ABSTRACT
Locally advanced and locally recurrent colon cancers pose a surgical challenge with tumors extending into surrounding structures and organs. Anticipation of the need for an extended surgical resection, often with multivisceral en bloc organ removal, is critical for surgical planning. For both primary and recurrent tumors, postsurgical long-term survival is achievable but only after complete resection. The role of neoadjuvant and adjuvant therapy continues to be redefined in this era of biologic chemotherapeutics, and multimodality therapy holds promise in aiding resection and improving postsalvage survival.
Keywords: Locally advanced, recurrent, colon cancer, salvage, locoregional, surgery
The management of locally advanced and locally recurrent colon cancer poses a surgical challenge as these lesions often extend into surrounding structures and organs. Complete, margin-negative resection is critical to achieving long-term survival and generally entails multivisceral en bloc organ resection. This review focuses on the clinicopathologic features, associated morbidity, and predictors of survival in patients undergoing surgery for locally advanced and locally recurrent colon cancer. Finally, the emerging roles of chemotherapy and radiation are discussed.
LOCALLY ADVANCED COLON CANCER
Of the estimated 100,000 cases of colon cancer that present each year in the United States, 10% to 20% represent locally advanced disease, with tumors extending through the colon wall with perforation and/or invasion of adjacent organs or structures.1,2,3,4,5,6,7,8,9,10,11,12,13,14 These are classified as T4 lesions by the American Joint Committee on Cancer staging schema.15
Identifying patients with advanced lesions is important in surgical planning. The majority of patients with locally advanced tumors present with symptoms including back or flank pain; nausea or emesis, indicating some degree of obstruction; or frank hematochezia.2,16 Often, symptoms indicate the area of disease; for example, invasion of the bladder is associated with dysuria and hematuria. As expected, the most commonly involved organs are anatomically close to the primary lesion: cecum and sigmoid carcinomas generally involve the ovaries, fallopian tubes, uterus, or small bowel, whereas hepatic flexure, transverse colon, or splenic flexure carcinomas are more likely to invade the gallbladder, duodenum, stomach, pancreas, or spleen. The abdominal wall is more likely to be invaded by tumors in the intraperitoneal portions of the colon, and the retroperitoneum is more likely to be invaded by lesions located at the hepatic/splenic flexures and ascending and descending colon.
Advanced lesions tend to be larger and are often palpable on physical examination. Colonoscopy may reveal annular or constrictive lesions. Radiographic imaging with computed tomography (CT) frequently indicates possible malignant fistula (Fig. 1); however, the finding may be subtle (Fig. 2). CT scan cannot differentiate peritumoral inflammation from direct tumor infiltration. Ultimately, the surgeon needs to decide intraoperatively whether or not an extended resection is necessary.
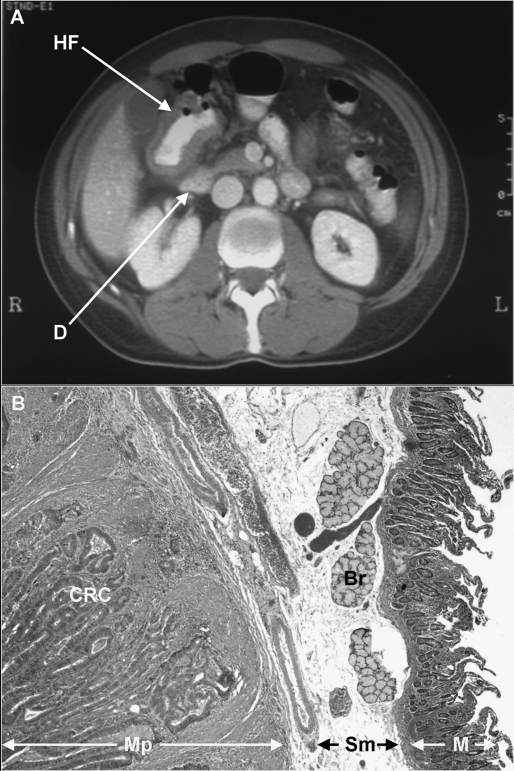
Figure 1.
(A) Computed tomography scan of a 52-year-old patient with a hepatic flexure (HF) colon adenocarcinoma adjacent to the duodenum (D). The patient underwent right colectomy and en bloc partial duodenectomy. (B) A photomicrograph of the lesion (H&E stain, ×20) demonstrating moderately differentiated colonic adenocarcinoma (CRC) invading into the muscularis propria (Mp) of the duodenum. Sm, small bowel submucosa; Br, Brunner’s glands; M, mucosa.
