed] [Google Scholar]
- 52.Lennard-Jones J E, Misiewicz J J, Connell A M, Baron J H, Avery Jones F. Prednisone as maintenance treatment for ulcerative colitis in remission. Lancet. 1965;191:188–189. doi: 10.1016/s0140-6736(65)90973-6. [DOI] [PubMed] [Google Scholar]
- 53.Watkinson G. Treatment of ulcerative colitis with topical hydrocortisone hemisuccinate sodium A controlled trial employing restricted sequential analysis. BMJ. 1958:1077–1082. doi: 10.1136/bmj.2.5104.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lennard-Jones J E, Baron J H, Connell A M, et al. A double blind controlled trial of prednisolone-21-phosphate suppositories in the treatment of idiopathic proctitis. Gut. 1962;3:207–210. doi: 10.1136/gut.3.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Truelove S. Treatment of ulcerative colitis with local hydrocortisone hemisuccinate sodium. A report on a controlled therapeutic trial. BMJ. 1958:1072–1077. doi: 10.1136/bmj.2.5104.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lindgren S, Lofberg R, Bergholm L, et al. Effect of budesonide enema on remission and relapse rate in distal ulcerative colitis and proctitis. Scand J Gastroenterol. 2002;37:705–710. doi: 10.1080/00365520212512. [DOI] [PubMed] [Google Scholar]
- 57.Cohen R D, Woseth D M, Thisted R A, Hanauer S B. A meta-analysis and overview of the literature on treatment options for left-sided ulcerative colitis and ulcerative proctitis. Am J Gastroenterol. 2000;95:1263–1276. doi: 10.1111/j.1572-0241.2000.01940.x. [DOI] [PubMed] [Google Scholar]
- 58.Farmer R G, Schumacher O P. Treatment of ulcerative colitis with hydrocortisone enemas. Comparison of absorption and clinical response with hydrocortisone alcohol and hydrocortisone acetate. Am J Gastroenterol. 1970;54:229–236. [PubMed] [Google Scholar]
- 59.Elliott P R, Powell-Tuck J, Gillespie P E, et al. Prednisolone absorption in acute colitis. Gut. 1980;21:49–51. doi: 10.1136/gut.21.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Truelove S C, Jewell D P. Intensive intravenous regimen for severe attacks of ulcerative colitis. Lancet. 1974;1:1067–1070. doi: 10.1016/s0140-6736(74)90552-2. [DOI] [PubMed] [Google Scholar]
- 61.Rizzello F, Gionchetti P, Venturi A, Campieri M. Medical treatment of severe ulcerative colitis. Aliment Pharmacol Ther. 2003;17(suppl 2):7–10. doi: 10.1046/j.1365-2036.17.s2.18.x. [DOI] [PubMed] [Google Scholar]
- 62.Meyers S, Sachar D B, Goldberg J D, Janowitz H D. Corticotropin versus hydrocortisone in the intravenous treatment of ulcerative colitis. A prospective, randomized, double-blind clinical trial. Gastroenterology. 1983;85:351–357. [PubMed] [Google Scholar]
- 63.Powell-Tuck J, Buckell N A, Lennard-Jones J E. A controlled comparison of corticotropin and hydrocortisone in the treatment of severe proctocolitis. Scand J Gastroenterol. 1977;12:971–975. doi: 10.3109/00365527709181359. [DOI] [PubMed] [Google Scholar]
- 64.Kaplan H P, Portnoy B, Binder H J, Amatruda T, Spiro H. A controlled evaluation of intravenous adrenocorticotropic hormone and hydrocortisone in the treatment of acute colitis. Gastroenterology. 1975;69:91–95. [PubMed] [Google Scholar]
- 65.Talar-Williams C, Sneller M C. Complications of corticosteroid therapy. Eur Arch Otorhinolaryngol. 1994;251:131–136. doi: 10.1007/BF00181824. [DOI] [PubMed] [Google Scholar]
