Abstract
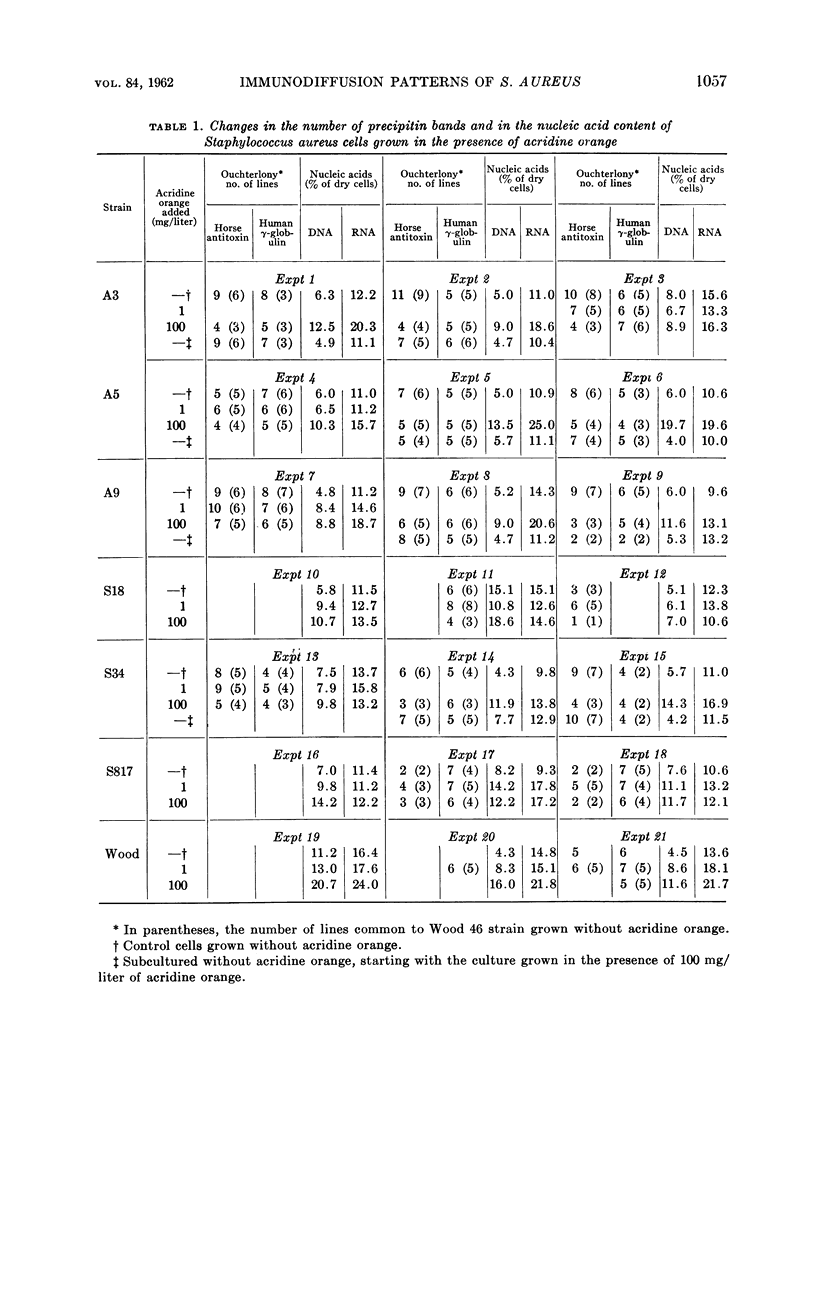
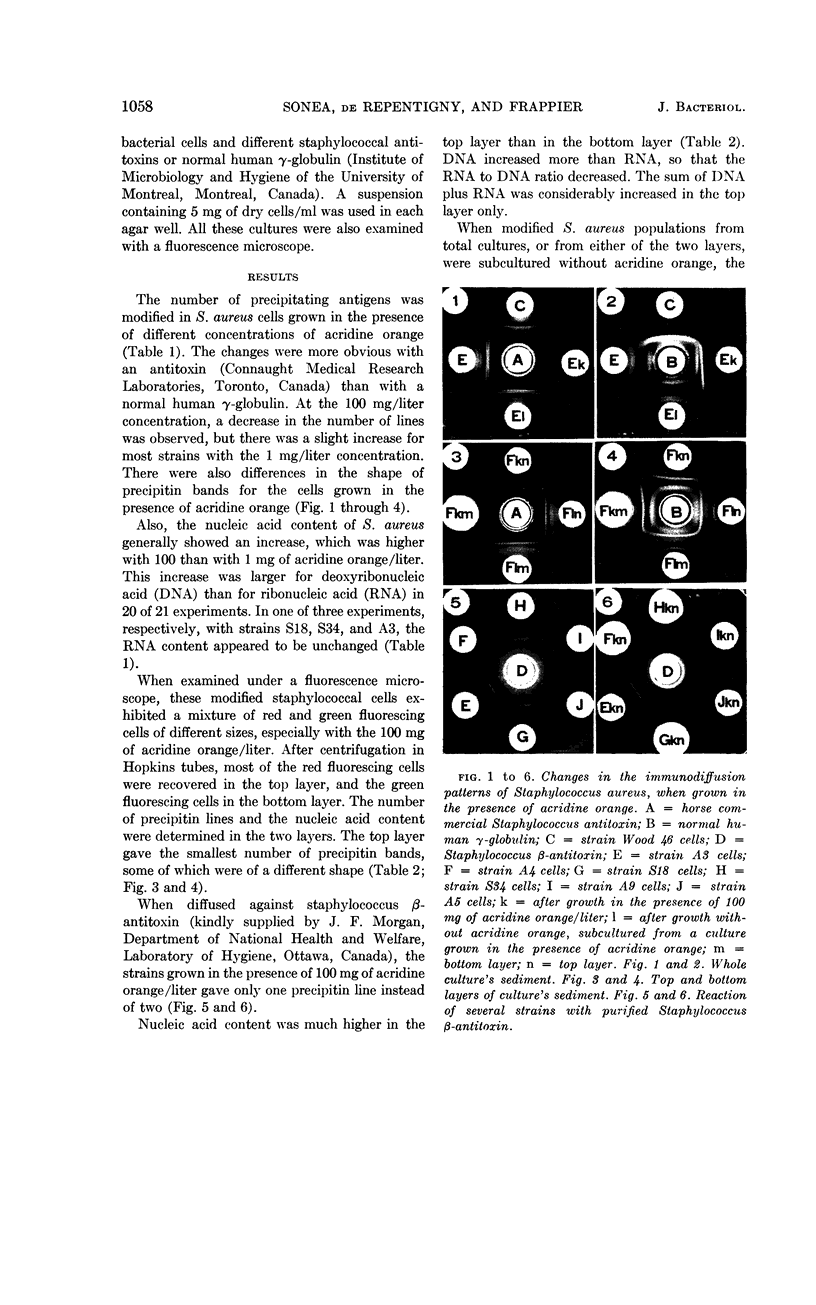
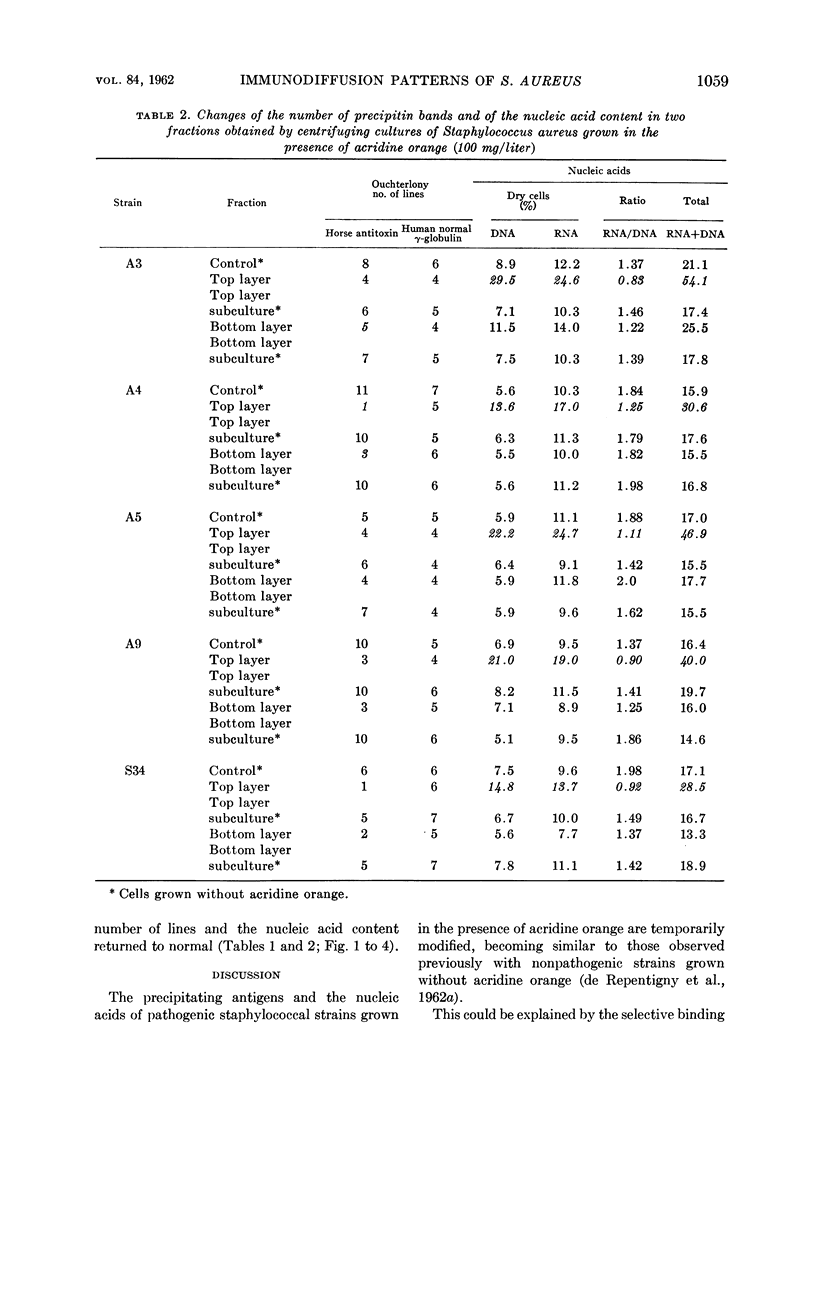
Sonea, S. (University of Montreal, Montreal, Canada), J. de Repentigny, and A. Frappier. Changes in immunodiffusion patterns and in nucleic acid content of Staphylococcus aureus grown in the presence of a nucleic acid fluorochrome. J. Bacteriol. 84:1056–1060. 1962.—When grown in the presence of acridine orange, coagulase-positive Staphylococcus aureus exhibits changes in the number and the shape of its precipitin bands; these are more pronounced with commercial horse staphylococcal antitoxin than with normal human γ-globulin. Simultaneously, there is an increase in the content of nucleic acids, especially deoxyribonucleic acid. Consequently, when grown in the presence of this nucleic acid fluorochrome, pathogenic staphylococci become similar to nonpathogenic strains in their antigenic structure and nucleic acid content. The