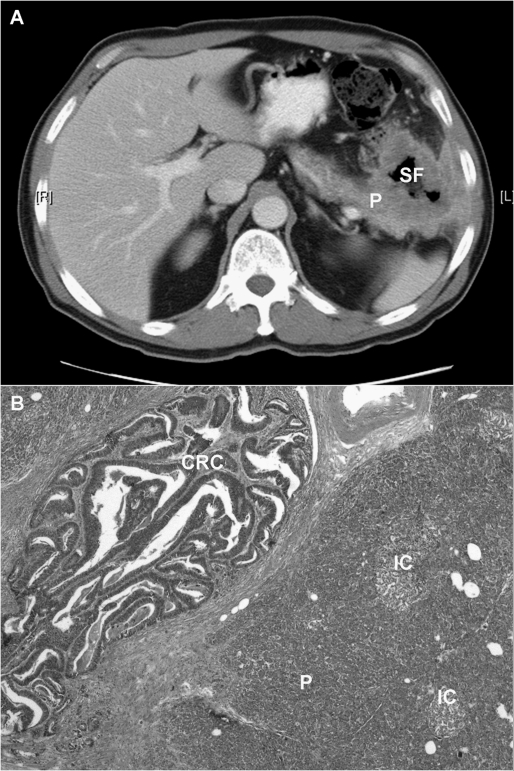
Figure 2.
(A) Computed tomography scan of a 62-year-old patient with a splenic flexure (SF) colon adenocarcinoma invading the tail of the pancreas (P) and into the abdominal wall. The patient underwent left hemicolectomy with en bloc resection of the pancreatic tail, spleen, abdominal wall, and portion of the left hemidiaphragm. (B) A photomicrograph of the lesion (H&E stain, ×20) revealing moderately differentiated colonic adenocarcinoma (CRC) invading into the pancreas (P). IC, Islet cells of Langerhans surrounded by pancreatic acinar cells.
In a recent report of 201 cases of colorectal cancer with multivisceral resection for suspected local tumor infiltration, Lehnert el al17 addressed the issue of intraoperative assessment. In this cohort of 139 locally advanced colon lesions, a total of 225 organs were adherent to tumor and were resected in continuity with the colon lesion (Table 1).12,17,18,19 Histologic evidence of tumor infiltration was noted in only 50% of cases. Interestingly, the surgeons involved in this study were not able to discern malignant fistula from benign inflammation intraoperatively.
Table 1.
Multivisceral Organ Resection for Locally Advanced Colon Cancer
| Organs/Structures* | Montesani, 199119 | Gebhardt, 199918 | Lehnert, 200217 | Taylor, 200212 |
|---|---|---|---|---|
| Patients (n) | 38 | 173 | 139 | 25 |
| Abdominal wall/diaphragm/peritoneum | 11 | 40 | 32 | 7 |
| Retroperitoneum | 3 | 2 | ||
| Duodenum/small bowel/appendix | 9 | 67 | 59 | 8 |
| Pancreas | 1 | 11 | 4 | |
| Prostate/seminal vesicle | 16 | |||
| Ureter/bladder | 9 | 46 | 40 | 9 |
| Uterus/tubes/ovary | 9 | 67 | 28 | 6 |
| Stomach/spleen | 2 | 13 | 26 | 2 |
| Liver | 2 | 2 | 6 | 1 |
| Kidney/adrenal | 1 | 6 | 13 | 2 |
| Gallbladder/bile duct | 10 | |||
| Multiple | 3 | 71 | 13 |
Not mutually exclusive.