- 66.Singleton J W, Law D H, Kelley M L, Jr, Mekhjian H S, Sturdevant R A. National Cooperative Crohn's Disease Study: adverse reactions to study drugs. Gastroenterology. 1979;77:870–882. [PubMed] [Google Scholar]
- 67.Kusunoki M, Moeslein G, Shoji Y, et al. Steroid complications in patients with ulcerative colitis. Dis Colon Rectum. 1992;35:1003–1009. doi: 10.1007/BF02253508. [DOI] [PubMed] [Google Scholar]
- 68.Turunen U M, Farkkila M A, Hakala K, et al. Long-term treatment of ulcerative colitis with ciprofloxacin: a prospective, double-blind, placebo-controlled study. Gastroenterology. 1998;115:1072–1078. doi: 10.1016/s0016-5085(98)70076-9. [DOI] [PubMed] [Google Scholar]
- 69.Mantzaris G J, Archavlis E, Christoforidis P, et al. A prospective randomized controlled trial of oral ciprofloxacin in acute ulcerative colitis. Am J Gastroenterol. 1997;92:454–456. [PubMed] [Google Scholar]
- 70.Mantzaris G J, Petraki K, Archavlis E, et al. A prospective randomized controlled trial of intravenous ciprofloxacin as an adjunct to corticosteroids in acute, severe ulcerative colitis. Scand J Gastroenterol. 2001;36:971–974. doi: 10.1080/003655201750305503. [DOI] [PubMed] [Google Scholar]
- 71.Burke D A, Axon A T, Clayden S A, Dixon M F, Johnston D, Lacey R W. The efficacy of tobramycin in the treatment of ulcerative colitis. Aliment Pharmacol Ther. 1990;4:123–129. doi: 10.1111/j.1365-2036.1990.tb00456.x. [DOI] [PubMed] [Google Scholar]
- 72.Lobo A J, Burke D A, Sobala G M, Axon A T. Oral tobramycin in ulcerative colitis: effect on maintenance of remission. Aliment Pharmacol Ther. 1993;7:155–158. doi: 10.1111/j.1365-2036.1993.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 73.Mantzaris G J, Hatzis A, Kontogiannis P, Triadaphyllou G. Intravenous tobramycin and metronidazole as an adjunct to corticosteroids in acute, severe ulcerative colitis. Am J Gastroenterol. 1994;89:43–46. [PubMed] [Google Scholar]
- 74.Dickinson R J, O'Connor H J, Pinder I, Hamilton I, Johnston D, Axon A T. Double blind controlled trial of oral vancomycin as adjunctive treatment in acute exacerbations of idiopathic colitis. Gut. 1985;26:1380–1384. doi: 10.1136/gut.26.12.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chapman R W, Selby W S, Jewell D P. Controlled trial of intravenous metronidazole as an adjunct to corticosteroids in severe ulcerative colitis. Gut. 1986;27:1210–1212. doi: 10.1136/gut.27.10.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gionchetti P, Rizzello F, Ferrieri A, et al. Rifaximin in patients with moderate or severe ulcerative colitis refractory to steroid-treatment: a double-blind, placebo-controlled trial. Dig Dis Sci. 1999;44:1220–1221. doi: 10.1023/a:1026648812439. [DOI] [PubMed] [Google Scholar]
- 77.Gionchetti P, Rizzello F, Helwig U, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–1209. doi: 10.1016/s0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 78.Gionchetti P, Rizzello F, Venturi A, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–309. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 79.Rembacken B J, Snelling A M, Hawkey P M, Chalmers D M, Axon A T. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354:635–639. doi: 10.1016/s0140-6736(98)06343-0. [DOI] [PubMed] [Google Scholar]
