ABSTRACT
The majority of hepatic metastases in the United States occur in patients with a primary colorectal malignancy. Advances in technology combined with increasing surgeon experience have broadened the treatment options available for hepatic metastases from colorectal cancer. Surgical resection is the most effective therapy for metastatic colorectal cancer isolated to the liver. The aim of this article is to discuss the role of locally aggressive treatment options including resection, ablation, and regional chemotherapy in the management of patients with metastases from colorectal cancer.
Keywords: Hepatic metastases, colorectal cancer, hepatic resection, hepatic ablation
Colon and rectal cancer is currently the third most commonly diagnosed cancer in the United States. Approximately 145,000 new cases of colorectal cancer are diagnosed each year. The survival from colorectal cancer has improved over the past few decades, primarily because of earlier detection and treatment. However, nearly half of these patients eventually succumb to their malignancy. Annually, more than 56,000 deaths are attributable to colon and rectal cancer.1
The liver is the most common site of metastases for tumor sites that drain initially via the portal circulation. Metastatic liver disease is found in 10% to 25% of patients having surgery for primary colorectal cancer.2,3,4 If hepatic interrogation is specifically pursued with computed tomography (CT) and intraoperative ultrasonography, the incidence appears to be close to 35% in patients with otherwise curable primary disease.5,6 Extrahepatic metastases consisting of pulmonary, distant nodal (portal, celiac), and/or peritoneal disease are present in at least half of patients found to have hepatic metastases.7 Thus, of the 145,000 patients with newly diagnosed colorectal cancer, ~30,000 to 40,000 are expected to develop either synchronous or metachronous metastases that are apparently confined to the liver.
RISK ASSESSMENT
It is important to acknowledge that not all hepatic lesions in a patient with a history of colorectal cancer are metastatic disease, although a majority are. Many other mass lesions are known to occur with some frequency, and it is important to rule these out. Such lesions include benign liver cysts, hemangiomas, adenomas, focal nodular hyperplasia, hepatocellular carcinoma, and metastases from other sites. The ability to distinguish between these disorders and colorectal metastases relies on an adequate history of the patient, laboratory studies, and imaging studies. This combination allows accurate preoperative diagnosis in most patients. Preoperative biopsy is rarely necessary in patients with resectable disease. As the chemotherapeutic regimens in the adjuvant and metastatic settings currently differ, patients who are not candidates for resection usually require a tissue diagnosis or definitive imaging before beginning chemotherapy. In this setting, percutaneous biopsy may be appropriate. The presence of a new mass lesion, increasing levels of carcinoembryonic antigen (CEA), and a history of colorectal cancer should provide enough evidence of disease to justify treatment. Biopsy carries risk for tumor dissemination (which should be enough to discourage unwarranted biopsies) as well as minor risks for bleeding and pneumothorax.
Goals for management of metastatic colorectal cancer are to provide the patient with an optimum quality of live for the longest duration possible. Resection of hepatic metastases is now associated with long-term survival and low mortality such that patients who would have been denied surgical treatment in previous eras are now routinely offered locally aggressive treatment options. However, it is important to realize that cure is not a realistic goal for most patients with hepatic metastases, and we should strive to determine which patients can be provided with more quality time while sparing the patients with relatively poor functional levels and limited survival time from treatments that are unlikely to provide effective survival benefit. In determining an appropriate form of treatment for a particular patient, a thorough evaluation aimed at providing a diagnosis, establishing the extent of disease, and estimating the functional level of the patient are critical.
Although liver metastases have been documented to occur in patients with early-stage colorectal cancer, the likelihood of developing hepatic metastases is correlated with the original primary tumor and nodal staging. In patients with node-negative primary disease without extension beyond the muscularis propria (T1–2N0M0, stage I), the 5-year survival rate is 75% to 90%8,9; in patients with node-negative primary disease involving the serosa (T3N0M0, stage IIA, AJCC [American Joint Committee on Cancer], 6th ed.), the figure is ~65% to 80%; and those with node-positive primary disease (T1–3N1–3M0) may expect a 45% to 65% likelihood of survival beyond 5 years. Adjuvant chemotherapy in patients with stage III disease has been shown in prospective randomized trials to diminish risk for recurrence by 40% (relative to watchful waiting) after curative resection of the primary disease.10 Overall 5-year survival of patients with rectal cancer has been reported to be 72% for stage I, 54% for stage II, and 39% for stage III.11 Although the group most likely to benefit from adjuvant therapy for rectal cancer remains controversial, chemotherapy for patients with stage III disease and radiation therapy (before or after surgery) in patients with T3 to T4 primary disease are generally recommended and have been shown to reduce the risk for distant metastases.
PRESENTATION AND DIAGNOSIS
Presentation of liver metastases may be either synchronous or metachronous. Synchronous disease, commonly defined as liver metastasis occurring within 12 months of the colon or rectal primary, represents 13% to 25% of newly diagnosed colorectal liver metastases.3,12,13 Disease is found primarily on preoperative imaging or intraoperative exploration. In these patients, the original presentation is often related to the primary neoplasm, whereas liver metastases are incidental findings. Common presenting symptoms generally include fever, fatigue, weight loss, and anorexia. Patients may also describe a sense of abdominal fullness or even upper abdominal and right flank pain. Physical examination may reveal a palpable liver mass or hepatomegaly, jaundice, and ascites. More commonly, the physical examination is unremarkable. The optimal timing for synchronous liver metastases is controversial. Although a staged approach with initial resection of the primary lesion followed by hepatic resection 3 months later has been practiced, an increasing number of hepatic surgeons are utilizing a simultaneous, collaborative approach with the colorectal surgeon during the initial operation. A prospective analysis revealed that simultaneous colon and liver resection is safe and effective.12 By avoiding a second laparotomy, the overall complication rate is reduced and the treatment time is shortened.
