ABSTRACT
Colorectal cancer is the third most common malignancy in men and women and accounts for 10% of all cancer deaths. The primary risk factor for colorectal cancer is advancing age, but other factors also play a role in its development, including genetic predisposition, smoking, alcohol consumption, obesity, and high-fat, low-fiber diet. Colon cancer survival is primarily related to the stage of disease at diagnosis. The main screening tests for colon cancer are fecal occult blood testing, flexible sigmoidoscopy, double-contrast barium enema, and colonoscopy. The pre-operative evaluation should include a complete blood count, carcinoembryonic antigen (CEA), colonoscopy, and chest radiograph. Other preoperative evaluations are patient specific or of unproven benefit. The operative procedure should include a bowel preparation, parenteral antibiotics, and deep venous thrombosis prophylaxis. The procedure performed must be tailored to the location of the colon cancer but should include complete, en bloc resection of the cancer and its lymphatic drainage, including locally invaded structures. The bowel margins of resection should be at least 5 cm from the tumor to minimize anastomotic recurrences. Laparoscopic colectomy has been shown to be as safe and effective as open colectomy for the treatment of colon cancer. The use of sentinel lymph node biopsy is feasible but has not yet been proved clinically useful. Surveillance after surgery for colon cancer is necessary to monitor for metastatic disease or local recurrence. Several groups have made surveillance recommendations including office visits, colonoscopy, and CEA monitoring.
Keywords: Colon cancer, screening, colectomy
Colorectal cancer is the third most common malignancy in both men and women. There were an estimated 147,500 new cases of colorectal cancer diagnosed in 2003. An estimated 57,100 people died in 2003 from colorectal cancer, representing10% of all cancer deaths. The overall 1- and 5-year survival rates for colorectal cancer are 83% and 62%, respectively. The survival rate continues to decline beyond 5 years to 55% at 10 years. Men have a 1 in 17 lifetime probability, whereas women have a 1 in 18 lifetime probability of developing colon or rectal cancer.1,2
RISK FACTORS
The primary risk factor for developing colorectal cancer is advanced age. In fact, 90% of colorectal cancers are diagnosed in patients older than 50. There are other associated risk factors as well. There are genetic predispositions to colorectal cancer including a personal or family history of colorectal polyps or cancer, familial adenomatous polyposis syndrome (FAP), hereditary nonpolyposis colorectal cancer syndrome (HNPCC), and history of inflammatory bowel disease (IBD). Behavioral factors that have been associated with development of colorectal cancer include smoking, alcohol consumption, obesity, and high-fat, low-fiber diet.
Individuals with a family history of colon cancer are at an increased risk for developing colon cancer themselves. The risk is associated not only with the rare, inherited, high-risk syndromes but also with only a family history of colon cancer. These individuals are at approximately twofold increased risk compared with individuals without a family history.3
FAP syndrome is due to a rare inherited mutation of the APC gene located on chromosome 5q that accounts for 1% of colon cancers. The frequency of this mutation is 1 in 10,000, and the lifetime penetrance of this mutation is 100%. Sporadic mutations in the APC gene are frequently seen in other colon cancers. FAP is characterized by multiple colorectal adenomas. These adenomas sometimes number into the thousands and begin appearing as early as childhood. If untreated, the affected individual invariably progresses to colon cancer during his or her lifetime. Gardner's syndrome is a variant of FAP with extracolonic manifestations of osteomas, fibromas, and epidermoid cysts. Turcot's syndrome is another FAP variant with fewer adenomas but an association with medulloblastoma. The treatment for FAP is prophylactic resection of the large bowel.
