ABSTRACT
Until recently, peritoneal carcinomatosis from colorectal cancer was a universally fatal manifestation of this cancer. However, two innovations in treatment have improved outcome for these patients. The new surgical interventions are collectively referred to as peritonectomy procedures. During these procedures, all visible cancer is removed in an attempt to leave the patient with only microscopic residual disease. Perioperative intraperitoneal chemotherapy, the second innovation, is employed to eradicate small-volume residual disease. The intraperitoneal chemotherapy is administered in the operating room with moderate hyperthermia and is referred to as heated intraoperative intraperitoneal chemotherapy. If tolerated, additional intraperitoneal chemotherapy can be administered during the first 5 postoperative days. The use of these combined treatments, cytoreductive surgery and intraperitoneal chemotherapy, improves survival, optimizes quality of life, and maximally preserves function. This article describes the natural history of colorectal cancer with carcinomatosis, the patterns of dissemination within the peritoneal cavity, and the benefits and limitations of intraperitoneal chemotherapy. Peritonectomy procedures are defined and described. Also presented are the mechanics of delivering perioperative intraperitoneal chemotherapy and the clinical assessments used to select patients who will benefit from combined treatment. The results of combined treatment for colorectal carcinomatosis are presented.
Keywords: Peritonectomy, intraperitoneal chemotherapy, mitomycin C, 5-fluorouracil, hyperthermia, peritoneal surface malignancy, colorectal cancer
The quality of care directed toward patients with colorectal cancer has a profound effect upon survival.1 The treatments that have evolved over the past several decades have become increasingly complex. Currently, the use of radiotherapy and systemic chemotherapy combined with surgery continues to improve survival, optimize quality of life and maximally preserve function. Surgical procedures also continue to evolve toward new standards of care.2 Undoubtedly, the increasing complexity of gastrointestinal cancer management as a result of the integration of surgery, radiation therapy, and systemic chemotherapy has improved patients' care.
A better understanding of the natural progression of surgically treated colorectal cancer has also evolved over the past several decades. The emphasis in clinical research on “anatomic sites of surgical treatment failure” has provided oncologists with a target for radiation and chemotherapeutic clinical investigations. One aspect of surgical treatment failure that presents itself as a prominent need for better understanding and for concentrated research activities is peritoneal surface dissemination. Until recently, peritoneal carcinomatosis has been a universally fatal manifestation of colorectal cancer.
TREATMENT INNOVATIONS
Despite the grim outlook for patients with this disease, laboratory and clinical research efforts have continued (Table 1).3,4,5,6,7,8,9,10,11,12,13,14,15,16,17 Recent success with a curative approach stems from two treatment innovations—one surgical and the second chemotherapeutic—specifically developed for management of peritoneal carcinomatosis. The new surgical interventions are collectively referred to as peritonectomy procedures.3 Using high-voltage electrosurgery and a thorough knowledge of the distribution patterns of peritoneal carcinomatosis, the surgeon resects the lining of the abdomen and pelvis at all sites with visible evidence of cancerous implants. It should be emphasized that not all peritoneum is stripped; only visibly diseased tissue is resected.
Table 1.
Evolution of Treatments for Peritoneal Carcinomatosis from Gastrointestinal Cancer
| Authors | Year | Event |
|---|---|---|
| From Gertsch.17 Reprinted with permission. | ||
| Spratt et al4 | 1980 | Suggested a hyperthermic peritoneal perfusion system with the administration of intraperitoneal chemotherapy. |
| Speyer et al5 | 1981 | Pharmacology of intraperitoneal 5-fluorouracil (5-FU) in humans. |
| Koga et al6 | 1984 | Experimental study with prophylactic continuous hyperthermic peritoneal perfusion with mitomycin; significant prolongation of survival was obtained when 41.5°C hyperthermia was combined with mitomycin. |
| Flessner et al7 | 1984 | Pharmacokinetic studies established the peritoneal plasma barrier. |
| Sugarbaker et al8 | 1985 | Randomized controlled study of intravenous versus intraperitoneal 5-FU documented a diminished incidence of peritoneal carcinomatosis in colon cancer patients. |
| Koga et al9 | 1988 | First study of adjuvant intraoperative hyperthermic peritoneal perfusion with mitomycin in gastric cancer. |
| Fujimoto et al10 | 1988 | Used intraoperative hyperthermic peritoneal perfusion with mitomycin combined with extended surgery in patients with gastric cancer and established peritoneal carcinomatosis; after the treatment, 12.8% survived 1 year as compared with 0% after surgery alone. |
| Sugarbaker and Jablonski11 | 1995 | Trial of early postoperative intraperitoneal mitomycin and 5-FU in the management of carcinomatosis. |
| Sugarbaker3 | 1995 | Peritonectomy procedures. |
| Yonemura et al12 | 1996 | Suggested peritoneal cavity expander for optimization of intraoperative intraperitoneal hyperthermic chemotherapy delivery in patients with gastric cancer. |
| Yu et al13 | 1998 | Positive results of randomized study of adjuvant early postoperative intraperitoneal chemotherapy for gastric cancer. |
| Moran and Cecil14 | 2003 | Pseudomyxoma peritonei treatment center designated for the United Kingdom in North Hampshire Hospital, Basingstoke, England. |
| Urano et al15 | 1999 | In vivo chemohyperthermia parameters defined. |
| Verwaal et al16 | 2003 | Randomized trial showing superiority of comprehensive treatment for carcinomatosis from colon cancer. |
The second innovation, the use of perioperative intraperitoneal chemotherapy, is employed to eradicate small-volume residual disease. This intraperitoneal chemotherapy must be a planned part of the surgery for peritoneal carcinomatosis because the perioperative timing of intraperitoneal drug administration is crucial for success.18 In a majority of peritoneal surface malignancy treatment centers, the intraperitoneal chemotherapy is administered in the operating room with moderate hyperthermia19; this treatment is referred to as heated intraoperative intraperitoneal chemotherapy. Additional chemotherapy may be used as an abdominal lavage for the first 5 postoperative days, and such treatment is referred to as early postoperative intraperitoneal chemotherapy.
Because these combined treatment modalities have been employed in large numbers of patients, selection factors associated with improved long-term survival and acceptable morbidity and mortality have become established. The purpose of this article is to present the management plans and results of treatment—that is, cytoreductive surgery with peritonectomy procedures plus perioperative intraperitoneal chemotherapy—in patients with peritoneal carcinomatosis from colorectal cancer.
NATURAL HISTORY STUDIES
Surgeons, especially those involved in reoperative surgery for gastrointestinal cancer, have repeatedly observed the intracoelomic dissemination of cancer. Nevertheless, little was done to clarify the impact of peritoneal seeding upon survival until a report by Chu and colleagues was published.20 These investigators studied 100 patients with nongynecologic malignancy who had biopsy-proven peritoneal carcinomatosis. The mean survival of 45 colorectal cancer patients was 8.5 months. The presence or absence of ascites was an important prognostic variable in these patients.
In 2000, Sadeghi and coworkers reported on 370 patients with peritoneal carcinomatosis from nongynecologic malignancies who were enrolled in a European prospective multicenter trial (EVOCAPE 1).21 These patients had the benefit of fluorouracil-based systemic chemotherapy, but the results were remarkably similar to those reported by Chu a decade earlier. The mean survival of 118 patients with carcinomatosis from colorectal cancer was 6.9 months.