Metachronous disease develops in 20% to 25% of patients.14,15,16 The presentation of metachronous hepatic metastases varies according to the method by which they are detected. In unscreened populations, hepatic metastases can be detected only if the patient is having symptoms referable to the metastatic process. Other more commonly used methods of early detection in patients who previously had curative treatment for primary colorectal cancer include serial serum CEA determination and serial surveillance with cross-sectional imaging and/or ultrasonography. Presently, most metachronous presentations are not amenable to resection because of the late presentation or detection. Thus, dedicated, systematic follow-up for patients with a resected colorectal primary would lead to identification of patients who would benefit from hepatic resection.
The natural history of colorectal hepatic metastases is not encouraging. Although no randomized studies have compared survival in treated populations and untreated populations, several retrospective reviews have documented the outcomes in patients with unresected liver metastases. Among patients with unresected, apparently resectable disease, 5-year survivors are rare, and median survival is ~14 to 21 months.17,18 Current chemotherapeutic regimens report a median survival of 20 months. Patients with unresectable metastases appear to have a median survival of 4 to 12 months.3,17,18,19,20,21,22 Although 1-year survival ranges from 6% to 46% in these series, 5-year survivors are very rare (0% to 2%). Autopsy studies have suggested that the metastatic disease remains confined to the liver in 10% to 30% of patients who die of metastatic colorectal carcinoma.23,24 This observation is also supported by the demonstration that 20% to 25% of patients undergoing curative resection remain free of disease at 10-year follow-up.14,25 Therefore, it is desirable to identify patients who may be benefit from some form of interventional therapy.
Diagnostic tests for hepatic colorectal metastases include serum CEA levels, CT, magnetic resonance imaging (MRI), positron emission tomography (PET), ultrasonography, and laparoscopy. CEA level has a sensitivity of ~75% and a specificity of 90% to 95% in detecting hepatic recurrence. CEA levels also have prognostic significance in patients undergoing evaluation for hepatic metastasectomy. High preoperative levels of CEA have been shown to predict less successful outcomes following hepatic metastasectomy.14 In a review of 1001 patients having hepatic resection for colorectal metastases, a CEA level greater than 200 ng/mL was found to be a predictor of adverse outcome. Patients with a preoperative CEA level less than 200 ng/mL had a median survival of 38 months, and those with a level greater than 200 ng/mL had a median survival of 24 months. Despite this correlation, the preoperative CEA level in isolation is not a reason to preclude potentially curative hepatic resection. Triphasic CT scan has been shown to have a sensitivity greater than 90% in detecting liver lesions compared with the 75% sensitivity of standard contrast-enhanced CT.26,27 MRI should be reserved for patients who cannot receive the contrast load for CT or those with an equivocal CT study. PET is an appropriate second test in patients with increasing levels of CEA and no clear abnormalities on CT of the chest, abdomen, or pelvis. Combined CT-PET devices have also been developed that provide both physiologic and anatomical detail in the same setting. PET can also be useful for detecting extrahepatic disease that would preclude hepatic resection. In one review, when PET was used for screening or staging prior to hepatic resection for metastatic colorectal cancer, the 5-year overall survival was 58.6%. This is higher than that reported in other large studies in which PET is not routinely used. This signifies that PET may afford the advantage of selecting those who may benefit significantly from major surgery. PET has also been shown to change management in ~25% of patients, sparing these patients the risk of significant morbidity and mortality from major operations.28
The most important use of ultrasonography is during surgery. Intraoperative ultrasonography can detect occult colorectal metastases not seen on CT or transabdominal ultrasonography and has an overall sensitivity of 96%.29 Intraoperative ultrasonography is also useful in demonstrating segmental hepatic anatomy. This is particularly important when the tumor is in proximity to the inflow or outflow vessels. The value of intraoperative ultrasonography is operator dependent but in well-trained hands has been shown to alter the preoperative surgical plan in nearly 20% of patients.30
Diagnostic laparoscopy is useful prior to planned hepatic resection for colorectal metastases. It can aid in identifying lesions that may have been missed on preoperative cross-sectional imaging studies. Performance of laparoscopy does add time, expense, and its unique morbidities and has, therefore, not been universally practiced. A clinical risk score (CRS) has been described to clarify the role of pre-resectional laparoscopy yield.31 The CRS comprises five variables: CEA level, lymph node status of primary tumor, disease-free interval (time from colorectal primary to diagnosis of liver metastasis), number of hepatic tumors, and size of hepatic tumors. It has been demonstrated that the yield of laparoscopy increases with increasing CRS. Application of clinical parameters such as these can aid in determining the utility of laparoscopy in settings where it is not routinely applied. This preoperative index is useful in stratifying patients into risk for early recurrence and may help to direct higher risk patients to a neoadjuvant approach. It is important for the clinician to be cognizant of the various modalities available to delineate the extent of disease before embarking on aggressive operative intervention.