bacterial population becomes a mixture of red and green fluorescing cells of different sizes. After centrifugation, most of the large and red fluorescing cells are found in the top layer, which shows more changes in antigenic composition and in nucleic acid content than the bottom layer. In subcultures grown in the absence of acridine orange, the cells revert to the original composition of the pathogenic strains.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, HENDLEY D. D., STEINER R. F. Inhibition and activation of polynucleotide phosphorylase through the formation of complexes between acridine orange and polynucleotides. Nature. 1958 Jul 26;182(4630):242–244. doi: 10.1038/182242a0. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATLIN B. W., CUNNINGHAM L. S. Studies of extracellular and intracellular bacterial deoxyribonucleic acids. J Gen Microbiol. 1958 Dec;19(3):522–539. doi: 10.1099/00221287-19-3-522. [DOI] [PubMed] [Google Scholar]
- CERIOTTI G. Determination of nucleic acids in animal tissues. J Biol Chem. 1955 May;214(1):59–70. [PubMed] [Google Scholar]
- FRAPPIER A., SONEA S., PANISSET M. Le pouvoir pathogène des Staphylocoques. I. Etude comparative de 40 souches à l'aide de quatre méthodes d'infection expérimentale. Rev Can Biol. 1955 Sep;14(2):152–172. [PubMed] [Google Scholar]
- FUKUI G. M., LAWTON W. D., LAWTON W. D., MORTLOCK R. P. Synthesis of virulence factors by Pasteurella pestis. J Bacteriol. 1961 Apr;81:656–660. doi: 10.1128/jb.81.4.656-660.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LERMAN L. S. Structural considerations in the interaction of DNA and acridines. J Mol Biol. 1961 Feb;3:18–30. doi: 10.1016/s0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- de REPENTIGNY, SONEA S., FRAPPIER A. [Microfluorometric study of microorganisms. I. Primary fluorescence (autofluorescence)]. Ann Inst Pasteur (Paris) 1961 Sep;101:353–366. [PubMed] [Google Scholar]