- 80.Kruis W, Schutz E, Fric P, Fixa B, Judmaier G, Stolte M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 1997;11:853–858. doi: 10.1046/j.1365-2036.1997.00225.x. [DOI] [PubMed] [Google Scholar]
- 81.Fedorak R, Gionchetti P, Campieri M, Madsen K, Isaacs K. VSL3 probiotic mixture induces remission in patients with active ulcerative colitis. Gastroenterology. 2003;124:A-377. doi: 10.1111/j.1572-0241.2005.41794.x. [DOI] [PubMed] [Google Scholar]
- 82.Beaugerie L, Massot N, Carbonnel F, Cattan S, Gendre J P, Cosnes J. Impact of cessation of smoking on the course of ulcerative colitis. Am J Gastroenterol. 2001;96:2113–2116. doi: 10.1111/j.1572-0241.2001.03944.x. [DOI] [PubMed] [Google Scholar]
- 83.Green J T, Richardson C, Marshall R W, et al. Nitric oxide mediates a therapeutic effect of nicotine in ulcerative colitis. Aliment Pharmacol Ther. 2000;14:1429–1434. doi: 10.1046/j.1365-2036.2000.00847.x. [DOI] [PubMed] [Google Scholar]
- 84.Sandborn W J. Nicotine therapy for ulcerative colitis: a review of rationale, mechanisms, pharmacology, and clinical results. Am J Gastroenterol. 1999;94:1161–1171. doi: 10.1111/j.1572-0241.1999.01059.x. [DOI] [PubMed] [Google Scholar]
- 85.Pullan R D, Rhodes J, Ganesh S, et al. Transdermal nicotine for active ulcerative colitis. N Engl J Med. 1994;330:811–815. doi: 10.1056/NEJM199403243301202. [DOI] [PubMed] [Google Scholar]
- 86.Sandborn W J, Tremaine W J, Offord K P, et al. Transdermal nicotine for mildly to moderately active ulcerative colitis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1997;126:364–371. doi: 10.7326/0003-4819-126-5-199703010-00004. [DOI] [PubMed] [Google Scholar]
- 87.Thomas G A, Rhodes J, Mani V, et al. Transdermal nicotine as maintenance therapy for ulcerative colitis. N Engl J Med. 1995;332:988–992. doi: 10.1056/NEJM199504133321503. [DOI] [PubMed] [Google Scholar]
- 88.Guslandi M. Long-term effects of a single course of nicotine treatment in acute ulcerative colitis: remission maintenance in a 12-month follow-up study. Int J Colorectal Dis. 1999;14:261–262. doi: 10.1007/s003840050221. [DOI] [PubMed] [Google Scholar]
- 89.Green J T, Thomas G A, Rhodes J, et al. Nicotine enemas for active ulcerative colitis—a pilot study. Aliment Pharmacol Ther. 1997;11:859–863. doi: 10.1046/j.1365-2036.1997.00220.x. [DOI] [PubMed] [Google Scholar]
- 90.Sandborn W J, Tremaine W J, Leighton J A, et al. Nicotine tartrate liquid enemas for mildly to moderately active left-sided ulcerative colitis unresponsive to first-line therapy: a pilot study. Aliment Pharmacol Ther. 1997;11:663–671. doi: 10.1046/j.1365-2036.1997.00208.x. [DOI] [PubMed] [Google Scholar]
- 91.Bean R. The treatment of chronic ulcerative colitis with 6-mercaptopurine. Med J Aust. 1962;49:592–593. [PubMed] [Google Scholar]
- 92.Jewell D P, Truelove S C. Azathioprine in ulcerative colitis: final report on controlled therapeutic trial. BMJ. 1974;4:627–630. doi: 10.1136/bmj.4.5945.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kirk A P, Lennard-Jones J E. Controlled trial of azathioprine in chronic ulcerative colitis. Br Med J (Clin Res Ed) 1982;284:1291–1292. doi: 10.1136/bmj.284.6325.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hawthorne A B, Logan R F, Hawkey C J, et al. Randomised controlled trial of azathioprine withdrawal in ulcerative colitis. BMJ. 1992;305:20–22. doi: 10.1136/bmj.305.6844.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ardizzone S, Molteni P, Imbesi V, Bollani S, Bianchi Porro G, Molteni F. Azathioprine in steroid-resistant and steroid-dependent ulcerative colitis. J Clin Gastroenterol. 1997;25:330–333. doi: 10.1097/00004836-199707000-00007. [DOI] [PubMed] [Google Scholar]