In 2002, Jayne and colleagues from Singapore used a database of 3019 colorectal cancer patients to identify 349 patients (13%) with peritoneal carcinomatosis.22 Of special interest were the 125 patients (58%) who had synchronous primary colorectal cancer and peritoneal implants. The median survival of those patients was only 7 months. These authors reported that survival was adversely affected by the extent of the peritoneal carcinomatosis and the stage of the primary cancer.
These survival statistics as they relate to the natural history of peritoneal surface dissemination demonstrate the aggressive behavior of colorectal cancer with carcinomatosis. These studies also show that peritoneal carcinomatosis can occur along with lymph node and liver metastases or as isolated peritoneal surface dissemination. In the Sadeghi et al study, 91 of the 118 colorectal cancer patients (77%) had no liver or lung metastases at the time carcinomatosis was diagnosed.21 In the Jayne et al study, 80% of the carcinomatosis patients in the synchronous group had no liver or systemic metastases.22
These natural history studies have proved to be most helpful in understanding the lethal nature of peritoneal carcinomatosis. However, the full impact of the profound deterioration of quality of life that accompanies disease progression has not been adequately communicated. Intestinal obstruction, bowel perforation with fistula formation, and nutritional deprivation cause immeasurable prolonged suffering in this group of patients. One of the most agonizing cancer deaths occurs from the progression of peritoneal carcinomatosis.23
PATHOBIOLOGY OF PERITONEAL DISSEMINATION OF CANCER
Although metastases through lymphatic channels to local lymph nodes and through the portal blood to the liver have been intensively studied, the dissemination of cancer cells on peritoneal surfaces has received less attention. In 1931, Sampson may have been the first to describe this type of cancer dissemination in humans.24 He observed that cancer cells escaped from primary ovarian cancer into the free peritoneal cavity, that they adhered to the mesothelial surface, that invasion occurred, and that a visible cancer nodule became apparent. He also distinguished cancer dissemination by implantation (spread) within the coelomic space from dissemination by way of lymphatic channels (metastases).
Sampson described the “life history of peritoneal carcinomatosis implants” as follows: (1) escape of the cancer cells from the primary ovarian tumor into the free peritoneal cavity, (2) migration of these cells to their site of implantation, (3) reaction of the peritoneal surface injured by the cancer cells so that fixation of the cancer in fibrin and organization of the fibrin occurred, and (4) progression of the cancerous implant at that site.
An important concept in tumor biology that has great relevance to the understanding of carcinomatosis derives from the studies of Weiss.25 He described the phenomenon of “metastatic inefficiency,” recognizing that the bloodstream may “teem with cancer cells” and yet no metastases develop. In other words, even though the portal vasculature of the liver may receive innumerable cells from a primary colorectal malignancy on a daily basis, less than half of these patients develop liver metastases. Thus, hematogenous dissemination of gastrointestinal cancer is rightfully characterized as metastatically inefficient. In contrast, cancer cells implant and grow with great efficiency within the peritoneal cavity.26
Peritoneal cancer implantation is spontaneous in 20% to 30% of patients with primary colorectal cancer as a result of full-thickness invasion of the bowel wall. In addition, there can be an iatrogenic component of carcinomatosis. The profound impact of a fresh wound induced by surgery on the likelihood of cancer cell implantation was clearly demonstrated by Zoetmulder (Amsterdam) in a thesis presentation. He showed in an experimental colon cancer model that a fresh surgical wound would increase the likelihood of tumor growth by a factor of 100; the peritoneal wound was observed to be a cancer promoter.27 The smallest amount of tumor contamination at a surgically traumatized site readily progresses to clinical symptoms as a result of metastatic efficiency and tumor growth enhancement from healing tissues.
PATTERNS OF INTRAPERITONEAL CANCER DISSEMINATION
The general surgical literature, especially manuscripts dealing with the spread of intraperitoneal infection, has described a characteristic pattern for the intracoelomic distribution of particles, bacterial organisms, or cancer cells. Autio identified six major compartments within the peritoneal cavity that would act as a reservoir for intracoelomic contaminants.28 He also showed that there was a flow of peritoneal fluid from the lower abdomen along the right paracolic sulcus to the upper abdomen. Meyers studied the distribution of intraperitoneal contrast material radiologically and documented that intra-abdominal cancer cells in fluid disseminated by well-defined routes.29 He emphasized that cancer dissemination in the presence of ascitic fluid was neither random nor limited to the immediate area of the primary neoplasm.
The role of surface lymphoid tissue within the peritoneal surface and associated peritoneal fluid resorption at these sites was described by Shimotsuma and colleagues.30 They found a close correlation of infiltrating cancer cells and the density of lymphoid aggregates; these aggregates were shown to be foci of lymphoid tissue on the peritoneal surface through which fluid and small particles were absorbed from the peritoneal cavity into the subperitoneum. The lymphoid aggregates were abundant within the greater omentum, perigonadal tissue, and mesentery and could be identified by their uptake of activated carbon particles. These authors suggested that not only the flow of peritoneal fluid but also its absorption at specific anatomic sites, such as beneath the hemidiaphragms and within the greater omentum, were important mechanisms of intraperitoneal cancer dissemination.
IMPACT OF INTRAPERITONEAL FLUID ON DISSEMINATION PATTERNS
Sugarbaker described the profound impact of intraperitoneal fluid on the patterns of cancerous dissemination within the peritoneal cavity.31 From observations collected from reoperative surgical procedures, he contrasted three important mechanisms of peritoneal cancer dissemination. In the absence of intraperitoneal fluid and surgical intervention, colorectal cancer cells metastasize in a random fashion immediately adjacent to the primary neoplasm that has penetrated the serosal surface. A pattern of “random and proximal” spread is expected from invasive nonmucinous cancers. The cells adhere, implant, and then progress at the initial site of cell contact with the peritoneal surface.
However, such a distribution pattern contrasts with that of cancers that invade the colon wall but also produce ascitic fluid or mucus. The fluid causes a characteristic redistributed pattern of implants. In this model, both the peritoneal compartments and the flow of intraperitoneal fluid determine the pattern of implant distribution. Mucinous adenocarcinoma migrates as cancer cells move with the flow of peritoneal fluid and become trapped within the large crevices between stationary surfaces or fluid pools created by gravity.
The dominant regions for mucinous adenocarcinoma progression would be the space between the right diaphragm and liver, the lower part of the left paracolic sulcus, and the cul-de-sac of Douglas. Of course, fluid drawn to milky spots within the greater or lesser omentum, gonadal or perigonadal tissue, and mesenteric border of the small bowel would also accumulate a proportion of the intraperitoneal malignant cells. Data documenting the profound impact that intraperitoneal mucin has on the patterns of intraperitoneal cancer dissemination were presented by Carmignani and colleagues.32
TUMOR CELL ENTRAPMENT
The third pattern of intraperitoneal cancer dissemination is referred to as tumor cell entrapment. The anatomic sites associated with an increased incidence of cancerous implants would be all traumatized peritoneal surfaces. Cancer implants would be observed at anastomotic sites, at sites where bowel was repeatedly handled, within the abdominal closure, and within the raw tissues created by a retroperitoneal dissection.31 Also, cancer progression within the ovary as a result of implantation in the corpus hemorrhagicum results from tumor cell entrapment. In summary, fibrin plus cancer cells result in cancer implants at specific traumatized sites; ascites plus cancer cells result in a redistributed pattern of dissemination.