SURGICAL RESULTS FOR LOCALLY ADVANCED COLON CANCER
The results of surgery for locally advanced colon cancer are well characterized in the study reported by Lehnert et al.17 Complete resections, with microscopic negative margins (R0), were possible in 91 of the 139 cases, and this factor was the greatest predictor of survival. Outcome following complete resections was stratified by pathologic stage with 5-year survival reported as 69%, 36%, and 13% for stage II, III, and IV, respectively. Locally advanced tumors did not have a poor outcome when completely resected. When controlled for stage, survival for the cohort requiring multivisceral organ resection was not significantly different from that of 773 concurrently treated colon cancer patients without local tumor infiltration. Furthermore, in the cohort of completely resected patients, multivisceral organ resection and malignant fistula were not predictors of poor outcome on multivariate analysis. Morbidity and mortality for patients undergoing extended resection (complete and incomplete resection) included 39 patients (28%) suffering complications and 13 perioperative deaths (9.4%). Over half of the perioperative mortality in the palliative surgery cohort was secondary to progression of disease. The authors concluded that en bloc resection of all adherent organs/structures should be performed, as the presence of malignant infiltration cannot be reliably predicted intraoperatively and dissection of a malignant fistula can lead to tumor spillage. In support of their hypothesis, the authors noted that their outcome is superior to that of reported series in which in-continuity resection was not performed.20,21,22,23
Other studies confirm the need for complete en bloc resection of locally advanced colon cancer. The Mayo Clinic reported a series of 25 patients treated with multimodal therapy.12 The majority of these patients (20 of 25) presented after surgical exploration elsewhere. All patients underwent resection of either one (n = 12), two (n = 10), or three (n = 3) adjacent organs in addition to the primary colon lesion. There were 11 patients with stage II (T4N0) disease and 14 with stage III (T4N1/2) disease. A total of 15 patients had complete resections (R0), 7 had positive microscopic margins (R1), and 3 had grossly incomplete resections (R2). For the entire cohort the median survival was 38 months, with a 5-year disease-specific survival of 49%. Distant metastasis was the most common mode of failure, and local relapse was noted in 12% of patients. The authors concluded that extended resection can result in long-term survival but advocated liberal use of adjuvant systemic chemotherapy.
Curley et al24 reported a primary colon cancer series involving genitourinary symptoms. Malignant invasion of the ureter or bladder was documented in 71 of 101 patients. For the entire cohort of patients, the 5-year actuarial survival rate was 54%. Not surprisingly, positive resection margins had a negative impact on survival.
A more challenging situation arises when extended resection includes higher risk procedures such as partial duodenectomy and/or pancreatectomy. Koea et al25 reported eight cases of T4 right colon lesions requiring either pancreatic or duodenal resections. The patients required right colectomy and en bloc duodenectomy (n = 4) or pancreaticoduodenectomy (n = 4) to ensure complete resection. There were only two minor complications and no deaths. Six patients remained alive without evidence of disease at a median follow-up of 26 months, and one survivor was free of disease at 84 months. In another study reported by Curley et al,26 12 patients underwent en bloc lateral duodenectomies (n = 5) or pancreaticoduodenectomies (n = 7) at the time of colon resection. Eight patients were described as alive with no evidence of disease at a median of 42 months. A similar report by Berrospi et al27 noted disease-free survival ranging from 10 to 113 months. These series provide evidence supporting aggressive resection of adjacent organs, including the pancreas, for locally advanced colon cancer, provided this can be performed with acceptable morbidity and mortality. When a surgeon is not prepared to undertake an extended resection, the patient is better served by referral to a tertiary center for reoperation rather than by incomplete resection.
LOCALLY RECURRENT COLON CANCER
Approximately 40% of patients with resected colon cancer have recurrences, and the majority have a relapse initially at distant sites. Locoregional recurrence, as the first site of disease, is much less common, constituting 10% to 20% of all recurrences.9 The mechanism of local recurrence includes incomplete resection of transmural, mural, or lymphatic disease; tumor shedding; and local implantation.28 Surgery remains the preferred treatment modality; however, it is clear that complete resection is necessary to achieve long-term survival.
The largest series of attempted salvage surgery for locoregional recurrences have been reported by Memorial Sloan-Kettering Cancer Center2 (MSKCC) and the Mayo Clinic.12 The MSKCC series described 100 patients, and the Mayo Clinic series described 73 patients. In both series, patients initially underwent curative colectomy, developed locoregional recurrence, and subsequently underwent laparotomy for resection with curative intent. The primary lesions that led to recurrence in the MSKCC series were generally advanced tumors extending through the intestinal wall: 85% were T3 or T4, 11% were obstructing, and 13% had evidence of perforation. Both studies concurred that the majority of primary tumors were distal to the splenic flexure. Not surprisingly, lymph node metastases were noted in only 50% of patients in the MSKCC study and 60% in the Mayo Clinic study, indicating that the mechanism of locoregional recurrence generally includes incomplete resection of extensively infiltrating tumor. This hypothesis is supported by large retrospective series of resected colon cancer patients in which locoregional recurrence is not predicted by the nodal stage of the primary tumor.7,9,29
As has been described for the majority of gastrointestinal tumor relapses, locoregional recurrence generally occurs within 3 years of resection. Both the MSKCC and Mayo Clinic series reported a median time to failure of 24 months. Approximately 50% of patients had symptoms heralding recurrence in the MSKCC series; the most common complaint was pain in 35%, followed by bleeding and symptoms of partial obstruction including nauseas, emesis, and malaise. Carcinoembryonic antigen (CEA) levels were elevated at the time of detection of relapse in 51% of cases. The most frequent methods utilized to diagnose and confirm recurrence included CT scan in 62%, colonoscopy in 45%, and positron emission tomography in 11%. In the MSKCC series, 26% of patients had synchronous and resectable distant disease, most commonly noted in liver or lung.