SYSTEMIC CHEMOTHERAPY
With ~80% of patients with colorectal hepatic metastases presenting with nonresectable disease, systemic chemotherapy represents the main if not the only form of therapy for many patients. Chemotherapy may also play a role in transforming a portion of patients with unresectable disease into resection candidates. Randomized trials of systemic chemotherapy in patients with unresectable hepatic metastases have been done.32,33 Data from these trials suggest that median survival can be extended by ~4 to 6 months and that adjuvant chemotherapy may at least improve the quality of life. Long-term survival is rare in patients with unresectable disease despite treatment with systemic chemotherapy. Systemic agents have been introduced that may elicit a better response than standard 5-fluorouracil (FU)-leucovorin (LV)–based treatment for advanced disease. Irinotecan (IR) and oxaliplatin (OX) combined with FU-LV appear to have a higher response rate than FU-LV alone.34 IR has also been shown to facilitate resectability in patients initially deemed unresectable.35
Current first-line therapy for metastatic colorectal cancer now includes OX or IR in combination with FU-LV. These combination chemotherapies have demonstrated superior response rates (50% to 60%) compared with the traditional FU-LV regimen (20% to 30%).36
Addition of the monoclonal antibody against vascular endothelial growth factor, bevacizumab, to the combination chemotherapy has been shown to improve survival significantly.37 The success of these newer chemotherapeutic agents has supported the use of neoadjuvant chemotherapy for patients with unresectable disease in the hope of converting them to resection candidates. Reports demonstrate that 13% to 16% of patients could be rendered resectable using neoadjuvant chemotherapy.36 The success of the modern chemotherapeutic agents for metastatic colorectal cancer is creating new opportunities for long-term survival in nonresectable patients. The application of neoadjuvant therapy for resectable disease also warrants investigation in clinical trials.
OPERATIVE MANAGEMENT
Hepatic resection is currently the most effective form of therapy for colorectal metastases confined to the liver. It is important to have a definition of resectability to maintain a standard for evaluation. We define resectability as complete gross resection while retaining a sufficient liver remnant with intact biliary drainage and vasculature. Better understanding of liver anatomy and physiology, complemented by advances in operative technique and postoperative care has resulted in the ability to perform large-volume liver resections with low morbidity and mortality. With nearly 80% of hepatic parenchyma being safe to remove, hepatic resection should be considered in many patients. It is equally important to identify patients who have conditions and/or factors that would preclude a resectional strategy. Accepted contraindications to metastasectomy include poor overall health, inadequate liver reserve, inability to achieve margin-negative resection, and the presence of extrahepatic disease. Some exceptions can be made, such as for patients with limited, resectable pulmonary metastases or isolated portal lymph node metastases. In the absence of any effective alternative, these higher risk patients should be considered on a case-by-case basis and usually within the context of an adjuvant/neoadjuvant protocol. Patients with multiple, small, synchronous metastases represent a difficult population. Most researchers advocate a trial period of systemic chemotherapy for these patients to assess the “biology” of disease because the pace of disease may not be apparent at initial presentation. Patients with large, bulky disease and a short disease-free interval also represent difficult cases in which the overall prognosis may not be altered by an extensive operation.
Anatomic or segmental resections are currently favored over large wedge resections, although there are currently scant data on this subject. The oncologic principle of getting an adequate negative margin should be applied. Anatomic resections offer the best chance for achieving negative margins while maintaining maximum liver parenchyma. In a review of 267 patients with predominantly solitary disease (80%), one study showed that the incidence of positive margins after wedge resection (16%) was significantly higher than that for segmental resection (2%). Median survival was significantly increased in patients having segmental resection (38 versus 55 months). The resection margin is also critical in trying to achieve a cure. A positive histologic margin has been shown to be associated with poor long-term survival. The optimal surgical margin width, however, remains debatable. There have been no definitive studies showing that 1 cm or greater is favorable to a grossly negative margin. Thus, the importance of achieving a negative margin favors anatomic resections over wedge resections. It is likely, however, that a wedge resection is oncologically equivalent in the event that a negative margin can be reliably obtained.
The success of resection of hepatic colorectal metastases has resulted in looking for ways to extend resectability. One technique is through preoperative portal vein embolization. The procedure is based on the physiologic phenomenon of liver atrophy of the embolized lobe and liver hypertrophy of the contralateral lobe. This augments the volume of the remaining liver and allows safe large-volume resectional strategies. The portal vein is usually reached through a percutaneous transhepatic route under ultrasound and fluoroscopic guidance. Increases of up to 50% in the size of the nonembolized lobe in 4 to 6 weeks have been reported.38 The majority of patients, however, should have normal preoperative liver function and do not require such preoperative portal vein embolization.38,39,40
The overall 5-year survival reported following hepatic resection for curative intent for metastatic colorectal cancer in most series ranges from 28% to 50%.14,41,42,43,44,45 Table 1 presents the results of several single-institution series involving more than 150 patients.14,25,41,42,43,44,45 Perioperative mortality ranges from 0% to 4.4% in these series, and overall morbidity rates are ~20% to 33%. Actuarial rates of 5- and 10-year survival following resection are reported to be 24% to 58% and 17% to 24%, respectively. Actual long-term survivors are well documented and are consistent with older autopsy studies showing that a significant proportion of patients die with the liver as the only evident site of disease.23,24 The median survival time shown in these studies ranges from 23 to 46 months.