- 96.Lobo A J, Foster P N, Burke D A, Johnston D, Axon A T. The role of azathioprine in the management of ulcerative colitis. Dis Colon Rectum. 1990;33:374–377. doi: 10.1007/BF02156261. [DOI] [PubMed] [Google Scholar]
- 97.Steinhart A H, Baker J P, Brzezinski A, Prokipchuk E J. Azathioprine therapy in chronic ulcerative colitis. J Clin Gastroenterol. 1990;12:271–275. doi: 10.1097/00004836-199006000-00007. [DOI] [PubMed] [Google Scholar]
- 98.Korelitz B I, Adler D J, Mendelsohn R A, Sacknoff A L. Long-term experience with 6-mercaptopurine in the treatment of Crohn's disease. Am J Gastroenterol. 1993;88:1198–1205. [PubMed] [Google Scholar]
- 99.George J, Present D H, Pou R, Bodian C, Rubin P H. The long-term outcome of ulcerative colitis treated with 6-mercaptopurine. Am J Gastroenterol. 1996;91:1711–1714. [PubMed] [Google Scholar]
- 100.Pearson D C, May G R, Fick G H, Sutherland L R. Azathioprine and 6-mercaptopurine in Crohn's disease. A meta-analysis. Ann Intern Med. 1995;123:132–142. doi: 10.7326/0003-4819-123-2-199507150-00009. [DOI] [PubMed] [Google Scholar]
- 101.Mahadevan U, Tremaine W J, Johnson T, et al. Intravenous azathioprine in severe ulcerative colitis: a pilot study. Am J Gastroenterol. 2000;95:3463–3468. doi: 10.1111/j.1572-0241.2000.03362.x. [DOI] [PubMed] [Google Scholar]
- 102.Black A J, McLeod H L, Capell H A, et al. Thiopurine methyltransferase genotype predicts therapy-limiting severe toxicity from azathioprine. Ann Intern Med. 1998;129:716–718. doi: 10.7326/0003-4819-129-9-199811010-00007. [DOI] [PubMed] [Google Scholar]
- 103.Colombel J F, Ferrari N, Debuysere H, et al. Genotypic analysis of thiopurine S-methyltransferase in patients with Crohn's disease and severe myelosuppression during azathioprine therapy. Gastroenterology. 2000;118:1025–1030. doi: 10.1016/s0016-5085(00)70354-4. [DOI] [PubMed] [Google Scholar]
- 104.Snow J L, Gibson L E. A pharmacogenetic basis for the safe and effective use of azathioprine and other thiopurine drugs in dermatologic patients. J Am Acad Dermatol. 1995;32:114–116. doi: 10.1016/0190-9622(95)90195-7. [DOI] [PubMed] [Google Scholar]
- 105.Dubinsky M C, Lamothe S, Yang H Y, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705–713. doi: 10.1016/s0016-5085(00)70140-5. [DOI] [PubMed] [Google Scholar]
- 106.Cuffari C, Theoret Y, Latour S, Seidman G. 6-mercaptopurine metabolism in Crohn's disease: correlation with efficacy and toxicity. Gut. 1996;39:401–406. doi: 10.1136/gut.39.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lowry P W, Franklin C L, Weaver A L, et al. Measurement of thiopurine methyltransferase activity and azathioprine metabolites in patients with inflammatory bowel disease. Gut. 2001;49:665–670. doi: 10.1136/gut.49.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Belaiche J, Desager J P, Horsmans Y, Louis E. Therapeutic drug monitoring of azathioprine and 6-mercaptopurine metabolites in Crohn's disease. Scand J Gastroenterol. 2001;36:71–76. doi: 10.1080/00365520150218084. [DOI] [PubMed] [Google Scholar]