Locoregional recurrence can be categorized into four groups on the basis of location and may be related to mechanism of failure.2,4,9 These four groups are perianastomotic (mural disease), mesenteric (regional nodal disease), retroperitoneal (drop metastases, distant nodal disease, or residual disease transmural disease), and peritoneal. At the time that relapse is detected, there is often considerable overlap and ambiguity in these categories; however, this schema does have prognostic significance.2 In the MSKCC series, perianastomotic single-site recurrence was the most prevalent (36%), followed by peritoneal (16%), mesenteric (15%), and retroperitoneal (12%). Not surprisingly, two sites of recurrence were noted in 21% of cases, with the most common combination involving the anastomosis and peritoneum. Interestingly, site of recurrence was related to clinicopathologic features, with left-sided primary tumors more commonly associated with anastomotic recurrence and clinical obstruction associated with peritoneal disease.
The Mayo Clinic series attempted to delineate more accurately the various types of nodal recurrence. Although some cases of recurrence were attributable to inadequate mesenteric resections, most nodal relapses in this series were at sites not included in standard colon oncologic resections (i.e., para-aortic, celiac, and iliac).
SURGICAL SALVAGE RESULTS FOR LOCALLY RECURRENT COLON CANCER
In the MSKCC series of 100 patients taken to surgery with curative intent, 56 patients underwent complete resection. Thirty patients had incomplete resection, with 11 and 19 having microscopic and macroscopic residual disease, respectively. Fourteen patients were found to be unresectable at exploration. In an attempt at complete resection, 41 patients required extended resection with en bloc removal of adjacent organs or structures; most commonly the abdominal wall, ureter, kidney, stomach, uterus, and pancreas. Nine patients required resection of multiple organs. Twenty-six patients had synchronous distant disease, thought to be resectable at the time of salvage surgery. Overall, 21 of these patients had complete, distant metastasectomy. The median hospital stay was 9 days, operative mortality was 1%, and perioperative morbidity was 24%. Morbidity was highest (36%) in the 14 patients deemed unresectable at time of surgery. Thirty-one patients received external beam and/or intraoperative radiation therapy as part of the treatment plan.
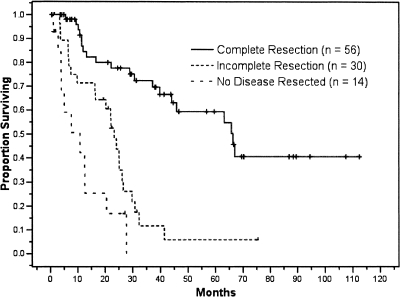
Outcome following salvage surgery for locoregional recurrence is closely associated with completeness of resection (Fig. 3). In the MSKCC series, postsalvage actuarial 5-year survival for the entire cohort was 35%, with a median survival of 30 months. In the 56 patients who were able to undergo complete (R0) resection, 5-year survival was 58% and median survival was 66 months. Incomplete resection, resulting in either microscopic (R1) or macroscopic (R2) residual disease, was associated with a significantly worse outcome. Median survival for patients undergoing R1 (n = 11) and R2 (n = 19) resections was 25 months and 23 months, respectively. There were no 5-year survivors in either of the incomplete resection cohorts. In this series, 14 patients were explored but were found to be unresectable, and these patients had a median survival of less than 12 months.
Figure 3.
Kaplan-Meier survival curves divided by resection type. The 5-year disease-specific survival for patients who underwent a curative R0 resection, incomplete R1 and R2 resection, or no disease resection was 58%, 5%, and 0%, respectively (p < 0.0001).