Table 1.
Prognosis after Hepatic Metastasectomy: Large, Single-Institution Experience
| Author/Year | Patients (n) | Median Survival (mo) | 5-Year Survival (%) | 10-Year Survival (%) | Mortality Rate (%) |
|---|---|---|---|---|---|
| Minagawa 200041 | 235 | 37 | 26 | 24 | 0 |
| Fong 199914 | 1001 | 42 | 37 | 22 | 2.8 |
| Ambiru 199942 | 168 | 23 | 26 | 18 | 3.6 |
| Jamison 199743 | 280 | 32 | 27 | 20 | 4 |
| Scheele 199525 | 434 | 40 | 33 | 20 | 4.4 |
| Doci 199545 | 224 | 30 | 24 | 17 | 2.2 |
| Choti 200250 | 226 | 46 | 40 | 26 | 0.9 |
Overall, the perioperative mortality of liver resections for colorectal metastases is less than 3% in most series. The potential for complications and the complexity of the procedure warrant that they be performed in centers with significant experience. Morbidity from major hepatic resection results from hemorrhage (1% to 3%), bile leak (2% to 5%), perihepatic abscess (1% to 5%), pleural effusion (1% to 5%), and hepatic failure (1% to 3%).
Although surgical resection results in prolonged survival and perhaps even a cure in a few patients, the majority develop recurrence. Analyses of potential prognostic factors (Table 2) have also been reported.14,25,41,42,45 The prognostic factors can be roughly divided into patient-related factors, primary tumor characteristics, and metastatic features. Age, sex, primary tumor location, and the presence of bilateral metastases do not appear to be important predictors of recurrence following metastasectomy. Primary tumor stage is a constant predictor of patients' outcome, and the preoperative CEA level, disease-free interval (time from primary to metastatic disease), number of metastases, and size of the largest metastasis also appear to be important. In much of the older literature, resection was reserved for those with truly solitary metastases while calling for restraint in patients with multiple tumors. A bilateral distribution was often felt to be a predictor of recurrence, yet on the multivariate analyses of these larger studies, this factor was not significant. A CRS based on an experience with 1001 patients having hepatic resection has also been developed.14 This system takes into account five variables that can be ascertained before surgery. These variables are disease-free interval less than 12 months, tumor size greater than 5 cm, more than one metastasis, preoperative CEA level greater than 200 ng/mL, and the presence of a node-positive primary tumor. One point is assigned to each of these adverse prognostic variables. In the study, a group without any adverse features (score = 0) had a median survival time of 74 months and a 5-year survival rate of 60%. Survival diminished as the score increased, such that patients with a score of five had a median survival of 22 months and an actuarial survival of 14%. There appeared to be a breakpoint beyond a score of 2, where 5-year survival greatly diminished from 40% to 20%. Even in the group with the most adverse effects (score = 5), long-term survival and median survival were much greater than would be expected based on series of unresected “resectable” disease.17,18
Table 2.
Factors Affecting Prognosis after Hepatic Metastasectomy
| Author/Year | Patients (n) |
Primary Tumor |
Lobar |
CEA |
Extrahepatic Disease |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | Location | Stage | Synchronous | Size | Distribution | ||||
| CEA, carcinoembryonic antigen. | ||||||||||
| Minagawa 200041 | 235 | No | No | No | Yes | No | No | No | No | No |
| Fong 199914 | 1001 | No | No | No | Yes | Yes | Yes | No | Yes | Yes |
| Ambiru 199942 | 168 | No | No | No | Yes | No | No | No | No | N/A |
| Scheele 199525 | 434 | No | No | No | Yes | No | Yes | No | No | Yes |
| Doci 199545 | 224 | No | No | No | Yes | No | Yes | No | No | N/A |
| Choti 200250 | 226 | N/A | N/A | No | No | No | N/A | No | Yes | N/A |
Recurrence following hepatic metastasectomy generally portends a poor outcome as the distribution of disease tends to be systemic (one third) or extensive within the liver (one third). In ~20% to 30% of patients, recurrent disease is limited and may be amenable to re-resection.46,47,48 Re-resection is technically more difficult given the extensive adhesions and hypertrophy of the liver remnant. The inflow structures are distorted in location and can be treacherous if unrecognized. Nonetheless, the morbidity and mortality and overall survival for re-resection, when done in experienced units, mirror those of primary hepatic metastasectomy. In well-selected patients, further resection has been demonstrated to provide prolonged survival after recurrence of colorectal liver metastases.49,50
ABLATIVE OPTIONS
Ablative therapies are also available for patients with unresectable disease who do not have apparent extrahepatic metastases. Patients with inadequate hepatic reserve despite technically resectable lesions are also candidates. Local ablative techniques such as radiofrequency ablation (RFA) and cryoablation treat tumors in situ, effecting tumor killing by thermal mechanisms. Ablative techniques have rapidly proliferated and are now commonly performed in a percutaneous fashion. Radiofrequency generators produce a rapidly alternating low-voltage current at the treatment electrode. As the current alternates, ions in proximity to the electrode rapidly change directions, resulting in frictional heating. As the current rapidly decreases with increasing distance from the electrode, it is conduction and not frictional heat that enables heating remote from the electrode. Newer generation RFA generators are now easily capable of creating a 5-cm zone of necrosis in ~20 minutes. Cryoablation effects cell death through two mechanisms depending on the rate of cooling. Slow cooling leads to a gradual loss of intracellular fluid and ions as the extracellular fluid freezes, which leads to protein denaturation and membrane disruption. Rapid freezing that occurs in proximity to the cryoprobe leads to intracellular ice formation that disrupts intracellular organelles and membranes. Repeated freeze-thaw cycles increase the radius of effective killing as the previously frozen liver demonstrates increased thermoconductivity.