- 109.Sandborn W J, Tremaine W J, Wolf D C, et al. Lack of effect of intravenous administration on time to respond to azathioprine for steroid-treated Crohn's disease North American Azathioprine Study Group. Gastroenterology. 1999;117:527–535. doi: 10.1016/s0016-5085(99)70445-2. [DOI] [PubMed] [Google Scholar]
- 110.Present D H, Meltzer S J, Krumholz M P, Wolke A, Korelitz B I. 6-mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann Intern Med. 1989;111:641–649. doi: 10.7326/0003-4819-111-8-641. [DOI] [PubMed] [Google Scholar]
- 111.Connell W R, Kamm M A, Dickson M, Balkwill A M, Ritchie J K, Lennard-Jones J E. Long-term neoplasia risk after azathioprine treatment in inflammatory bowel disease. Lancet. 1994;343:1249–1252. doi: 10.1016/s0140-6736(94)92150-4. [DOI] [PubMed] [Google Scholar]
- 112.Fraser A G, Orchard T R, Robinson E M, Jewell D P. Long-term risk of malignancy after treatment of inflammatory bowel disease with azathioprine. Aliment Pharmacol Ther. 2002;16:1225–1232. doi: 10.1046/j.1365-2036.2002.01297.x. [DOI] [PubMed] [Google Scholar]
- 113.Dayharsh G A, Loftus E V, Jr, Sandborn W J, et al. Epstein-Barr virus-positive lymphoma in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine. Gastroenterology. 2002;122:72–77. doi: 10.1053/gast.2002.30328. [DOI] [PubMed] [Google Scholar]
- 114.Feagan B G, Fedorak R N, Irvine E J, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn's disease. North American Crohn's Study Group Investigators. N Engl J Med. 2000;342:1627–1632. doi: 10.1056/NEJM200006013422202. [DOI] [PubMed] [Google Scholar]
- 115.Feagan B G, Rochon J, Fedorak R N, et al. Methotrexate for the treatment of Crohn's disease. North American Crohn's Study Group Investigators. N Engl J Med. 1995;332:292–297. doi: 10.1056/NEJM199502023320503. [DOI] [PubMed] [Google Scholar]
- 116.Egan L J, Tremaine W J, Mays D C, Lipsky J J, Sandborn W J. Clinical outcome and pharmacokinetics after addition of low-dose cyclosporine to methotrexate: a case study of five patients with treatment-resistant inflammatory bowel disease. Inflamm Bowel Dis. 2000;6:286–289. doi: 10.1002/ibd.3780060406. [DOI] [PubMed] [Google Scholar]
- 117.Baron T H, Truss C D, Elson C O. Low-dose oral methotrexate in refractory inflammatory bowel disease. Dig Dis Sci. 1993;38:1851–1856. doi: 10.1007/BF01296109. [DOI] [PubMed] [Google Scholar]
- 118.Kozarek R A, Patterson D J, Gelfand M D, Botoman V A, Ball T J, Wilske K R. Methotrexate induces clinical and histologic remission in patients with refractory inflammatory bowel disease. Ann Intern Med. 1989;110:353–356. doi: 10.7326/0003-4819-110-5-353. [DOI] [PubMed] [Google Scholar]
- 119.Oren R, Arber N, Odes S, et al. Methotrexate in chronic active ulcerative colitis: a double-blind, randomized, Israeli multicenter trial. Gastroenterology. 1996;110:1416–1421. doi: 10.1053/gast.1996.v110.pm8613046. [DOI] [PubMed] [Google Scholar]
- 120.Paoluzi O A, Pica R, Marcheggiano A, et al. Azathioprine or methotrexate in the treatment of patients with steroid-dependent or steroid-resistant ulcerative colitis: results of an open-label study on efficacy and tolerability in inducing and maintaining remission. Aliment Pharmacol Ther. 2002;16:1751–1759. doi: 10.1046/j.1365-2036.2002.01340.x. [DOI] [PubMed] [Google Scholar]