In addition to completeness of resection, the MSKCC series described several factors that predicted outcome following salvage surgery, including number of sites of recurrence, location of recurrence, presalvage CEA, age, evidence of distant disease, and stage of primary tumor. The locally recurrent classification scheme predicted outcome. Patients with more than one site of locoregional recurrence and patients with evidence of mesenteric recurrence rather than anastomotic, retroperitoneal, or peritoneal relapse had worse outcomes. The presence of synchronous distant metastases also predicted poor outcome. However, 12% of the patients with distant and local recurrence were alive more than 5 years after resection, indicating that the presence of synchronous distant relapses is not a contraindication to salvage surgery. Time to recurrence was not a significant predictor of outcome, which is somewhat surprising, as an extended disease-free interval suggests a more indolent cancer biology.5,12,30 This may represent a selection bias inherent in surgical retrospective series.2,12,31 Not surprisingly, extent of resection and number of adjacent organs removed at surgery did not influence outcome.2
The Mayo Clinic series includes 73 patients who underwent surgical exploration for locoregional recurrence. Complete resection was achieved in 52% of these patients. Incomplete resection included microscopic and macroscopic residual disease in 26% and 22%, respectively. Overall, 51% of patients had complications; 70% were minor and 30% major, including one perioperative mortality. All patients received either external beam radiation and/or intraoperative radiation in the course of their treatment.12 For the entire cohort, the actuarial 5-year survival was 25%, and the median survival was 33 months (reported from time of recurrent disease diagnosis). Complete (R0) resection was accomplished in 38 patients (52%) and was associated with a significantly improved 5-year survival of 37%. Thirty-five patients had incomplete resection: 19 with microscopic (R1) and 16 with macroscopic (R2) residual disease. There was no statistical difference in outcome in the incompletely resected cohorts, with a 25% 5-year survival noted in the R1 cohort and no 5-year survivors in the R2 group.
These results emphasize the well-described relationship between completeness of resection and outcome. Long-term survival is rarely, if ever, achieved with partial resection. It appears that any residual disease, either microscopic or macroscopic, adversely affects outcome. The challenge for clinicians is to determine which patients may be amenable to complete surgical resection and avoid incomplete resection, which only delays initiation of other treatment modalities.
The MSKCC series was large enough to identify factors associated with complete, R0 resection of locoregional recurrence. Patients with a single site of disease, perianastomotic (versus mesenteric, retroperitoneal, or peritoneal) recurrence, low presalvage CEA, and absence of distant disease were more likely to be rendered free of disease with salvage surgery. Although not absolute, these factors may be identified on preoperative studies and help stratify patients with regard to surgical success. The patients with peritoneal disease and nodal/mesenteric recurrence, two sites of local recurrence, elevated CEA, and synchronous distant disease are unlikely to be completely resected and may be better served with neoadjuvant therapy prior to attempted surgical resection.
CHEMOTHERAPY AND RADIATION FOR LOCALLY ADVANCED AND LOCOREGIONAL COLON CANCER RECURRENCE
Adjuvant chemotherapy following complete resection of primary colon cancer, including 5-fluorouracil and leucovorin, is well proved to reduce recurrence and mortality by 30% in patients with stage III colon cancer.32 The addition of a third drug, oxaliplatin, to this regimen has been shown to improve outcome further.33 The use of adjuvant therapy in stage II disease is considerably more controversial because the vast majority of these patients are cured as a result of surgery alone. However, up to 20% of stage II colon cancer patients have recurrences and presumably would have benefited from postoperative chemotherapy. Therefore, adjuvant chemotherapy is administered selectively for stage II patients considered high risk for relapse. Poor prognostic features include locally advanced disease with tumor obstruction, perforation, or evidence of malignant fistulas was well as histologic evidence of lymphovascular invasion or poor differentiation.34
For patients with recurrent and metastatic colon cancer, the addition of biologic therapy such as bevacizumab (an antibody against vascular endothelial growth factor) to conventional chemotherapy has proved effective, extending median survival to greater than 20 months.34a The use of these agents prior to surgery for locoregional recurrence to aid in resection and improve outcome is an exciting treatment option but needs to be studied in a prospective manner.35
Radiation therapy is widely employed to treat locally advanced rectal cancer where disease is situated in the pelvis. This modality is less commonly utilized for colon cancer, where the deliverable dose to the abdomen is limited by the risk of small bowel toxicity.36 In a retrospective series utilizing historical controls, Willett et al37 reported improved survival in patients treated with adjuvant external beam radiotherapy following surgery for locally advanced colon cancer. Other centers have supplemented external beam radiotherapy with intraoperative radiotherapy to achieve a more effective dose while avoiding toxicity to surrounding structures. The Mayo Clinic reported two retrospective series utilizing this technique for locally advanced colon cancer, with acceptable toxicity and encouraging results.36,38 However, a prospective randomized trial designed to answer the question of whether chemoradiation improved outcome for patients with locally advanced colon cancer failed to accrue sufficient patients.39 Of the 187 patients analyzed, 93 received bolus 5-fluorouracil, oral levamisole and concomitant 4500 cGy in 25 fractions; the remaining 94 received weekly chemotherapy only. Grade 3 leukopenia was significantly greater in the chemoradiation cohort, but there was no difference in nonhematologic toxicity. There was no difference in disease-free or overall survival; however, the series was severely underpowered.