RFA can be employed through percutaneous, laparoscopic, thoracoscopic, and open procedures. Size and location of the tumor dictate choice of treatment. Ultrasound guidance facilitates accurate and safe probe placement. Small lesions(< 3 cm) located on the periphery are best suited for percutaneous approach. The laparoscopic approach with intraoperative ultrasonography has higher accuracy for detecting hepatic lesions than transcutaneous ultrasonography and is safer for mobilizing and ablating tumors that are close to or in contact with surrounding organs. A thoracoscopic approach is useful for tumors situated on the dome of the liver that are difficult to reach percutaneously or laparoscopically. When a question of efficacy or safety arises, an open approach should be used.
As attractive as ablative therapy may seem, it appears to be inferior to formal hepatic resection in the treatment of colorectal metastases. In addition, there is the issue of recurrence. Hepatic recurrence rates following RFA of 40% to 50% are commonly reported.51 Certain predictors of failure or recurrence have emerged and are listed in Table 3. Size (> 3.5 cm) is a particularly strong indicator for early recurrence at the ablation site. A large series of 418 consecutive patients who underwent treatment for hepatic colorectal metastases with resection or RFA, or both, demonstrated that overall survival was highest after resection (58% at 5 years) compared with the RFA-only group, whose survival tracked close to that of patients treated with chemotherapy alone (less than 20% at 5 years). Recurrence was more common after RFA (84% versus 52% for resection).52
Table 3.
Factors Associated with Recurrence following Radiofrequency Ablation
| Size of lesion > 3.5 cm |
| Proximity to hepatic vasculature |
| Multiple lesions |
| Subcapsular location |
| Tumor histology: adenocarcinoma > neuroendocrine |
| Percutaneous ablation |
The majority of complications from ablation therapy are related to infection or biliary injury, or both. Infectious complications arise from pulmonary, gastrointestinal, and indwelling catheter-related sources that may seed the necrosed, ablated tissue. Biliary injury is most often seen in patients with peribiliary tumors. Visceral damage and cardiac and renal complications can be associated with RFA. Liver failure or insufficiency is rare from ablative measures, although cases have been reported in the literature.53,54,55
HEPATIC ARTERIAL INFUSION
The use of hepatic arterial infusion pump (HAIP) placement and administration of chemotherapy is based on the observation that hepatic tumors derive their blood supply primarily from the hepatic artery, in contrast to normal hepatic parenchyma, which is principally supplied by the portal vein. Therefore, infusion of chemotherapeutic agents through the hepatic arterial circulation should lead to high concentrations of the agents within tumor cells while sparing the normal hepatic parenchyma. In addition, several agents are efficiently extracted within the liver such that systemic concentrations and concomitant toxicities are further reduced. Fluorodeoxyuridine is the most common agent administered by the intra-arterial route, although FU-LV has also been delivered in this fashion. The data on the efficacy of HAIP are mixed, and improved survival cannot be reliably demonstrated.56(Table 4) Furthermore, technical complications and toxicity in the form of biliary sclerosis remain formidable. The rate of biliary sclerosis vary from 1% to 35%.57,58 There is also no survival benefit for HAIP therapy when compared with systemic chemotherapy alone in reported data.58 Given the abundance of newer systemic agents, such as IR and OX, that afford similar response rates with less overall toxicity, the role for HAIP in hepatic colorectal metastases continues to evolve. The role of HAIP in resectable candidates is also being revisited in the era of modern chemotherapy.
Table 4.