MOTION HYPOTHESIS
The paper by Carmignani et al documented a fourth mechanism influencing the distribution of intraperitoneal cancer cells in mucinous or serous ascitic fluid.32 These investigators described the motion hypothesis in which the movement of an intra-abdominal structure largely determines the volume of malignancy associated with its peritoneal surface. Most structures within the abdomen are largely stationary. In contrast, the surfaces of the small bowel and its mesentery are in continuous motion by peristalsis. This motion greatly influences the distribution of tumor and, therefore, the surgical management of mucinous carcinomatosis. If the small bowel is largely clear of tumor nodules, parietal peritonectomy procedures can remove the remainder of the disease from other peritoneal surfaces.
The thin wall of the smooth muscle tube that constitutes the small bowel creates a difficult anatomic site for peritonectomy. Nodules of invasive cancer present on the small bowel surface must be left behind by the surgeon or a small bowel resection performed. Nodules of cancer on the liver, stomach, undersurface of the diaphragm, or pelvic sidewalls can be peritonectomized with negative margins. The observation that mucinous adenocarcinomas spare the small bowel surfaces but are located in large volume at other sites, especially within the omental cake and in dependent areas, is the original observation that led to a rationale for curative approach to mucinous peritoneal carcinomatosis.33
DISTRIBUTION OF MUCINOUS/NONMUCINOUS ADENOCARCINOMA
The studies by Carmignani et al quantitatively documented differences in the distribution of mucinous adenocarcinoma and nonmucinous adenocarcinoma throughout the abdomen and pelvis.32 The lesser omentum was involved with mucinous tumors in a majority of patients and was almost never involved with nonmucinous tumors. The undersurface of the right hemidiaphragm was nearly always involved with mucinous tumors but was rarely involved with the nonmucinous tumors. The same could be said for the surface of the liver. For the high-grade nonmucinous malignancies from colorectal cancer, the proximity of the tissue to the primary cancer was very important.
RATIONALE FOR INTRAPERITONEAL CHEMOTHERAPY IN GASTROINTESTINAL CANCER
Conceptual Changes in Chemotherapy Administration
Improvements in systemic chemotherapy have produced increasingly high response rates in patients with gastrointestinal cancer, and chemotherapy has become a standard part of the treatment for unresectable metastatic disease and of adjuvant treatment following complete resection of the primary malignancy. To modify the use of chemotherapy in patients with carcinomatosis, conceptual changes regarding its use have been proposed.
First, a change in the route of drug administration is required. Chemotherapy is administered intraperitoneally or perhaps with multidrug therapy, both intraperitoneally and intravenously. Intravenous chemotherapy for carcinomatosis has not been shown to prolong survival. Currently, systemic chemotherapy is sometimes used as induction therapy in carcinomatosis patients with a poor prognosis to reduce the volume of disease prior to definitive cytoreductive surgery plus intraperitoneal chemotherapy.
A second conceptual change in the use of cancer chemotherapy is the timing of drug administration. With carcinomatosis, the only successful management plans employ perioperative intraperitoneal chemotherapy. Usually, the drug administration is initiated in the operating room with a heated chemotherapy solution. The drugs selected for intraoperative use are augmented by hyperthermia, and those most frequently used include mitomycin (Mutamycin), doxorubicin, cisplatin, and oxaliplatin (Eloxatin). In the early postoperative period, drugs that require cell replication are most appropriate. These drugs are administered in a large volume of fluid for the first 5 to 7 days postoperatively and include5-fluorouracil, paclitaxel (Taxol), and docetaxel (Taxotere).
A third conceptual change involves criteria for selection of patients. The greatest benefit is observed in patients with small lesion size and limited distribution of the peritoneal implants. These patients can sometimes be made visibly free of disease by surgical resection with peritonectomy. A proportion of these patients show long-term benefits when cytoreductive surgery is combined with perioperative intraperitoneal chemotherapy. However, aggressive treatments for a large-volume and widely distributed invasive cancer on peritoneal surfaces are unlikely to produce any long-term benefits; the cytoreduction is incomplete and intraperitoneal chemotherapy ineffective.
Also, these surgically heroic procedures result in a high incidence of morbidity and mortality. From a technical perspective, treatment for peritoneal carcinomatosis must be initiated as early as possible in the natural history of the disease to achieve the greatest benefit (Fig. 1).
Figure 1.
Selection of patients for a complete cytoreduction based on implant size and invasive nature. Noninvasive peritoneal surface malignancy can be adequately cytoreduced even though tumor volume is extensive. Invasive cancer implants need to be removed before they invade the small bowel surface. The earlier the combined treatment, the more favorable the expected result. (From Sugarbaker PH. Review of a personal experience in the management of carcinomatosis and sarcomatosis. 2001;31:573–583, with permission.)
Peritoneal Space-to-Plasma Barrier
Selected chemotherapy agents demonstrate prolonged retention within the abdominopelvic space. Therefore, the exposure of peritoneal surfaces is much greater than the systemic drug exposure. This marked difference in drug exposure results in a much higher response rate at the peritoneal surface. For intraperitoneal chemotherapy, the differences in exposure at the peritoneal surface versus systemic exposure in the treatment of colorectal cancer are shown in Table 2.
Table 2.
Area under the Curve Ratios of Peritoneal Fluid to Plasma for Drugs Commonly Used to Treat Gastrointestinal Cancer
| Drug | Molecular Weight (Da) | AUC Ratio |
|---|---|---|
| AUC, concentration of drug times the duration of exposure. | ||
| 5-Fluorouracil | 130 | 250 |
| Mitomycin C | 334 | 75 |
| Doxorubicin | 544 | 500 |
| Cisplatin | 300 | 20 |
| Paclitaxel | 808 | 1000 |
| Gemcitabine | 263 | 50 |
| Oxaliplatin | 397 | 17 |
The common adverse effects of chemotherapy, even when delivered by the intraperitoneal route, are bone marrow and gastrointestinal mucosal damage. Indeed, one should not assume that the intraperitoneal administration of chemotherapy eliminates systemic toxicity. Although the drugs are sequestered for prolonged periods within the peritoneal space, they are cleared into the systemic circulation. For this reason, the safe dose of most drugs instilled into the peritoneal cavity is similar to the intravenous dose. The exceptions are drugs with hepatic metabolism such as 5-fluorouracil and gemcitabine (Gemzar). The dose of 5-fluorouracil can be increased by ~50% with intraperitoneal versus intravenous administration. The intravenous dose of 5-fluorouracil for 5 consecutive days is ~500 mg/m2/day; for intraperitoneal 5-fluorouracil, it is 750 mg/m2/day.
PERITONECTOMY PROCEDURES
If a surgical team elects to manage patients with peritoneal carcinomatosis from colorectal cancer, it is imperative that the technical skills required for completion of the peritonectomy be mastered. During the peritonectomy, all visible cancer is removed in an attempt to leave the patient with only microscopic residual disease. Knowledge of the dissemination patterns of gastrointestinal cancer spread is essential. Unless all sites are rigorously inspected and all foci of cancerous implants removed, patients will be left with gross disease and a poor long-term outcome.