Chemoradiation is widely utilized for recurrent rectosigmoid cancer when locoregional disease is limited to the pelvis but is less commonly used to treat colon cancer recurrence when disease is situated in the abdomen because of dose limitations. The few reports describing this modality for abdominal disease note acceptable toxicity31,40; however, the added benefit of radiotherapy in the completely resected patient is difficult to determine without data from a randomized trial. Whether radiotherapy can downsize tumor, thereby assisting complete resection of locoregional recurrence or improving outcome in the patients with incomplete resection, has yet to be proved.
CONCLUSION
Locally advanced primary colon cancer and locoregional recurrent colon cancer pose similar treatment challenges for the surgeon. Complete resection is a requisite for long-term survival. In the case of locally advanced colon cancer, differentiating malignant invasion from benign adhesion is often not possible in the operating room. Because dissection of a malignant fistula and violating tumor planes are associated with tumor spillage and a worse outcome, en bloc resection of involved structures is recommended. In some cases this may require multiorgan resection; however, cure is quite possible if all disease is excised. Proper anticipation of multivisceral organ involvement from physical signs, symptoms, and preoperative imaging ensures that a comprehensive surgical team is assembled to pursue extended resection when necessary.
Similarly, complete resection of locally recurrent colon cancer is necessary for long-term survival. Dissection is often challenging, as anatomic planes are distorted by previous surgery and multivisceral organ resection is commonly required. Radiopaque clips can be useful in outlining the area of dissection if postoperative radiotherapy is to be considered. Patients with a single site of disease; with perianastomotic recurrence rather than mesenteric, retroperitoneal, or peritoneal relapse; with low presalvage CEA; and with absence of distant disease are most likely to be completely resected at salvage. On the other hand, patients with peritoneal disease, with nodal/mesenteric recurrence, with two or more sites of local recurrence, with elevated CEA, and with synchronous distant disease are less likely to be completely resected. Neoadjuvant protocols utilizing conventional and biologic chemotherapy hold promise for downsizing tumors, facilitating complete resection, and prolonging survival.
REFERENCES
- 1.Boey J, Cheung H C, Lai C K, Wong J. A prospective evaluation of serum carcinoembryonic antigen (CEA) levels in the management of colorectal carcinoma. World J Surg. 1984;8:279–286. doi: 10.1007/BF01655052. [DOI] [PubMed] [Google Scholar]
- 2.Bowne W B, Lee B, Wong W D, et al. Operative salvage for locoregional recurrent colon cancer after curative resection: an analysis of 100 cases. Dis Colon Rectum. 2005;48:897–909. doi: 10.1007/s10350-004-0881-8. [DOI] [PubMed] [Google Scholar]
- 3.Gunderson L L, Sosin H, Levitt S. Extrapelvic colon—areas of failure in reoperation series: implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1985;11:731–741. doi: 10.1016/0360-3016(85)90305-0. [DOI] [PubMed] [Google Scholar]
- 4.Galandiuk S, Wieand H S, Moertel C G, et al. Patterns of recurrence after curative resection of carcinoma of the colon and rectum. Surg Gynecol Obstet. 1992;174:27–32. [PubMed] [Google Scholar]
- 5.Gwin J L, Hoffman J P, Eisenberg B L. Surgical management of nonhepatic intra-abdominal recurrence of carcinoma of the colon. Dis Colon Rectum. 1993;36:540–544. doi: 10.1007/BF02049858. [DOI] [PubMed] [Google Scholar]
- 6.Malcolm A W, Perencevich N P, Olson R M, et al. Analysis of recurrence patterns following curative resection for carcinoma of the colon and rectum. Surg Gynecol Obstet. 1981;152:131–136. [PubMed] [Google Scholar]