HNPCC syndrome, or Lynch syndrome, is due to a rare, autosomal-dominant, germline mutation of the DNA mismatch repair system. This syndrome accounts for 1% to 3% of all colon cancers.4,5 According to the Amsterdam criteria developed in 1991, the clinical diagnosis of HNPCC requires colorectal cancer to be diagnosed in at least three family members (with one being the first-degree relative of the other two), the cases span at least two successive generations, and one case is diagnosed before 50 years of age.6 The estimated lifetime risk for developing colon cancer with HNPCC is 80%.7 The average age of diagnosis is ~45 years, and the cancers are frequently right sided. There are two types of HNPCC syndrome. In HNPCC type I, individuals have only colorectal cancer. In HNPCC type II, individuals have extracolonic tumors (uterus, ovary, kidney, stomach, biliary tract, or small bowel) in addition to colorectal cancer.
IBD encompasses two entities: ulcerative colitis and Crohn's disease. Colorectal cancer associated with IBD accounts for 1% to 2% of all cases of colorectal cancer and for 15% of all deaths in IBD patients. Both ulcerative colitis and Crohn's disease carry an increased risk of colorectal cancer, but more is known about the association between colorectal cancer and ulcerative colitis. Colon cancer risk is increased with extent of disease, duration of disease, early age of onset, and primary sclerosing cholangitis. Eaden et al performed a meta-analysis based on 116 studies and found the overall prevalence of colorectal cancer with ulcerative colitis was 3.7%. The cumulative probabilities were found to be 2% at 10 years, 8% at 20 years, and 18% at 30 years.8 Gillen et al showed that patients with extensive colonic Crohn's disease also have an increased incidence of colorectal cancer, 8% at 20 years, which is similar to that of patients with extensive ulcerative colitis.9
SCREENING
Colon cancer survival is primarily related to the stage of the disease at diagnosis. As previously stated, the overall 5-year survival is 62%, but the 5-year survival for stage I colon cancer is 93.2%.10 Only 37% of patients are diagnosed with colon cancer when the disease is still localized, stage I or IIa.1,2 This is due to the fact that only a small fraction of people have symptoms early in the course of the disease. Thus, it is necessary to screen for the disease to effect early detection, when there is a better long-term prognosis.
Four tests are widely used in some capacity for colon cancer screening. These are fecal occult blood testing (FOBT), flexible sigmoidoscopy, double-contrast barium enema (DCBE), and colonoscopy. FOBT of three consecutive stools has been shown to be effective in detecting early, asymptomatic colon cancer and can decrease colon cancer mortality.11 The specificity of FOBT is reported to be 90% to 99% and the sensitivity 30% to 92%.12,13 Patients should avoid broccoli, turnips, radishes, cantaloupe, nonsteroidal anti-inflammatory drugs, and vitamin C for 2 days prior to FOBT to reduce the rate of false positives. Flexible sigmoidoscopy is useful for direct visualization of the left colon and is more sensitive and specific than FOBT in detecting left-sided colon cancers. The reported sensitivity of flexible sigmoidoscopy is 91.7% with a specificity of 85%.14 DCBE has not been evaluated for routine colon cancer screening but is generally regarded as less effective than colonoscopy for detecting polyps and colon cancer. DCBE may be adequate for screening in individuals with contraindications to endoscopic evaluation. Colonoscopy is considered the most effective screening test for early detection of colon cancer. It allows direct visualization of the entire colon and removal of polyps for pathologic evaluation. Colonoscopy has a reported sensitivity of 95% in the detection of colon cancer.15 Flexible sigmoidoscopy and colonoscopy do have some unique risks associated with their use, including perforation secondary to excessive insufflation or resection of polyps and bleeding from the excision of polyps.
Several groups have developed guidelines for colon cancer screening utilizing the aforementioned screening tests. The following recommendations are for individuals with an average risk for developing colon cancer and should begin at 50 years of age. Individuals with an average risk for colon cancer are asymptomatic, have no first-degree relatives with colon cancer, no personal history of colon cancer or polyps, no history of IBD, and no genetic predisposition for colon cancer. The U.S. Preventive Services Task Force (USPSTF) recommends yearly FOBT, periodic flexible sigmoidoscopy, or the combination of both. They could not make recommendations for or against DCBE or colonoscopy.16 The American Cancer Society (ACS) has its own set of colon cancer screening guidelines that include annual FOBT, flexible sigmoidoscopy every 5 years, or both. In addition, the ACS recommends a colonoscopy beginning at 50 years of age and every 10 years thereafter or if there is a positive FOBT or flexible sigmoidoscopy result. The ACS allows that DCBE every 5 years is an acceptable alternative to colonoscopy.1,2 The American College of Gastroenterology (ACG) recommends colonoscopy every 10 years beginning at 50 years of age. As an alternative strategy for screening, the ACG recommends annual FOBT with sigmoidoscopy every 5 years.17 At present, neither the USPSTF nor the ACG can recommend the use of computed tomography (CT) colography for screening for colon cancer. The screening recommendations for individuals at average risk are summarized in Table 1.