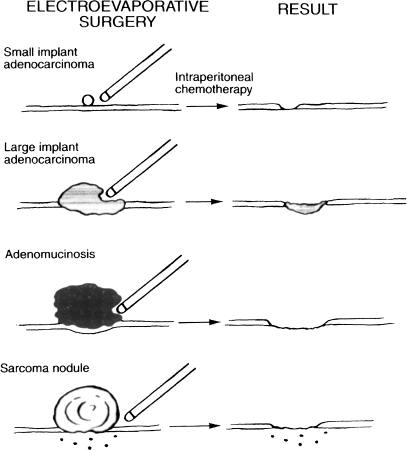
Isolated tumor nodules are removed using electroevaporation. Normal peritoneum is not excised; only the peritoneum involved by the malignant process is electrosurgically resected. If the visceral peritoneum requires removal and if a complete cytoreduction is contemplated, resection of portions of the small bowel, colorectum, or stomach is indicated.
SURGICAL OVERVIEW
To perform adequately cytoreductive surgery with peritonectomy, the surgeon must use an electroevaporative technology. Electroevaporative surgery involves a high voltage from an electrosurgical generator, a pure cut mode, and a ball electrosurgical tip. An attempt at peritonectomy using the traditional scissor-and-knife dissection will result in unnecessary blood loss. Also, the high-voltage electrosurgery creates a margin of heat necrosis that is devoid of viable tumor cells and less likely to develop recurrence.
The peritonectomy procedures can be briefly described as follows.3 After abdominal incision and placement of a self-retaining retractor, a greater omentectomy and splenectomy are performed. If the spleen and undersurface of the left hemidiaphragm are layered by tumor, a left subphrenic peritonectomy is necessary. This dissection elevates the spleen and distal pancreas prior to the division of the splenic artery and vein. The third peritonectomy is usually a right subphrenic peritoneal stripping. Electroevaporative surgery is also used to strip away Glisson’s capsule and the tumor layered on the liver surface.
Following these upper abdominal dissections, the surgeon generally initiates a complete pelvic peritonectomy, that is, a peritoneal stripping of the pelvic sidewalls and the bladder and resection of the female internal genitalia and the rectosigmoid colon along with the adjacent cul-de-sac. The vaginal cuff is copiously irrigated and must be closed prior to initiating heated intraoperative intraperitoneal chemotherapy, or leakage of the chemotherapy solution will occur. Usually, the final peritonectomy involves a cholecystectomy, lesser omentectomy, and stripping of the omental bursa.
It should be emphasized that no intestinal suturing is performed prior to the completion of heated intraoperative intraperitoneal chemotherapy. The only closure that is indicated is closure of the vaginal cuff to eliminate the loss of chemotherapy solution through this dependent site.
TECHNIQUES FOR PERIOPERATIVE INTRAPERITONEAL CHEMOTHERAPY
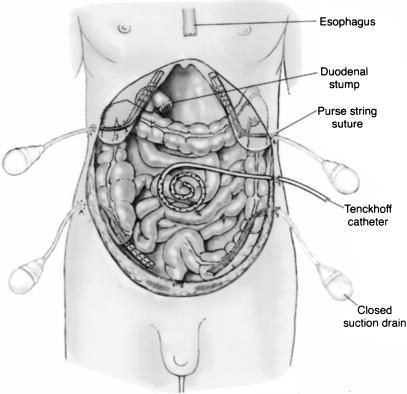
To initiate hyperthermic intraoperative intraperitoneal chemotherapy, a series of tubes and drains (four drainage tubes and a single inflow catheter) must be placed within the peritoneal cavity through the abdominal wall (Fig. 2). To prevent leakage of these tubes as they exit the abdominal skin, a purse string suture is used. Generally, the inflow catheter is placed at a site thought to be at highest risk for recurrent disease because the greatest heat is generated at this site. Placement on the pancreas should be avoided.
Figure 2.
Tubes and drains used for heated intraoperative intraperitoneal chemotherapy. There are four drainage tubes and a single inflow catheter.
Following tube placement, the self-retaining retractor is partially dismantled. It is reassembled to construct a frame ~4 inches above the anterior abdominal wall. A heavy gauge monofilament suture is used to elevate the skin edges on the self-retaining retractor and thereby create a reservoir for chemotherapy solution within the abdomen.
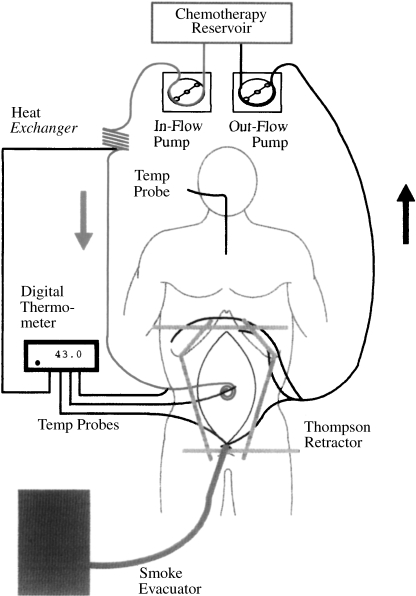
A perfusion circuit is necessary to maintain a temperature of ~41.5°C within the peritoneal cavity (Fig. 3). Inflow and outflow tubes are connected to roller pumps and a heat exchanger. A heater/cooler, which maintains a temperature of 48°C of water flowing through the heat exchanger, is part of the apparatus. For most drugs, a 90-minute perfusion is indicated to achieve a maximal cytotoxic effect.
Figure 3.
Perfusion circuit for heated intraoperative intraperitoneal chemotherapy. Four closed suction drains are positioned, one beneath each hemidiaphragm and within the pelvis. A Tenckhoff catheter is placed at the site that the surgeon thinks is at greatest risk for recurrent disease, which is the area within the abdomen to receive the greatest heat. Some dose intensification occurs with this approach, from both heat and chemotherapy exposure. Roller pumps, a heat exchanger, and thermometry allow the perfusion to proceed. A smoke evacuator tube pulls air from beneath the plastic sheet, keeping the airflow moving from operating theater to peritoneal cavity to smoke evacuator and through a charcoal filter.
The drugs used intraoperatively for hyperthermic chemotherapy are mitomycin (Mutamycin), cisplatin and doxorubicin, or oxaliplatin (Eloxatin). Following the completion of the intraoperative chemotherapy, the self-retaining retractor is again positioned. At this time, all intestinal anastomoses are completed, seromuscular repairs of the bowel are performed, and the abdomen is closed. Usually, a fifth closed-suction drain is placed within the subcutaneous space. The skin is closed so that no leakage of fluid occurs from the abdomen postoperatively.
If stable postoperatively, patients who receive heated intraoperative intraperitoneal chemotherapy are also given early postoperative intraperitoneal 5-fluorouracil. The catheters for drug instillation and abdominal drainage must be kept clear of blood clot, fibrin clot, and tissue debris.