- 7.Michelassi F, Vannucci L, Ayala J J, et al. Local recurrence after curative resection of colorectal adenocarcinoma. Surgery. 1990;108:787–792; discussion 792–793. [PubMed] [Google Scholar]
- 8.Olson R M, Perencevich N P, Malcolm A W, et al. Patterns of recurrence following curative resection of adenocarcinoma of the colon and rectum. Cancer. 1980;45:2969–2974. doi: 10.1002/1097-0142(19800615)45:12<2969::aid-cncr2820451214>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Read T E, Mutch M G, Chang B W, et al. Locoregional recurrence and survival after curative resection of adenocarcinoma of the colon. J Am Coll Surg. 2002;195:33–40. doi: 10.1016/s1072-7515(02)01224-3. [DOI] [PubMed] [Google Scholar]
- 10.Russell A, Tong D, Dawson L E, Wisbeck W. Adenocarcinoma of the proximal colon: sites of initial dissemination and patterns of recurrence following surgery alone. Cancer. 1984;53:360–367. doi: 10.1002/1097-0142(19840115)53:2<360::aid-cncr2820530232>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 11.Safi F, Link K H, Beger H G. Is follow-up of colorectal cancer patients worthwhile? Dis Colon Rectum. 1993;36:636–644. doi: 10.1007/BF02238589. [DOI] [PubMed] [Google Scholar]
- 12.Taylor W E, Donohue J H, Gunderson L L, et al. The Mayo Clinic experience with multimodality treatment of locally advanced or recurrent colon cancer. Ann Surg Oncol. 2002;9:177–185. doi: 10.1007/BF02557371. [DOI] [PubMed] [Google Scholar]
- 13.Umpleby H C, Bristol J B, Rainey J B, Williamson R CN. Survival of 727 patients with single carcinomas of the large bowel. Dis Colon Rectum. 1984;27:803–810. doi: 10.1007/BF02553944. [DOI] [PubMed] [Google Scholar]
- 14.Willett C G, Tepper J F, Cohen A M, Orlow E, Welch C E. Failure patterns following curative resection of colonic carcinoma. Ann Surg Oncol. 1984;200:685–690. doi: 10.1097/00000658-198412000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Joint Committee on Cancer Colon and rectum. AJCC Cancer Staging Manual. 6th ed. New York: Springer; 2002. pp. 113–124.
- 16.Lopez-Kostner F, Fazio V W, Vignali A, et al. Locally recurrent rectal cancer: predictors and success of salvage surgery. Dis Colon Rectum. 2001;44:173–178. doi: 10.1007/BF02234289. [DOI] [PubMed] [Google Scholar]
- 17.Lehnert T, Methner M, Pollok A, et al. Multivisceral resection for locally advanced primary colon and rectal cancer: an analysis of prognostic factors in 201 patients. Ann Surg. 2002;235:217–225. doi: 10.1097/00000658-200202000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gebhardt C, Meyer W, Ruckriegel S, Meier U. Multivisceral resection of advanced colorectal carcinoma. Langenbecks Arch Surg. 1999;384:194–199. doi: 10.1007/s004230050191. [DOI] [PubMed] [Google Scholar]
- 19.Montesani C, Ribotta G, De Milito R, et al. Extended resection in the treatment of colorectal cancer. Int J Colorectal Dis. 1991;6:161–164. doi: 10.1007/BF00341238. [DOI] [PubMed] [Google Scholar]
- 20.Gall F P, Tonak J, Altendorf A. Multivisceral resections in colorectal cancer. Dis Colon Rectum. 1987;30:337–341. doi: 10.1007/BF02555450. [DOI] [PubMed] [Google Scholar]
- 21.Hermanek P. Multivisceral resection of colorectal cancer—experiences of the Colorectal Cancer Study Group. Langenbecks Arch Chir Suppl Kongressbd. 1992:95–100. [PubMed] [Google Scholar]
- 22.Hunter J A, Ryan J A, Jr, Schultz P. En bloc resection of colon cancer adherent to other organs. Am J Surg. 1987;154:67–71. doi: 10.1016/0002-9610(87)90292-3. [DOI] [PubMed] [Google Scholar]
- 23.Lopez M J. Multivisceral resections for colorectal cancer. J Surg Oncol. 2001;76:1–5. doi: 10.1002/1096-9098(200101)76:1<1::aid-jso1000>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 24.Curley S A, Carlson G W, Shumate C R, et al. Extended resection for locally advanced colorectal carcinoma. Am J Surg. 1992;163:553–559. doi: 10.1016/0002-9610(92)90554-5. [DOI] [PubMed] [Google Scholar]