Table 1.
Summary of Screening Recommendations for Average-Risk Individuals
| Test | USPSTF | ACS | ACG |
|---|---|---|---|
| ACG, American College of Gastroenterology; ACS, American Cancer Society; USPSTF, U.S. Preventive Services Task Force. | |||
| Fecal occult blood testing (FOBT) | At 50 and then annually | At 50 and then annually | At 50 and then annually |
| Flexible sigmoidoscopy | Periodically or in combination with FOBT | At 50 and every 5 years | At 50 and every 5 years in combination with FOBT, as an alternative to colonoscopy |
| Double-contrast barium enema | No recommendation | At 50 and every 5 years, as an alternative to colonoscopy | No recommendation |
| Colonoscopy | No recommendation | At 50 and every 10 years | At 50 and every 10 years |
Patients with an elevated risk for developing colon cancer may need to begin screening at an earlier age. Patients with a moderate risk are those with a first-degree relative with colon cancer or those with a personal history of colon cancer or adenomas. Patients with IBD and patients with FAP or HNPCC are considered at high risk for developing colon cancer. For patients with one first-degree relative with colon cancer, the ACS recommends the same screening as for patients with average risk but beginning at age 40. For patients with two or more first-degree relatives with colon cancer, the ACS recommends colonoscopy every 5 years and screening to begin at 40 or 10 years younger than the earliest diagnosis in the family.1,2 Eaden and Mayberry recommend that screening colonoscopy for patients with IBD be conducted when the disease is in remission, beginning after 8 to 10 years of disease and every 5 years after that. In patients with only left-sided disease, the screening may begin after 15 to 20 years of disease. For patients with pancolitis, colonoscopy should be performed every 3 years in the second decade of disease, every 2 years in the third decade of disease, and every year in the fourth decade of disease.18 Screening in patients with FAP should begin with counseling at puberty, and genetic screening may be used to reach a presymptomatic diagnosis of FAP. A negative genetic screen for FAP does not rule out the diagnosis, as only 80% to 90% of FAP families have an identifiable APC mutation.19 Cancer surveillance for FAP should begin at puberty with yearly sigmoidoscopy until the age of 35, when the interval can be increased to every 3 years.20 If HNPCC is suspected, genetic testing should be considered. If genetic testing is positive or the Amsterdam criteria are met for HNPCC, yearly colonoscopic surveillance should begin between the ages of 20 and 25, or 10 years younger than the age of the earliest diagnosis of colon cancer in the family.21 The ACS screening recommendations for individuals with moderate or high risk for colorectal cancer are summarized in Table 2.
Table 2.