EARLY POSTOPERATIVE INTRAPERITONEAL CHEMOTHERAPY
If the patient recovers well from the cytoreductive surgery and intraperitoneal heated chemotherapy, early postoperative intraperitoneal chemotherapy is initiated. At the Washington Hospital Center, early postoperative chemotherapy is initiated for all patients except those with very early disease and a low likelihood of tumor recurrence. The drug employed in this chemotherapy is a cell cycle–dependent drug, 5-fluorouracil. All the intra-abdominal catheters are withdrawn after fluid drainage is substantially reduced prior to the patient’s discharge from the hospital.
SELECTION OF PATIENTS FOR TREATMENT USING QUANTITATIVE PROGNOSTIC INDICATORS
The greatest impediment to achieving long-term benefits from combined treatment with cytoreductive surgery and perioperative intraperitoneal chemotherapy is improper selection of patients. Patients with advanced disease experience minimal benefit and significant morbidity and mortality. Given the risks and benefits for patients with a large volume of invasive cancer, elective cytoreductive surgery should be withheld in this subgroup of patients unless performed by the most experienced surgical teams.
Excluding pseudomyxoma peritonei and cystic mesothelioma, extensive cytoreductive surgery and aggressive intraperitoneal chemotherapy are not likely to produce a lasting benefit in patients with advanced peritoneal surface cancer from a gastrointestinal primary. Rapid recurrence of the peritoneal surface disease combined with progression of lymph nodal, liver, or systemic disease is likely to interrupt long-term benefit. Asymptomatic patients with small-volume peritoneal carcinomatosis should be referred for treatment.
In the past, peritoneal carcinomatosis was a fatal disease process. The only assessment required was the presence or absence of carcinomatosis. Currently, three important clinical assessments of peritoneal surface malignancy are used to select colorectal cancer patients who will benefit from the combined treatment: (1) preoperative computed tomogram (CT) of the chest, abdomen and pelvis with maximal oral and intravenous contrast, (2) peritoneal cancer index determination, and (3) the completeness of cytoreduction (CC) score.
Preoperative Computed Tomography Scan
A preoperative CT scan of the chest, abdomen, and pelvis is required in planning treatment of a peritoneal surface malignancy. This radiologic examination is essential to exclude liver or systemic metastases and pleural surface spread. Unfortunately, the CT scan is an inaccurate test by which to quantitate nonmucinous carcinomatosis distribution and volume. As described by Archer et al, the malignant tissue progresses as a layer on the peritoneal surfaces and conforms to the normal contours of the abdominopelvic structures—quite different from the metastatic process in the liver or lung, which shows up as three-dimensional spherical tumor nodules and can be accurately assessed by CT.33
Fortunately, CT is helpful in imaging mucinous adenocarcinoma on peritoneal surfaces.34 These tumors produce large volumes of mucoid material, readily distinguished by anatomic location, shape, and density. A knowledgeable radiologic interpretation can also distinguish patients with a high likelihood of complete cytoreduction from those who would be designated for incomplete resections. The CT scan excludes patients who are unlikely to receive the benefit of a potentially curative approach from an elective operative intervention. Interventions in patients with advanced disease would be performed for symptom management, and the surgery would be of a palliative, minimally aggressive nature.
The two radiologic criteria found to be most useful in excluding carcinomatosis patients from elective intervention are segmental obstruction of the small bowel and the presence of tumor nodules greater than 5 cm in diameter on small bowel surfaces or directly adjacent to small bowel mesentery in the jejunum or upper ileum. Tumor involvement of the small bowel at the terminal ileum is not thought to be a contraindication to elective surgery because disease at this site can be resected as part of the cytoreductive surgery.
These criteria reflect radiologically the pathobiology of carcinomatosis. Obstructed segments of bowel indicate an invasive character of the malignancy where portions of small bowel lack peristalsis or become narrowed. Large, greater than 5 cm, mucinous tumor nodules on the small bowel or its mesentery indicate that the cancer is not redistributed away from the intestinal surface by peristaltic motion. Difficulties with dissection of the mucinous tumor from small bowel signify the need for a palliative effort.
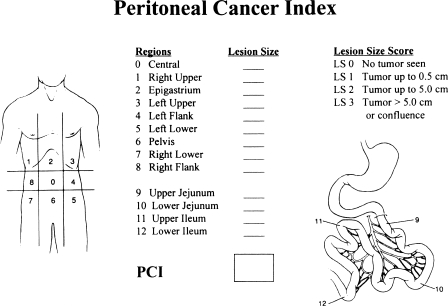
Peritoneal Cancer Index
The third prognostic assessment of peritoneal surface malignancy is the peritoneal cancer index, which is a quantitative prognostic indicator derived from the integration of the size of peritoneal implant and the distribution of nodules on the peritoneal surface (Fig. 4). This index should be used in the treatment decision-making process while the abdomen is being completely explored. The choice between a definitive cytoreduction and a palliative debulking is greatly influenced by the peritoneal cancer index.
Figure 4.
Peritoneal cancer index. This composite score is determined after complete exploration of the abdomen and pelvis. The lesion size score (0–3) and the involvement of abdominopelvic regions (0–12) are combined.
To arrive at a score, the size of the intraperitoneal nodules must be assessed in all abdominopelvic regions; the number of nodules is not scored, only the size of the largest nodule. A lesion score of zero (LS-0) means that no malignant deposits are visualized; an LS-1 signifies that tumor nodules less than 0.5 cm are present; an LS-2 indicates that tumor nodules between 0.5 and 5.0 cm are present; and an LS-3 indicates the presence of tumor nodules greater than 5.0 cm in any dimension. If there is a confluence (layering) of tumor, the lesion size is scored as 3.
To assess the distribution of peritoneal surface disease, an LS score is determined for each of these 13 abdominopelvic regions. The summation of the LS score is the peritoneal cancer index. A maximal score is 39 (13 × 3).
To date, the peritoneal cancer index has been validated in two separate situations for colorectal cancer. Portilla and coworkers showed that it could be used to predict long-term survival in patients with peritoneal carcinomatosis from colon cancer undergoing a second cytoreduction.35 Sugarbaker showed that it could be used to predict the likelihood of long-term survival in colon cancer patients undergoing combined treatment.36 In these studies, patients with a favorable prognosis had a peritoneal cancer index less than 13.
EXCEPTIONS TO THE RULE
Exception to the rules for using the peritoneal cancer index have been established. First, noninvasive malignancy on peritoneal surfaces may be completely cytoreduced even though the peritoneal cancer index is as high as 39. A disease such as pseudomyxoma peritonei falls into this category. With these minimally invasive tumors, the status of the abdomen and pelvis at completion of cytoreduction may have no relationship to the volume recorded at the time of abdominal exploration; that is, even though the surgeon explores an abdomen with a maximal peritoneal cancer index, it can be brought to an index of 0 by cytoreduction. In these diseases, the prognosis is related only to the condition of the abdomen after the cytoreduction (CC score).
A second caveat for using the peritoneal cancer index is related to cancer at crucial anatomic sites. For example, a small volume of invasive cancer incompletely resected from the common bile duct results in a poor prognosis despite a low peritoneal cancer index. Invasion of the base of the bladder or unresectable disease on a pelvic sidewall may, by itself, result in residual invasive cancer after maximal cytoreduction and eventuate in a poor prognosis. In other words, invasive cancer at crucial anatomic sites may function as systemic disease in the assessment of the prognosis with invasive cancer. Because only patients who undergo a complete cytoreduction can achieve long-term survival, residual disease at anatomically crucial sites supersedes a favorable peritoneal cancer index score.