- 25.Koea J B, Conlon K, Paty P B, et al. Pancreatic or duodenal resection or both for advanced carcinoma of the right colon: is it justified? Dis Colon Rectum. 2000;43:460–465. doi: 10.1007/BF02237187. [DOI] [PubMed] [Google Scholar]
- 26.Curley S A, Evans D B, Ames F C. Resection for cure of carcinoma of the colon directly invading the duodenum or pancreatic head. J Am Coll Surg. 1994;179:587–592. [PubMed] [Google Scholar]
- 27.Berrospi F, Celis J, Ruiz E, Payet E. En bloc pancreaticoduodenectomy for right colon cancer invading adjacent organs. J Surg Oncol. 2002;79:194–197; discussion 198. doi: 10.1002/jso.10072. [DOI] [PubMed] [Google Scholar]
- 28.Rich T A, Terry N H, Meistrich M, et al. Pathologic, anatomic, and biologic factors correlated with local recurrence of colorectal cancer. Semin Radiat Oncol. 1993;3:13–19. doi: 10.1053/SRAO00300013. [DOI] [PubMed] [Google Scholar]
- 29.Stipa S, Noclanti V, Botti C, et al. Local recurrence after curative resection for colorectal cancer: frequency, risk factors and treatments. J Surg OncoL Suppl. 1991;2:155–160. doi: 10.1002/jso.2930480532. [DOI] [PubMed] [Google Scholar]
- 30.Fong Y, Fortner J, Sun R L, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer. Analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318; discussion 318–321. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pezner R D, Chu D Z, Wagman L D, et al. Resection with external beam and intraoperative radiotherapy for recurrent colon cancer. Arch Surg. 1999;134:63–67. doi: 10.1001/archsurg.134.1.63. [DOI] [PubMed] [Google Scholar]
- 32.Moertel C G, Fleming T R, Macdonald J S, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322:352–358. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 33.Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 34.Benson A B, III, Schrag D, Somerfield M R, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408–3419. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 34a.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 35.Schrag D, Weiser M, Schattner M, et al. An increasingly common challenge: management of the complete responder with multi-focal metastatic colorectal cancer. J Clin Oncol. 2005;23:1799–1802. doi: 10.1200/JCO.2005.02.185. [DOI] [PubMed] [Google Scholar]
- 36.Gunderson L L, Nelson H, Martenson J A, et al. Intraoperative electron and external beam irradiation with or without 5-fluorouracil and maximum surgical resection for previously unirradiated, locally recurrent colorectal cancer. Dis Colon Rectum. 1996;39:1379–1395. doi: 10.1007/BF02054527. [DOI] [PubMed] [Google Scholar]
- 37.Willett C G, Fung C Y, Kaufman D S, et al. Postoperative radiation therapy for high-risk colon carcinoma. J Clin Oncol. 1993;11:1112–1117. doi: 10.1200/JCO.1993.11.6.1112. [DOI] [PubMed] [Google Scholar]
- 38.Haddock M G, Nelson H, Donohue J H, et al. Intraoperative electron radiotherapy as a component of salvage therapy for patients with colorectal cancer and advanced nodal metastases. Int J Radiat Oncol Biol Phys. 2003;56:966–973. doi: 10.1016/s0360-3016(03)00189-5. [DOI] [PubMed] [Google Scholar]
- 39.Martenson J A, Jr, Willett C G, Sargent D J, et al. Phase III study of adjuvant chemotherapy and radiation therapy compared with chemotherapy alone in the surgical adjuvant treatment of colon cancer: results of intergroup protocol 0130. J Clin Oncol. 2004;22:3277–3283. doi: 10.1200/JCO.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 40.Alektiar K M, Zelefsky M J, Paty P B, et al. High-dose-rate intraoperative brachytherapy for recurrent colorectal cancer. Int J Radiat Oncol Biol Phys. 2000;48:219–226. doi: 10.1016/s0360-3016(00)00634-9. [DOI] [PubMed] [Google Scholar]