Summary of American Cancer Society Recommendations for Screening of Moderate- or High-Risk Individuals
| Test | One First-Degree Relative with Colon Cancer | Two or More First-Degree Relatives with Colon Cancer | Familial Adenomatous Polyposis (FAP) | Hereditary Nonpolyposis Colon Cancer (HNPCC) |
|---|---|---|---|---|
| Fecal occult blood testing (FOBT) | At 40 and then annually | |||
| Flexible sigmoidoscopy | At 40 and every 5 years | At puberty and annually until 35, then every 3 years | ||
| Double-contrast barium enema | At 40 and every 5 years, as an alternative to colonoscopy | |||
| Colonoscopy | At 40 and every 10 years | At 40 or 10 years younger than first diagnosis and every 5 years | Annually at 20–25 or 10 years younger than earliest diagnosis |
PATIENT EVALUATION
Evaluation of a patient with colon cancer begins with a thorough history and physical examination. History should include symptoms to elicit evidence of bowel obstruction, weight loss and malnutrition, anemia, and somatic pain suggestive of local invasion of the parietal peritoneum. The history should include any family history of colon cancer so that familial syndromes can be considered, if appropriate. In addition, comorbidities need to be addressed to evaluate the patient's fitness to undergo a major abdominal surgery. On physical examination, cachexia and distant lymphadenopathy (cervical or inguinal) should be noted, as these are suspicious for metastatic disease. The abdomen and liver edge must be palpated as well to evaluate for masses and metastases. A digital rectal examination is an essential part of the physical examination as it allows assessment of gross blood, occult blood, a synchronous rectal carcinoma, or a Blumer's shelf metastasis (a sign of carcinomatosis). A synchronous rectal cancer or Blumer's shelf may significantly alter the operative plan. If the lesion is expected to be in the rectum or sigmoid colon, rigid proctoscopy to identify the exact location of the lesion is important.
The preoperative evaluation should also include a complete blood count (CBC), carcinoembryonic antigen (CEA), colonoscopy, and chest radiograph. A CBC should be obtained to evaluate the patient for anemia, which may be corrected prior to surgery. Preoperative CEA should be measured, as an elevated CEA (> 5 ng/mL) is an indicator of a poor prognosis.22 The CEA should be repeated after surgery as well to establish a new baseline for subsequent surveillance. If not done previously, a colonoscopy should be completed to evaluate the entirety of the colon for synchronous cancers and polyps. Colonoscopy is preferable to barium enema for this evaluation as colonoscopy allows biopsy of suspicious lesions and removal of polyps for pathologic examination. With advances in radiologic imaging, it has become possible to evaluate the colon with CT colonography, so-called virtual colonoscopy. This entity may be useful in patients who cannot undergo colonoscopy or who had incomplete colonoscopies. In 2002, Neri et al studied 34 patients and showed a sensitivity of 56% and a specificity of 92% for detection of colorectal cancer with an incomplete colonoscopy and a sensitivity of 100% and a specificity of 96% with CT colonography for the detection of colorectal cancer.23 Again, this imaging modality does not allow biopsy of suspicious lesions, but it may alter the operative procedure if synchronous lesions are seen. At present, CT colonography, if available, should be considered an alternative in a select group of patients with incomplete colonoscopy or with contraindications to colonoscopy. Plain films of the chest should be obtained preoperatively to evaluate for coexisting cardiac and pulmonary issues, and if advanced disease is suspected, the chest radiograph may demonstrate lung metastases.
The role of routine preoperative CT scans in patients with colon cancer is controversial. For patients with rectal cancer, a preoperative CT scan has become the standard. A CT scan does allow a detailed evaluation of the patient for synchronous lesions, especially liver metastases, and the surgeon can alter the operative plan accordingly. McAndrew and Saba did a retrospective review of 67 patients who underwent CT scan prior to resection of colon cancer. They showed a sensitivity of 75% and a specificity of 88% for detection of hepatic metastases by CT scan. Of these 67 patients, only 3 had findings on CT scan that changed the operative management of the patient.24 Barton et al evaluated all 70 patients diagnosed with colon cancer at the Puget Sound Veterans Affairs Healthcare System between 1997 and 2001 with preoperative CT scans. The information gained from these scans was used in treatment planning in 26 (37%), but if the scans had not been performed, the clinical management would have been altered in only 13 (19%).25 CT scanning does not seem to have a routine role in the preoperative assessment of most patients, but for patients with suspected advanced disease or metastases, it is an essential part of the preoperative work-up. This may be suspected in patients who have obstructive symptoms, somatic abdominal or back pain, or abnormal laboratory studies (elevated liver or kidney function tests, hematuria, or markedly elevated CEA).