- 121.Lichtiger S, Present D H, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330:1841–1845. doi: 10.1056/NEJM199406303302601. [DOI] [PubMed] [Google Scholar]
- 122.Svanoni F, Bonassi U, Bagnolo F, et al. Effectiveness of cyclosporoine A (CsA) in the treatment of active refractory ulcerative colitis. Gastroenterology. 1998;114:A1096. [Google Scholar]
- 123.D'Haens G, Lemmens L, Geboes K, et al. Intravenous cyclosporine versus intravenous corticosteroids as single therapy for severe attacks of ulcerative colitis. Gastroenterology. 2001;120:1323–1329. doi: 10.1053/gast.2001.23983. [DOI] [PubMed] [Google Scholar]
- 124.Van Assche G, D'Haens G, Noman M, et al. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology. 2003;125:1025–1031. doi: 10.1016/s0016-5085(03)01214-9. [DOI] [PubMed] [Google Scholar]
- 125.Kornbluth A, Present D H, Lichtiger S, Hanauer S. Cyclosporin for severe ulcerative colitis: a user's guide. Am J Gastroenterol. 1997;92:1424–1428. [PubMed] [Google Scholar]
- 126.Cohen R D, Stein R, Hanauer S B. Intravenous cyclosporin in ulcerative colitis: a five-year experience. Am J Gastroenterol. 1999;94:1587–1592. doi: 10.1111/j.1572-0241.1999.01149.x. [DOI] [PubMed] [Google Scholar]
- 127.Domenech E, Garcia-Planella E, Bernal I, et al. Azathioprine without oral ciclosporin in the long-term maintenance of remission induced by intravenous ciclosporin in severe, steroid-refractory ulcerative colitis. Aliment Pharmacol Ther. 2002;16:2061–2065. doi: 10.1046/j.1365-2036.2002.01385.x. [DOI] [PubMed] [Google Scholar]
- 128.Sandborn W J. Cyclosporine in ulcerative colitis: state of the art. Acta Gastroenterol Belg. 2001;64:201–204. [PubMed] [Google Scholar]
- 129.Latteri M, Angeloni T G, Silveri N G, Manna R, Gasbarrini G, Navarra P. Pharmacokinetics of cyclosporin microemulsion in patients with inflammatory bowel disease. Clin Pharmacokinet. 2001;40:473–483. doi: 10.2165/00003088-200140060-00006. [DOI] [PubMed] [Google Scholar]
- 130.Actis G C, Aimo G, Priolo G, Moscato D, Rizzetto M, Pagni R. Efficacy and efficiency of oral microemulsion cyclosporin versus intravenous and soft gelatin capsule cyclosporin in the treatment of severe steroid-refractory ulcerative colitis: an open-label retrospective trial. Inflamm Bowel Dis. 1998;4:276–279. doi: 10.1002/ibd.3780040404. [DOI] [PubMed] [Google Scholar]
- 131.Navazo L, Salata H, Morales S, et al. Oral microemulsion cyclosporine in the treatment of steroid-refractory attacks of ulcerative and indeterminate colitis. Scand J Gastroenterol. 2001;36:610–614. doi: 10.1080/003655201750163051. [DOI] [PubMed] [Google Scholar]
- 132.Sood A, Midha V, Sood N. Oral cyclosporine in patients with active severe ulcerative colitis not responding to steroids. Indian J Gastroenterol. 2002;21:155–156. [PubMed] [Google Scholar]
- 133.Sternthal M GJ, Kornbluth A. Toxicity associated with the use of cyclosporin in patients with inflammatory bowel disease. Gastroenterology. 1996;110:A1019. [Google Scholar]
- 134.Bousvaros A, Kirschner B S, Werlin S L, et al. Oral tacrolimus treatment of severe colitis in children. J Pediatr. 2000;137:794–799. doi: 10.1067/mpd.2000.109193. [DOI] [PubMed] [Google Scholar]