Completeness of Cytoreduction Score
The most definitive assessment of prognosis to be used with peritoneal surface malignancy is the CC score. This information, however, is of less value to the surgeon in planning treatment than the peritoneal cancer index because it is not available until after the cytoreduction is complete (the peritoneal cancer index is available at the time of abdominal exploration). If, during exploration, it becomes obvious that cytoreduction will not be complete, the surgeon may decide that a palliative debulking, which will provide temporary symptomatic relief, is appropriate and may discontinue plans for an aggressive cytoreduction with intraperitoneal chemotherapy.
In both noninvasive and invasive peritoneal surface malignancy, the CC score is the major prognostic indicator. It has been shown to function with accuracy in peritoneal carcinomatosis from colon cancer.36
For gastrointestinal cancer, the CC score has been defined as follows: A CC score of zero (CC-0) indicates that no peritoneal seeding occurred during the complete exploration. A CC-1 score indicates that tumor nodules persisting after cytoreduction are smaller than 2.5 mm (this is a nodule size thought to be penetrable by intracavitary chemotherapy). Both CC-0 and CC-1 scores would, therefore, be designated as a complete cytoreduction. A CC-2 score indicates residual tumor nodules between 2.5 mm and 2.5 cm. A CC-3 score indicates residual tumor nodules greater than 2.5 cm or a confluence of unresectable tumor nodules at any site within the abdomen or pelvis. CC-2 and CC-3 cytoreductions are considered incomplete (Fig. 5).
Figure 5.
Completeness of cytoreduction score. A CC score of zero (CC-0) indicates that no peritoneal seeding occurred during the complete exploration. A CC-1 score indicates that tumor nodules persisting after cytoreduction are smaller than 2.5 mm. A CC-2 score indicates residual tumor nodules between 2.5 mm and 2.5 cm. A CC-3 score indicates residual tumor nodules greater than 2.5 cm or a confluence of unresectable tumor nodules at any site within the abdomen or pelvis.
MODIFICATIONS OF COLON AND RECTAL CANCER SURGERY TO ACHIEVE COMPLETE CYTOREDUCTION
As the combined treatment of carcinomatosis becomes more widely used, major changes in the management of cancer patients with peritoneal seeding must be considered. With this new approach using peritonectomy for cytoreduction, one gains a concept of an intact peritoneum as the first line of defense against carcinomatosis. Opening large tissue planes in the presence of free intraperitoneal cancer cells will jeopardize subsequent attempts at curative treatment. Cancer cells will implant within the cancer resection site and beneath the peritoneal surfaces. This implantation and cancer progression will occur beneath the peritoneum and be inaccessible to peritonectomy, which means that “iatrogenic invasion” may occur into the pelvic sidewall, along the course of the ureter, in and around the structures of the porta hepatis, and at other surgically traumatized sites. A patient with cancer progressing deep within the recesses of the abdomen and pelvis is no longer a candidate for peritonectomy and is unlikely to have successful combined treatment.
One may conclude that the initial surgery in patients with peritoneal seeding should be modified. For example, in this new approach to colorectal carcinomatosis, a patient with a perforated mucinous colon malignancy who is found to have peritoneal seeding at the time the primary cancer is diagnosed should have a minimal surgical procedure. A limited exteriorization resection should be performed, the omental implants should be generously biopsied, and then the abdomen closed for definitive combined treatment at a later time.
As a second example, if the patient has an obstructing colonic malignancy, an ostomy above the primary cancer would be appropriate. In a patient without obstructive symptoms and a diagnosis of colon cancer with carcinomatosis, definitive biopsy of peritoneal implants may be the only recommended procedure. Only the most debilitated patient, who is not a candidate for cytoreduction with intraperitoneal chemotherapy, should undergo definitive resection.
The optimal treatment of colon cancer with carcinomatosis requires resection of the primary cancer, peritonectomy of implants on visceral and parietal peritoneum to remove all visible evidence of disease, and perioperative intraperitoneal chemotherapy. In the absence of an adequate management plan, minimal surgical intervention to avoid iatrogenic invasive disease is indicated. In an institution not adequately prepared to manage carcinomatosis, referral to a peritoneal surface treatment center would be appropriate.
RESULTS OF TREATMENT OF CARCINOMATOSIS FROM COLON CANCER
Reports from five institutions with at least 25 patients treated, for a total of 333 patients, are shown in Table 3.37,38,39,40,41 The combined mean follow-up was 33 months (range 6 to 99 months); the mean survival of all patients at 3 years was 31% (range: 23% to 47%). In all these reports, patients in whom complete cytoreductive surgery was possible had a median survival that far exceeded the survival in patients who had incomplete cytoreductive surgery.
Table 3.
Results of Combined Treatment of Peritoneal Carcinomatosis from Colon Cancer at Five Treatment Centers
| Year | Investigator | Number of Patients | 3-Year Survival |
|---|---|---|---|
| From Glehen O, Gilly FN, Sugarbaker PH. New perspectives in the management of colorectal cancer; what about peritoneal carcinomatosis? Scand J Surg 2003;92:178–179, with permission. | |||
| 2000 | Pestieau and Sugarbaker37 | 99 | 28% |
| 2001 | Elias et al38 | 64 | 47% |
| 2001 | Witkamp et al39 | 77 | 23% |
| 2003 | Shen et al40 | 40 | 25% |
| 2003 | Glehen et al41 | 53 | 18% |
| Total | 333 | 28% | |
A phase III, prospective randomized study by Verwaal and colleagues involving 105 patients deserves special attention.42 After cytoreductive surgery and peritonectomy, 54 patients were treated with heated intraoperative intraperitoneal chemotherapy with mitomycin C. In 51 patients, the administered treatment was the standard of care in Holland, a 5-fluorouracil/leucovorin regimen. Analyzing the data by an intention-to-treat principle at a median follow-up of 21.6 months (3 to 44 months), 30 patients were alive in the experimental arm and 20 in the control arm. The Kaplan-Meier survival analysis showed a mean survival of 22.4 months for patients receiving the combined treatment. The 2-year survival was 43% in the experimental group and 16% in the systemic chemotherapy group (p = 0.032).
Recently, a retrospective multi-institutional study of 506 patients from 28 institutions was published.43 Patients in whom cytoreductive surgery was complete had a median survival of 32.4 months, compared with 8.4 months for patients in whom complete cytoreductive surgery was not possible (p < 0.001). Positive independent prognostic indicators by multivariate analysis were complete cytoreduction, treatment by a second procedure, limited extent of peritoneal carcinomatosis, age younger than 65 years, and use of chemotherapy. The use of neoadjuvant chemotherapy, lymph node involvement, presence of liver metastasis, and poor histologic differentiation were negative independent prognostic indicators. These authors concluded that the therapeutic approach combining cytoreductive surgery with perioperative intraperitoneal chemotherapy achieved long-term survival in a selected group of patients with peritoneal carcinomatosis of colorectal origin with acceptable morbidity and mortality. The complete cytoreductive surgery was the most important prognostic indicator.