A group from South Korea reported on the use of hydrocolonic ultrasonography (HUS) for the preoperative staging of colon cancer. With HUS, the colon is filled with 1 to 2 L of saline and then ultrasonography is conducted at 3.75 MHz with an external ultrasound probe. They evaluated 17 patients preoperatively with HUS and compared these results with postoperative histologic findings. Tumor staging for colon cancer with hydrocolonic sonography was accurate in 88% of patients, and the accuracy was 71% for evaluating nodal involvement. HUS is inexpensive, noninvasive, and widely available. This technique seems useful for preoperative local staging of colon cancer.26
OPERATIVE TECHNIQUE
Bowel preparation prior to elective colon surgery for colon cancer is still accepted as essential, although the issue is being reevaluated. The classic bowel preparation consists of both mechanical preparation and administration of nonabsorbable oral antibiotics. The goal of the mechanical preparation is to rid the colon of solid stool. The removal can be accomplished with several types of oral lavage solutions. The clean colon has a lower bacterial load, which lessens the spillage of fecal contents into the peritoneal cavity and the wound and limits confusing solid stool for a mass during palpation of the colon. The mechanical preparation also eliminates the passage of solid stool across a new anastomosis, and, if need be, colonoscopy can be performed intraoperatively after mechanical bowel preparation.
The most common mechanical bowel preparation consists of either 4 L of polyethylene glycol (PEG) solution or two 45-mL doses of sodium phosphate (NaP). Oliveira et al randomly assigned 200 patients undergoing colorectal surgery to receive bowel preparation with either PEG or NaP. In these patients, they showed that both preparations gave adequate bowel preparation, 87% for NaP and 76% for PEG, but that NaP was better tolerated by patients.27 Slim et al conducted a meta-analysis of seven studies including 1454 patients to evaluate the efficacy of omitting bowel preparation with PEG. In their analysis, there were significantly more anastomotic leaks in the group receiving PEG bowel preparation, 5.6% versus 3.2% for no bowel preparation. Other differences that favored no bowel preparation included wound infections, septic complications, and nonseptic complications.28 There is currently a large, multicenter trial under way to address the continuing need for bowel preparation for elective colon surgery. Until the results of this study are published, it would be unwise to suggest that mechanical bowel preparation is no longer an essential part of elective colon surgery.
Bowel preparation also frequently includes treatment with nonabsorbable oral antibiotics. The theory is that nonabsorbable antibiotics further decrease the load of colonic mucosal bacteria at the time of surgery. The choice of antibiotics is based on surgeon preference but has historically been neomycin with either erythromycin base or metronidazole. There are no studies that demonstrate a difference in efficacy between the two combinations. Most colorectal surgeons, 86.5%, include preoperative parenteral antibiotics as well as oral antibiotics in preparing the patient for surgery.29 Historically, the choice of parenteral antibiotics included an aminoglycoside for gram-negative coverage, a penicillin for gram-positive coverage, and metronidazole or clindamycin for anaerobic coverage. The development of single broad-spectrum agents has made the use of three individual antibiotics obsolete. The parenteral antibiotic is also a choice for the individual surgeon, but it should cover both aerobic and anaerobic bacteria. The timing of administration of parenteral antibiotics should be such that tissue levels exceed the minimum inhibitory concentration for colonic flora at the time of incision and throughout the operation.
All patients should have prophylaxis to prevent deep venous thrombosis (DVT). Use of sequential compression devices is a simple and effective means of preventing DVT. Patients can also receive either heparin or low-molecular-weight heparin (LMWH) prior to induction of anesthesia for DVT prevention. McLeod et al studied 936 colorectal patients randomly assigned to either 5000 units of heparin subcutaneously three times daily or 40 mg of enoxaparin daily with injection of 0.9% normal saline twice daily. This study showed equivalent rates of DVT between the two groups and the same rate of bleeding complications.30 A meta-analysis of 33 studies of heparin versus LMWH for the prevention of DVT also showed no significant differences in the rate of DVT between the two treatments.31 LMWH probably has a better safety profile than heparin, but LMWH is also significantly more expensive than heparin. Thus, the choice of heparin versus LMWH for DVT prophylaxis is up to the individual surgeon. Once in the operating room, a Foley catheter should be placed to decompress the bladder to allow adequate access to the pelvis and allow monitoring of urine output through the case. In addition, an orogastric tube should be placed with anesthesia to decompress the stomach, and this can be removed at the end of the case.