- 135.Baumgart D C, Wiedenmann B, Dignass A U. Rescue therapy with tacrolimus is effective in patients with severe and refractory inflammatory bowel disease. Aliment Pharmacol Ther. 2003;17:1273–1281. doi: 10.1046/j.1365-2036.2003.01534.x. [DOI] [PubMed] [Google Scholar]
- 136.Matsuhashi N, Nakajima A, Watanabe K, et al. Tacrolimus in corticosteroid-resistant ulcerative colitis. J Gastroenterol. 2000;35:635–640. doi: 10.1007/s005350070065. [DOI] [PubMed] [Google Scholar]
- 137.Pascu M, Muller A R, Wiedenmann B, Dignass A U. Rescue therapy with tacrolimus in a patient with toxic megacolon. Int J Colorectal Dis. 2003;18:271–275. doi: 10.1007/s00384-002-0458-8. [DOI] [PubMed] [Google Scholar]
- 138.Fellermann K, Tanko Z, Herrlinger K R, et al. Response of refractory colitis to intravenous or oral tacrolimus (FK506) Inflamm Bowel Dis. 2002;8:317–324. doi: 10.1097/00054725-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 139.Hanauer S LG, Colombel J F, et al. Maintenance infliximab (Remicade) is safe, effective and steroid-sparing in Crohn's disease: preliminary results of the Accent I trial. Gastroenterology. 2001;120:A-21. [Google Scholar]
- 140.Sands B E, Tremaine W J, Sandborn W J, et al. Infliximab in the treatment of severe, steroid-refractory ulcerative colitis: a pilot study. Inflamm Bowel Dis. 2001;7:83–88. doi: 10.1097/00054725-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 141.Chey W Y, Hussain A, Ryan C, Potter G D, Shah A. Infliximab for refractory ulcerative colitis. Am J Gastroenterol. 2001;96:2373–2381. doi: 10.1111/j.1572-0241.2001.04039.x. [DOI] [PubMed] [Google Scholar]
- 142.Kaser A, Mairinger T, Vogel W, Tilg H. Infliximab in severe steroid-refractory ulcerative colitis: a pilot study. Wien Klin Wochenschr. 2001;113:930–933. [PubMed] [Google Scholar]
- 143.Probert C, Hearing S D, Shreiber S, Kuhbacher T, Ghosh S, Forbes A. Infliximab in steroid-resistant ulcerative colitis: a randomized controlled trial. Gastroenterology. 2002;122:A-99. doi: 10.1136/gut.52.7.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ochsenkuhn T, Sackmann M, Goeke B. Infliximab for acute severe uclerative colitis: a randomized pilot study in nonsteroid refractory patients. Gastroenterology. 2003;124:A62. doi: 10.1097/00042737-200411000-00014. [DOI] [PubMed] [Google Scholar]
- 145.Su C, Salzberg B A, Lewis J D, et al. Efficacy of anti-tumor necrosis factor therapy in patients with ulcerative colitis. Am J Gastroenterol. 2002;97:2577–2584. doi: 10.1111/j.1572-0241.2002.06026.x. [DOI] [PubMed] [Google Scholar]
- 146.Dhillon A P, Anthony A, Sim R, et al. Mucosal capillary thrombi in rectal biopsies. Histopathology. 1992;21:127–133. doi: 10.1111/j.1365-2559.1992.tb00360.x. [DOI] [PubMed] [Google Scholar]
- 147.Gaffney P R, Doyle C T, Gaffney A, Hogan J, Hayes D P, Annis P. Paradoxical response to heparin in 10 patients with ulcerative colitis. Am J Gastroenterol. 1995;90:220–223. [PubMed] [Google Scholar]
- 148.Evans R C, Wong V S, Morris A I, Rhodes J M. Treatment of corticosteroid-resistant ulcerative colitis with heparin—a report of 16 cases. Aliment Pharmacol Ther. 1997;11:1037–1040. doi: 10.1046/j.1365-2036.1997.00252.x. [DOI] [PubMed] [Google Scholar]
- 149.Panes J, Esteve M, Cabre E, et al. Comparison of heparin and steroids in the treatment of moderate and severe ulcerative colitis. Gastroenterology. 2000;119:903–908. doi: 10.1053/gast.2000.18159. [DOI] [PubMed] [Google Scholar]