Trials of Hepatic Arterial Infusion (HAI) Therapy for Resectable and Unresectable Patients
| Author/Year | Patients (n) | Control Systemic Therapy | HAI Therapy | Response Rate (%) Control vs HAI | Median Survival (mo) Control vs HAI |
|---|---|---|---|---|---|
| 5-FU, 5-fluorouracil; FUDR, fluorodeoxyuridine; LCV, leucovorin; Hep Rxn, hepatic resection. | |||||
| Formulated from data in Barber et al.56 | |||||
| Unresectable | |||||
| Kemeny 1987 | 99 | FUDR | FUDR | 19.6 vs 50 (p < .001) | 12 vs 17 |
| Chang 1987 | 64 | FUDR | FUDR | 17 vs 62 (p < .003) | 12 vs 17 |
| Hohn 1989 | 115 | 5-FU | FUDR | 10 vs 42 (p < .0001) | 16 vs 15.6 |
| Martin 1990 | 69 | 5-FU | FUDR | 21 vs 48 (p < .02) | 10.5 vs 12.6 |
| Rougier 1992 | 152 | 5-FU | FUDR | 9 vs 43 (p < .05) | 11 vs 15 |
| Allen-Mersh 1994 | 100 | Various | FUDR | N/A | 7.5 vs 13.4 |
| Kerr 2003 | 290 | 5-FU/LCV | 5-FU/LCV | 19 vs 22 (NS) | 13.4 vs 14.7 |
| Control Therapy | HAI Therapy + Resection | Median Survival (mo) Control vs HAI Therapy + Resection | |||
| Resectable | |||||
| Wagman 1990 | 11 | Hep. Rxn | FUDR | 28.3 vs 37.3 | |
| Lygidaskis 1995 | 40 | Hep. Rxn | Various | 11 vs 30 (p < .001) | |
| Lorenz 1998 | 211 | Hep. Rxn | 5-FU | 40.8 vs 34.5 | |
| Kemeny 2002 | 109 | Hep. Rxn | FUDR + systemic 5-FU | 49 vs 63.7 | |
| Control | Median Survival (mo) | ||||
| Hep Rxn + Systemic Therapy | HAI Therapy + Resection | Control vs HAI Therapy + Resection | |||
| Lorenz 2000 | 108 | Hep. Rxn + 5-FU/LCV | Hep. Rxn | 17.6 vs 12.7 | |
| FUDR HAI | |||||
| Tono 2000 | 19 | Hep. Rxn + 5-FU | Hep. Rxn | 39.9 vs 62.6 | |
| 5-FU HAI | |||||
| Kemeny 1999 | 156 | Hep. Rxn + 5-FU/LCV | Hep. Rxn | 59.3 vs 72.2 | |
| 5-FU/LCV systemic | 2 year survival 72% vs 86% (p < .05) | ||||
| FUDR HAI | |||||
CONCLUSION
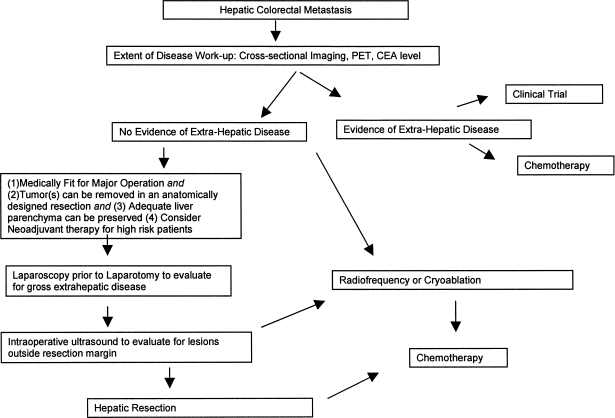
Surgical resection is currently the most effective form of therapy for colorectal metastases isolated to the liver and the only potentially curative modality. Most high-volume centers report a 5-year survival rate of 30% to 40% following resection for hepatic colorectal metastases. A thorough extent of disease work-up with laboratory tests (CEA), cross-sectional imaging, and nuclear and ultrasound imaging should be performed to identify patients with a good performance status who may benefit from hepatic metastasectomy. Adjuvant or neoadjuvant therapy provides a modest survival benefit and should be offered, especially with the optimism about emerging chemotherapeutic agents. Ablative and HAIP therapies are options best reserved for unresectable candidates or to complement resection-based strategies. Figure 1 depicts an algorithm for management of hepatic colorectal metastases.
Figure 1.
Algorithm for the management of hepatic colorectal metastases.
REFERENCES
- 1.Jemal A, Thomas A, Murray T, et al. Cancer statistics. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 2.Bengmark S, Hafstrom L. The natural history of primary and secondary malignant tumors of the liver. I. The prognosis for patients with hepatic metastases from colonic and rectal carcinoma by laparotomy. Cancer. 1969;23:198–202. doi: 10.1002/1097-0142(196901)23:1<198::aid-cncr2820230126>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 3.Cady B, Monson D, Swinton N. Survival of patients after colonic resection for carcinoma with simultaneous liver metastases. Surg Gynecol Obstet. 1970;131:697–700. [PubMed] [Google Scholar]
- 4.Scoggins C, Meszoely I, Blanke C, et al. Nonoperative management of primary colorectal cancer in patients with stage IV disease. Ann Surg Oncol. 1999;6:651–657. doi: 10.1007/s10434-999-0651-x. [DOI] [PubMed] [Google Scholar]
- 5.Ohlsson B, Tranberg K, Lundstedt C, et al. Detection of hepatic metastases in colorectal cancer: a prospective study of laboratory and imaging methods. Eur J Surg. 1993;159:275–281. [PubMed] [Google Scholar]
- 6.Finlay I, McArdle C S. Occult hepatic metastases in colorectal carcinoma. Br J Surg. 1986;73:732–735. doi: 10.1002/bjs.1800730918. [DOI] [PubMed] [Google Scholar]
- 7.Lefor A, Hughes K, Shiloni E, et al. Intra-abdominal extrahepatic disease in patients with colorectal hepatic metastases. Dis Colon Rectum. 1988;31:100–103. doi: 10.1007/BF02562637. [DOI] [PubMed] [Google Scholar]
- 8.Ries L, Miller B, Hankey B, et al. SEER Cancer Statistics Review, 1973–1991: Tables and Graphs. Report no. NIH-94–2789. Bethesda, MD: National Cancer Institute; 1994.