Positioning of the patient is dictated by the suspected location of the tumor. Patients with tumors proximal to the splenic flexure are usually placed in the supine position. With left-sided tumors, patients should generally be in the modified lithotomy position with the feet in stirrups. This positioning allows the surgeon to be between the patient's legs to take down the splenic flexure, allows intraoperative colonoscopy, allows rectal washout, and allows anal introduction of a stapling device.
The incision for colorectal resection should be a midline laparotomy. This incision allows adequate exposure of the entire colon, is easily extended, and does not compromise any possible stoma sites. The main disadvantage of the midline laparotomy is postoperative pain. Pain can be managed with several options including patient-controlled analgesia, epidural anesthesia, and/or instillation of long-acting local anesthetics near the incision. After the incision is made, some surgeons advocate the use of a wound protector. The proponents of wound protectors believe they assist with retraction and help to prevent postoperative infections. Kercher et al retrospectively studied 141 patients, 84 with wound protectors (ProtractorTM, Dexterity Surgical, San Antonio, TX) and 47 without wound protectors, who underwent laparoscopy-assisted colectomy from 1999 to 2002. This study showed no significant difference in the rate of wound infection, 10.7% with a wound protector and 14.0% without a wound protector.32 Certain wound protectors provide wound retraction, but their efficacy in prevention of wound infections needs further study.
There are basic surgical oncology tenets that must be followed for all colon cancer resections. These tenets include complete, en bloc resection of the cancer and its lymphatic drainage, including locally invaded structures. The bowel margins of resection should be at least 5 cm from the tumor to minimize anastomotic recurrences. The length of ileum resected does not seem to affect local recurrence. The actual extent of bowel resection is defined by removing the blood supply and lymphatics at the level of the origin of the primary feeding arterial vessel. If the tumor is equidistant from two primary arteries, both should be removed. A proper lymphadenectomy should extend to the level of the feeding artery. To effect a cure, all lymph nodes should be removed en bloc with the tumor-bearing segment of colon. The nodes at the origin of the feeding vessel should be tagged for pathologic analysis. Lymph nodes that appear or feel suspicious but lie outside the field of resection should be sampled. If these nodes are positive for malignancy, the entirety of that lymph node basin should also be resected. For entry into adjuvant trials, at least one lymph node must be examined, and for entry into adjuvant trials for negative lymph nodes, at least 12 lymph nodes must be examined.33
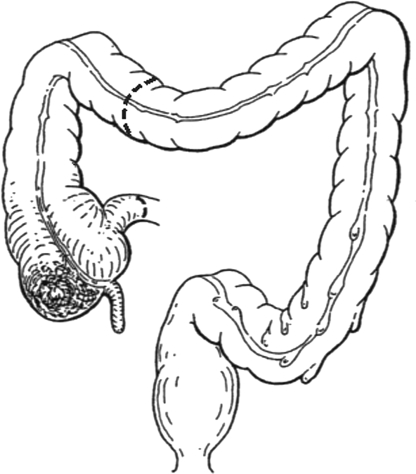
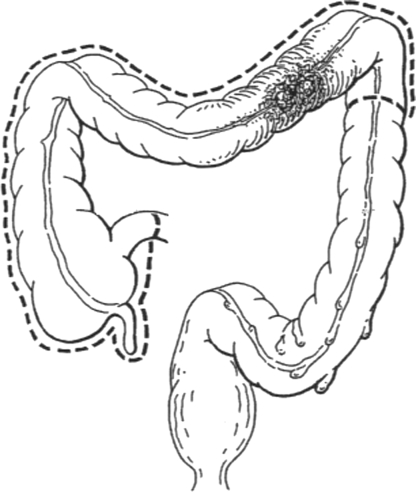