- 150.Ang Y S, Mahmud N, White B, et al. Randomized comparison of unfractionated heparin with corticosteroids in severe active inflammatory bowel disease. Aliment Pharmacol Ther. 2000;14:1015–1022. doi: 10.1046/j.1365-2036.2000.00802.x. [DOI] [PubMed] [Google Scholar]
- 151.Bloom S, Kiilerich S, Lassen M R, O'Morain C, Forbes A, Orm S. Randomized trial of tinzaparin, a low molecular weight heparin (LMWH), versus placebo in the treatment of mild to moderately active ulcerative colitis. Gastroenterology. 2003;124:A67. doi: 10.1111/j.1365-2036.2004.01926.x. [DOI] [PubMed] [Google Scholar]
- 152.Korzenik J, Miner P, Stanton D, et al. Multicenter, randomized, double-blind, placebo-controlled trial of Deligoparin (ultra low molecular weight heparin) for active ulcerative colitis. Gastroenterology. 2003;124:A67. [Google Scholar]
- 153.Tuvlin J A, Kane S V. Novel therapies in the treatment of ulcerative colitis. Expert Opin Investig Drugs. 2003;12:483–490. doi: 10.1517/13543784.12.3.483. [DOI] [PubMed] [Google Scholar]
- 154.Creed T, Hearing S, Probert C, Norman M, Dayan C. Basiliximab (IL-2 receptor antagonist) as a steroid sensitizing. Gastroenterology. 2003;124:A7. [Google Scholar]
- 155.Plevy S, Salzberg B A, Regueiro M, et al. A humanized anti-CD3 monoclonal antibody, visilizumab, for treatment of severe steroid-refractory ulcerative colitis: preliminary results of a phase I study. Gastroenterology. 2003;124:A7. doi: 10.1053/j.gastro.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 156.Feagan B, Greenberg G, Wild G, et al. A randomized controlled trial of a humanized α4β7 antibody in ulcerative colitis. Gastroenterology. 2003;124:Abstract [Google Scholar]
- 157.Musch E, Raedler A, Andus T, et al. A phase II placebo-controlled, randomized, multicenter study to evaluate efficacy and safety of interferon beta-1a in patients with ulcerative colitis. Gastroenterology. 2002;122:A431. [Google Scholar]
- 158.Sawada K, Ohnishi K, Fukui S, et al. Leukocytapheresis therapy, performed with leukocyte removal filter, for inflammatory bowel disease. J Gastroenterol. 1995;30:322–329. doi: 10.1007/BF02347507. [DOI] [PubMed] [Google Scholar]
- 159.Sawada K, Muto T, Shimoyama T, et al. Multicenter randomized controlled trial for the treatment of ulcerative colitis with a leukocytapheresis column. Curr Pharm Des. 2003;9:307–321. doi: 10.2174/1381612033391928. [DOI] [PubMed] [Google Scholar]
- 160.Sawada K, Kusugam K, Suzuki Y. Multicenter randomized double blind controlled trial for ulcerative colitis therapy with leukocytapheresis. Gastroenterology. 2003;124:A67. [Google Scholar]
- 161.Cottone M, Pietrosi G, Martorana G, et al. Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn's colitis. Am J Gastroenterol. 2001;96:773–775. doi: 10.1111/j.1572-0241.2001.03620.x. [DOI] [PubMed] [Google Scholar]
- 162.Mahadevan U, Loftus E V, Jr, Tremaine W J, et al. Azathioprine or 6-mercaptopurine before colectomy for ulcerative colitis is not associated with increased postoperative complications. Inflamm Bowel Dis. 2002;8:311–316. doi: 10.1097/00054725-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 163.Yang Y X, Lichtenstein G R. Corticosteroids in Crohn's disease. Am J Gastroenterol. 2002;97:803–823. doi: 10.1111/j.1572-0241.2002.05596.x. [DOI] [PubMed] [Google Scholar]