MORBIDITY AND MORTALITY
The early results of treatment in these carcinomatosis patients were associated with reasonable long-term survival when patients with peritoneal seeding were compared with other poor prognosis patients with pancreas cancer, liver metastases from colorectal cancer, or abdominopelvic sarcoma. However, the initial morbidity and mortality rates were high. Sugarbaker and Jablonski reported that 26% of patients (19 out of 72) developed a bowel perforation postoperatively if they presented for treatment with obstruction, prior radiotherapy, or prior intraperitoneal chemotherapy.44
As a result of continued efforts to reduce the complications, Stephens and colleagues reported a prospective study of morbidity and mortality in 200 consecutive patients who had undergone combined treatment.45 In these studies, three treatment-related deaths occurred (1.5%) and grade 3/4 complications developed in 27.0% of patients. Peripancreatitis was seen in 6% of patients, and the incidence of fistula decreased to 4.5%. In the registry report from 28 institutions, the morbidity was 23% and mortality was 4%.43
ETHICAL CONSIDERATIONS
The requirements for initiating a new program in peritoneal surface malignancy have been examined.46 Guidelines for the implementation of these complex new treatment strategies will vary from institution to institution and country to country. However, without exception, studies of adjuvant intraperitoneal chemotherapy in patients with primary gastrointestinal cancer must be randomized and reviewed by a research board. Also, when a group first attempts to initiate treatment plans for carcinomatosis, a steep learning curve is associated with the new surgical procedures and the new technology.
A “start-up protocol” approved by an institutional review board may prompt the members of the group to standardize the methods and familiarize themselves with the experience of others. Probably most important, selection criteria for treatment of patients would be standardized. An omnibus protocol that allows aggressive peritonectomy and perioperative intraperitoneal chemotherapy in patients without liver or systemic dissemination and with small-volume peritoneal seeding seems reasonable. This omnibus protocol should be utilized for a limited time to treat between 10 and 20 patients.
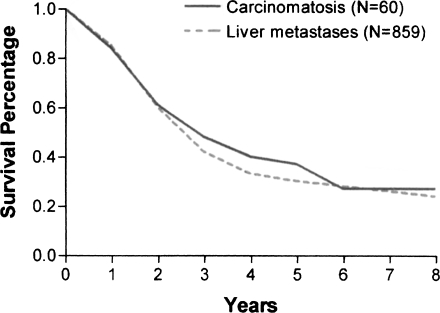
Formal institutional review board protocols should not be required for the treatment of debilitating ascites, in light of the marked quality-of-life benefits demonstrated by McQuellon and colleagues.47 Also, long-term survival of patients with peritoneal surface malignancy and a low peritoneal cancer index has been established. The survival of patients with resected liver metastases has been compared with that of patients with complete cytoreduction from carcinomatosis.17,48 Indeed, nearly identical survival has been shown for these two groups (Fig. 6). If liver resection for metastases has been accepted as standard of practice in the absence of phase III studies, perhaps this favorable comparison of treatment outcome suggests that further phase III studies may not be necessary for colorectal carcinomatosis. The ethical implications of these comparisons require careful thought.
Figure 6.
Comparison of the survival of a group of patients with colorectal metastases to the liver and a second group with carcinomatosis. In all liver metastases patients, the liver resection was scored R0; in all the carcinomatosis patients, the cytoreduction was scored as complete. (From Sugarbaker.48 Reprinted with permission.)
REFERENCES
- 1.Temple WJ, editor. Surgical Techniques and Outcomes. Philadelphia: Surgical Oncology Clinics of North America; WB Saunders; 2002.
- 2.Taylor I. What constitutes good practice in surgical oncology? Eur J Surg Oncol. 2001;27:517–520. doi: 10.1053/ejso.2001.1153. [DOI] [PubMed] [Google Scholar]
- 3.Sugarbaker P H. Peritonectomy procedures. Ann Surg. 1995;221:29–42. doi: 10.1097/00000658-199501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spratt J S, Adcock R A, Sherrill W, Tavathen S. Hyperthermia peritoneal perfusion system in canines. Cancer Res. 1980;40:253–255. [PubMed] [Google Scholar]
- 5.Speyer J L, Sugarbaker P H, Collins J M, Dedrick R L, Klecker R W, Myers C E. Portal levels and hepatic clearance of 5-fluorouracil after intraperitoneal administration in humans. Cancer Res. 1981;41:1916–1922. [PubMed] [Google Scholar]
- 6.Koga S, Hamazoe R, Maeta M, Shimizu N, Kanayama H, Osaki Y. Treatment of implanted peritoneal cancer in rats by continuous hyperthermic peritoneal perfusion in combination with an anticancer drug. Cancer Res. 1984;44:1840–1842. [PubMed] [Google Scholar]
- 7.Flessner M F, Dedrick R L, Schultz J S. A distributable model of peritoneal plasma transport: theoretical considerations. Am J Physiol. 1984;246:R597–R607. doi: 10.1152/ajpregu.1984.246.4.R597. [DOI] [PubMed] [Google Scholar]
- 8.Sugarbaker P H, Gianola F J, Speyer J L, Wesley R, Barofsky I, Myers C E. Prospective randomized trial of intravenous vs. peritoneal 5-FU in patients with advanced primary colon or rectal cancer. Semin Oncol. 1985;12(3 suppl 4):101–111. [PubMed] [Google Scholar]
- 9.Koga S, Hamazoe R, Maeta M, Shimizu N, Murakami A, Wakatsuki T. Prophylactic therapy for peritoneal recurrence of gastric cancer by continuous hyperthermic peritoneal perfusion with mitomycin C. Cancer. 1988;61:232–237. doi: 10.1002/1097-0142(19880115)61:2<232::aid-cncr2820610205>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 10.Fujimoto S, Shrestha R D, Kokubun M, et al. Intraperitoneal hyperthermic perfusion combined with surgery effective for gastric cancer patients with peritoneal seeding. Ann Surg. 1988;208:36–41. doi: 10.1097/00000658-198807000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugarbaker P H, Jablonski K A. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg. 1995;221:124–132. doi: 10.1097/00000658-199502000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yonemura Y, Fujimura T, Nishimura G, et al. Effects of intraoperative chemohyperthermia in patients with gastric cancer with peritoneal dissemination. Surgery. 1996;119:437–444. doi: 10.1016/s0039-6060(96)80145-0. [DOI] [PubMed] [Google Scholar]
- 13.Yu W, Whang I, Suh I, Averbach A, Chang D, Sugarbaker P H. Prospective randomized trial of early postoperative intraperitoneal chemotherapy as an adjuvant to resectable gastric cancer. Ann Surg. 1998;228:347–354. doi: 10.1097/00000658-199809000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moran B J, Cecil T D. The etiology, clinical presentation, and management of pseudomyxoma peritonei. Surg Oncol Clin N Am. 2003;12:585–603. doi: 10.1016/s1055-3207(03)00026-7. [DOI] [PubMed] [Google Scholar]
- 15.Urano M, Kuroda M, Nishimura Y. For the clinical application of thermo-chemotherapy given at mild temperatures. Int J Hyperthermia. 1999;15:79–107. doi: 10.1080/026567399285765. [DOI] [PubMed] [Google Scholar]
- 16.Verwaal V, Ruth S van, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 17.Gertsch P. A historical perspective on colorectal liver metastases and peritoneal carcinomatosis: similar results, different treatments. Surg Oncol Clin N Am. 2003;12:531–541. doi: 10.1016/s1055-3207(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 18.Sugarbaker P H, Graves T, DeBruijn E A, et al. Rationale for early postoperative intraperitoneal chemotherapy (EPIC) in patients with advanced gastrointestinal cancer. Cancer Res. 1990;50:5790–5794. [PubMed] [Google Scholar]
- 19.Jacquet P, Averbach A, Stephens A D, et al. Heated intraoperative intraperitoneal mitomycin C and early postoperative intraperitoneal 5-fluorouracil: pharmacokinetic studies. Oncology. 1998;55:130–138. doi: 10.1159/000011847. [DOI] [PubMed] [Google Scholar]
- 20.Chu D ZJ, Lang N P, Thompson C, Osteen P K, Westbrook K C. Peritoneal carcinomatosis in non-gynecologic malignancy. A prospective study of prognostic factors. Cancer. 1989;63:364–367. doi: 10.1002/1097-0142(19890115)63:2<364::aid-cncr2820630228>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies. Results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358–363. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 22.Jayne D G, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89:1545–1550. doi: 10.1046/j.1365-2168.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 23.Jones T. In: Erdrich L, Kenison K, editor. The Best American Short Stories 1993. New York: Houghton Mifflin; 1993. I want to live! pp. 127–145.