- 9.Jessup J, McGinnis L, Steele G J, et al. The national cancer data base report on colon cancer. Cancer. 1996;78:918–926. doi: 10.1002/(SICI)1097-0142(19960815)78:4<918::AID-CNCR32>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 10.Moertel C, Fleming T, MacDonald J, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med. 1995;122:321–326. doi: 10.7326/0003-4819-122-5-199503010-00001. [DOI] [PubMed] [Google Scholar]
- 11.Shelton A, Wong W. In: Cameron J, editor. St. Louis: Current Surgical Therapy; Mosby; 1998. Colorectal cancer. pp. 217–227.
- 12.Martin R, Paty P, Fong Y, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg. 2003;197:233–242. doi: 10.1016/S1072-7515(03)00390-9. [DOI] [PubMed] [Google Scholar]
- 13.Blumgart L H, Allison D J. Resection and embolization in the management of secondary hepatic tumors. World J Surg. 1982;6:32–45. doi: 10.1007/BF01656371. [DOI] [PubMed] [Google Scholar]
- 14.Fong Y, Fortner J, Sun R L, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fong Y, Blumgart L H. Hepatic colorectal metastases: current status of surgical therapy. Oncology. 1998;12:1489–1498. [PubMed] [Google Scholar]
- 16.Fong Y, Cohen A M, Fortner J G, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 17.Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. doi: 10.1002/bjs.1800771115. [DOI] [PubMed] [Google Scholar]
- 18.Wagner J, Adson M, Heerden J van, et al. The natural history of hepatic metastases from colorectal cancer. Ann Surg. 1984;199:502–508. doi: 10.1097/00000658-198405000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rougier P, Milan C, Lazorthes F, et al. Prospective study of prognostic factors in patients with unresected hepatic metastases from colorectal cancer. Br J Surg. 1995;82:1397–1400. doi: 10.1002/bjs.1800821034. [DOI] [PubMed] [Google Scholar]
- 20.Lahr C, Soong S, Cloud G, et al. A multifactorial analysis of prognostic factors in patients with liver metastases from colorectal carcinoma. J Clin Oncol. 1983;1:720–725. doi: 10.1200/JCO.1983.1.11.720. [DOI] [PubMed] [Google Scholar]
- 21.Wood C, Gillis C, Blumgart L. A retrospective study of the natural history of patients with liver metastases from colorectal cancer. Clin Oncol. 1976;2:285–288. [PubMed] [Google Scholar]
- 22.Pestana C, Reitemeier R, Moertel C, et al. The natural history of carcinoma of the colon and rectum. Am J Surg. 1964;108:826–829. doi: 10.1016/0002-9610(64)90041-8. [DOI] [PubMed] [Google Scholar]
- 23.Welch J, Donaldson G. The clinical correlation of an autopsy study of recurrent colorectal cancer. Ann Surg. 1979;189:496–502. doi: 10.1097/00000658-197904000-00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbert H, Kagan A. In: Weiss L, editor. Amsterdam: Fundamental Aspects of Metastasis; North-Holland Publishing; 1976. Metastases: incidence, detection, and evaluation without histologic confirmation. pp. 385–405.
- 25.Scheele J, Stang R, Atendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 26.Moran B, O'Rourke N, Plant G, Rees M. Computed tomographic portography in preoperative imaging of hepatic neoplasms. Br J Surg. 1995;82:669–671. doi: 10.1002/bjs.1800820534. [DOI] [PubMed] [Google Scholar]
- 27.McDermott V, Lawrance J, Paulson E, et al. CT during arterial portography: comparison of injection into the splenic versus superior mesenteric artery. Radiology. 1996;199:627–631. doi: 10.1148/radiology.199.3.8637977. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez F G, Drebin J A, Linehan D C, et al. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose(FDG-PET) Ann Surg. 2004;240:438–450. doi: 10.1097/01.sla.0000138076.72547.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soyer P, Levesque M, Elias D. Detection of liver metastases from colorectal cancer: comparison of intraoperative ultrasound and CT during arterial portography. Radiology. 1992;183:541–544. doi: 10.1148/radiology.183.2.1561365. [DOI] [PubMed] [Google Scholar]
- 30.Conlon R, Jacobs M, Dasgupta D, et al. The value of intraoperative ultrasound during hepatic resection compared with improved preoperative MRI. Eur J Ultrasound. 2003;16:211–216. doi: 10.1016/s0929-8266(02)00075-7. [DOI] [PubMed] [Google Scholar]
- 31.Grobmyer S R, Fong Y, D’Angelica M, et al. Diagnostic laparoscopy prior to planned hepatic resection for colorectal metastases. Arch Surg. 2004;139:1326–1330. doi: 10.1001/archsurg.139.12.1326. [DOI] [PubMed] [Google Scholar]
- 32.Expectancy or primary chemotherapy in patients with advanced asymptomatic colorectal cancer: a randomized trial. Nordic Gastrointestinal Tumor Adjuvant Therapy Group. J Clin Oncol. 1992;10:904–911. doi: 10.1200/JCO.1992.10.6.904. [DOI] [PubMed] [Google Scholar]
- 33.Scheithauer W, Rosen H, Kornek G, et al. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ. 1993;306:752–755. doi: 10.1136/bmj.306.6880.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saltz L, Shimada Y, Khayat D. CPT-11 (irinotecan) and 5-fluorouracil: a promising combination for therapy of colorectal cancer. Eur J Cancer. 1996;32A(suppl 3):S24–S31. doi: 10.1016/0959-8049(96)00294-8. [DOI] [PubMed] [Google Scholar]