- 24.Sampson J A. Implantation peritoneal carcinomatosis of ovarian origin. Am J Pathol. 1931;7:423–443. [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss L. Metastatic inefficiency: causes and consequences. Cancer Rev. 1986;3:1–24. [Google Scholar]
- 26.Spratt J S, Edward M, Kubota T, Lindberg R, Tseng M T. Peritoneal carcinomatosis: anatomy, physiology, diagnosis, management. Curr Probl Cancer. 1986;10:555–585. [Google Scholar]
- 27.Zoetmulder F AN. Modelstudies over het Colorectale Carcinoom. Amsterdam: Rodopi; 1982.
- 28.Autio V. The spread of intraperitoneal infection. Studies with Roentgen contrast medium. Acto Chir Scand. 1964;36(suppl 321):5–31. [PubMed] [Google Scholar]
- 29.Meyers M A. Distribution of intra-abdominal malignant seeding: dependency on dynamics of flow of ascitic fluid. Am J Roentgenol Radium Ther Nucl Med. 1973;119:198–206. doi: 10.2214/ajr.119.1.198. [DOI] [PubMed] [Google Scholar]
- 30.Shimotsuma M, Shields J W, Simpson-Morgan M W, et al. Morpho-physiological function and role of omental milky spots as omentum associated lymphoid tissue (OALT) in the peritoneal cavity. Lymphology. 1993;26:90–101. [PubMed] [Google Scholar]
- 31.Sugarbaker P H. In: Sugarbaker PH, editor. Boston: Peritoneal Carcinomatosis: Principles of Management; Kluwer; 1996. Observations concerning cancer spread within the peritoneal cavity and concepts supporting an ordered pathophysiology. pp. 79–100. [DOI] [PubMed]
- 32.Carmignani C P, Sugarbaker T, Bromley C M, Sugarbaker P H. Intraperitoneal cancer dissemination: mechanisms of the patterns of spread. Cancer Metastasis Rev. 2003;22:465–472. doi: 10.1023/a:1023791229361. [DOI] [PubMed] [Google Scholar]
- 33.Archer A G, Sugarbaker P H, Jelinek J S. In: Sugarbaker PH, editor. Boston: Peritoneal Carcinomatosis: Principles of Management; Kluwer; 1996. Radiology of peritoneal carcinomatosis. pp. 263–288. [DOI] [PubMed]
- 34.Jacquet P, Jelinek J S, Chang D, Koslowe P, Sugarbaker P H. Abdominal computed tomographic scan in the selection of patients with mucinous peritoneal carcinomatosis for cytoreductive surgery. J Am Coll Surg. 1995;181:530–538. [PubMed] [Google Scholar]
- 35.Portilla A G, Sugarbaker P H, Chang D. Second-look surgery after cytoreduction and intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer: analysis of prognostic factors. World J Surg. 1999;23:23–29. doi: 10.1007/s002689900560. [DOI] [PubMed] [Google Scholar]
- 36.Sugarbaker P H. Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol. 1999;43(suppl):S15–S25. doi: 10.1007/s002800051093. [DOI] [PubMed] [Google Scholar]
- 37.Pestieau S R, Sugarbaker P H. Treatment of primary colon cancer with peritoneal carcinomatosis: comparison of concomitant vs. delayed management. Dis Colon Rectum. 2000;43:1341–1346. doi: 10.1007/BF02236627. [DOI] [PubMed] [Google Scholar]
- 38.Elias D, Blot F, El Otmany A, et al. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer. 2001;92:71–76. doi: 10.1002/1097-0142(20010701)92:1<71::aid-cncr1293>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 39.Witkamp A J, de Bree E, Kaag M M, et al. Extensive cytoreductive surgery followed by intra-operative hyperthermia intraperitoneal chemotherapy with mitomycin C in patients with carcinomatosis of colorectal cancer. Eur J Surg. 2001;37:979–984. doi: 10.1016/s0959-8049(01)00058-2. [DOI] [PubMed] [Google Scholar]
- 40.Shen P, Levine E A, Hall J, et al. Factors predicting survival after intraperitoneal hyperthermic chemotherapy with mitomycin C after cytoreductive surgery for patients with peritoneal carcinomatosis. Arch Surg. 2003;138:26–33. doi: 10.1001/archsurg.138.1.26. [DOI] [PubMed] [Google Scholar]
- 41.Glehen O, Mithieux F, Osinsky D, et al. Surgery combined with peritonectomy procedures and intraperitoneal chemohyperthermia in abdominal cancers with peritoneal carcinomatosis: a phase II study. J Clin Oncol. 2003;21:799–806. doi: 10.1200/JCO.2003.06.139. [DOI] [PubMed] [Google Scholar]
- 42.Verwaal V J, Ruth S van, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2003;21:3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 43.Glehen O, Kwiatkowski F, Sugarbaker P H, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22:3284–3292. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 44.Sugarbaker P H, Jablonski K A. Prognostic factors of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg. 1995;221:124–132. doi: 10.1097/00000658-199502000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stephens A D, Alderman R, Chang D, et al. Morbidity and mortality of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the Coliseum technique. Ann Surg Oncol. 1999;6:790–796. doi: 10.1007/s10434-999-0790-0. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez-Bayon L, Sugarbaker P H, Gonzalez-Moreno S, de Lima Vazquez V, Alves S, Moran B J. Initiation of a program in peritoneal surface malignancy. Surg Oncol Clin N Am. 2003;12:741–753. doi: 10.1016/s1055-3207(03)00032-2. [DOI] [PubMed] [Google Scholar]
- 47.McQuellon R P, Loggie B W, Fleming R A, et al. Quality of life after intraperitoneal hyperthermic chemotherapy (IPHC) for peritoneal carcinomatosis. Eur J Surg Oncol. 2001;27:65–73. doi: 10.1053/ejso.2000.1033. [DOI] [PubMed] [Google Scholar]
- 48.Sugarbaker P H. Carcinomatosis, is cure an option? J Clin Oncol. 2003;21:762–764. doi: 10.1200/JCO.2003.12.071. [DOI] [PubMed] [Google Scholar]