- 35.Adam R. Chemotherapy and surgery: new perspectives on the treatment of unresectable liver metastases. Ann Oncol. 2003;14:13–16. doi: 10.1093/annonc/mdg731. [DOI] [PubMed] [Google Scholar]
- 36.Leonard G D, Brenner B, Kemeny N. Neoadjuvant chemotherapy before liver resection for patients with unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2005;23:2038–2048. doi: 10.1200/JCO.2005.00.349. [DOI] [PubMed] [Google Scholar]
- 37.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 38.Hamady Z Z, Kotru A, Nishio H, et al. Current techniques and results of liver resection for colorectal liver metastases. Br Med Bull. 2004;70:87–104. doi: 10.1093/bmb/ldh026. [DOI] [PubMed] [Google Scholar]
- 39.Elias D, Oullet J F, De Baere T, et al. Pre-operative selective portal vein embolization before hepatectomy for liver metastases. Surgery. 2002;131:294–299. doi: 10.1067/msy.2002.120234. [DOI] [PubMed] [Google Scholar]
- 40.Adam R, Laurent A, Azoulay D, et al. Two-stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777–785. doi: 10.1097/00000658-200012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minagawa M, Makuuchi M, Torzilli G, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg. 2000;231:487–499. doi: 10.1097/00000658-200004000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ambiru S, Miyazaki M, Isono T, et al. Hepatic resection for colorectal metastases: analysis of prognostic factors. Dis Colon Rectum. 1999;42:632–639. doi: 10.1007/BF02234142. [DOI] [PubMed] [Google Scholar]
- 43.Jamison R, Donohue J, Nagorney D, et al. Hepatic resection for metastatic colorectal cancer results in cure for some. Arch Surg. 1997;132:505–511. doi: 10.1001/archsurg.1997.01430290051008. [DOI] [PubMed] [Google Scholar]
- 44.Gayowski T, Iwatsuki S, Madariaga J, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery. 1994;116:703–711. [PMC free article] [PubMed] [Google Scholar]
- 45.Doci R, Bignami P, Montalto F, Gennari L. Prognostic factors for survival and disease-free survival in hepatic metastases from colorectal cancer treated by resection. Tumori. 1995;81(3 suppl):143–146. [PubMed] [Google Scholar]
- 46.Jaeck D, Bachellier P, Guiguet M, et al. Survival benefit of repeat liver resection for recurrent colorectal metastases: 143 cases. Wiad Lek. 1997;50(suppl 1):102–104. [PubMed] [Google Scholar]
- 47.Fong Y, Blumgart L, Cohen A, et al. Repeat hepatic resections for metastatic colorectal cancer. Ann Surg. 1994;220:657–662. doi: 10.1097/00000658-199411000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandez-Trigo V, Shamsa F, Sugarbaker P. Repeat liver resections from colorectal metastasis. Repeat Hepatic Metastases Registry. Surgery. 1995;117:296–304. doi: 10.1016/s0039-6060(05)80205-3. [DOI] [PubMed] [Google Scholar]
- 49.Petrowsky H, Gonen M, Jarnigan W, et al. Second liver resections are safe and effective treatment for recurrent hepatic metastases from colorectal cancer: a bi-institutional analysis. Ann Surg. 2002;235:863–871. doi: 10.1097/00000658-200206000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choti M A, Sitzmann J V, Tiburi M F, et al. Trends in long term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ng K K, Poon R T. Radiofrequency ablation for malignant liver tumor. Surg Oncol. 2005;14:41–52. doi: 10.1016/j.suronc.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Abdalla E K, Vauthey J N, Ellis L M, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–827. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wood T F, Rose D M, Chung M, et al. Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limits, and complications. Ann Surg Oncol. 2000;7:593–600. doi: 10.1007/BF02725339. [DOI] [PubMed] [Google Scholar]
- 54.Bowles B J, Machi J, Limm W ML, et al. Safety and efficacy of radiofrequency thermal ablation in advanced liver tumors. Arch Surg. 2001;136:864–869. doi: 10.1001/archsurg.136.8.864. [DOI] [PubMed] [Google Scholar]
- 55.Curley S A, Izzo F, Ellis L M, et al. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381–391. doi: 10.1097/00000658-200009000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barber F D, Mavligit G, Kurzock R. Hepatic arterial infusion chemotherapy for metastatic colorectal cancer: a concise overview. Cancer Treat Rev. 2004;30:425–436. doi: 10.1016/j.ctrv.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Onaitis M, Morse M, Hurwitz H, et al. Adjuvant hepatic arterial chemotherapy following metastasectomy in patients with isolated liver metastases. Ann Surg. 2003;237:782–788. doi: 10.1097/01.SLA.0000071561.76384.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin R C, Edwards M J, McMasters K M. Morbidity of adjuvant hepatic arterial infusion pump chemotherapy in the management of colorectal cancer metastatic to the liver. Am J Surg. 2004;188:714–721. doi: 10.1016/j.amjsurg.2004.08.042. [DOI] [PubMed] [Google Scholar]