The extent of bowel resection is determined by the location of the tumor. For tumors in the ascending colon, a right hemicolectomy is the accepted treatment, and for tumors in the proximal transverse colon, an extended right hemicolectomy is the accepted treatment. A right hemicolectomy (Fig. 1) begins with mobilization of the right colon and ileocecal region by dividing the peritoneum lateral to the ascending colon and cecum. The right colon and mesocolon is mobilized from lateral to medial, and the ureter is identified. During mobilization of the hepatic flexure care must be taken to avoid injuring the duodenum, which lies beneath this portion of the colon. If the tumor is in the transverse colon, the entire omentum overlying the tumor must be taken. At this point, the entire right colon and transverse colon can be lifted with their vascular supply from the retroperitoneum, if the tumor is not adherent to adjacent structures. If the tumor is adherent to adjacent structures, these must be taken en bloc with the tumor. The transverse colon is divided at the bifurcation of the middle colic artery, and terminal ileum can now be divided with a linear stapler to isolate the tumor-bearing segment of colon. After isolation, attention is turned to the vascular supply. For tumors in the ascending colon, the origin of the ileocolic artery must be identified and ligated near its origin from the superior mesenteric artery. The right branch of the middle colic artery is taken at the distal end of the resection. For lesions in the transverse colon, where an extended right hemicolectomy (Fig. 2) is indicated, the middle colic artery must be identified and ligated at its origin as well. At this point, the blood supply to the distal colon is dependent on the patency of the inferior mesenteric artery and collateral pathways (marginal artery of Drummond and the arcade of Riolan). If the collateral pathways do not provide adequate blood flow, it may be necessary to anastomose the ileum to viable descending or sigmoid colon. The ileocolic anastomosis can be created in a stapled or hand-sewn fashion, depending on the preference of the surgeon.
Figure 1.
Right hemicolectomy. (From Keighley MRB. Atlas of Colorectal Surgery. New York: Churchill Livingstone; 1996.) Reprinted with permission.
Figure 2.
Extended right hemicolectomy. (From Keighley MRB. Atlas of Colorectal Surgery. New York: Churchill Livingstone; 1996.) Reprinted with permission.
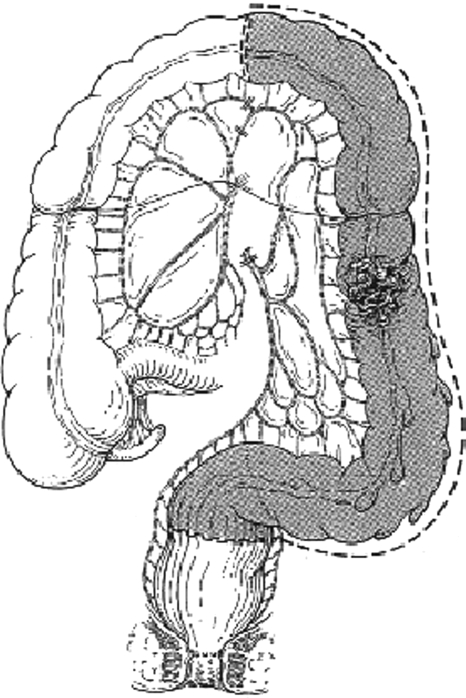
Tumors in the distal transverse colon, splenic flexure, descending colon, or proximal sigmoid colon are all surgically treated with a left hemicolectomy (Fig. 3). The left colon is mobilized by incising the peritoneum lateral to the descending colon. This incision is carried proximally toward the splenic flexure and distally to the sigmoid colon. The splenic flexure is then taken down with care not to damage the marginal artery. The left colon can now be lifted on its vascular pedicle. The inferior mesenteric artery is divided at its origin from the aorta. The inferior mesenteric vein is identified as it separates from the inferior mesenteric artery. The inferior mesenteric vein joins the splenic vein behind the pancreas. Ligation of the inferior mesenteric vein high up under the inferior border of the pancreas greatly lengthens the reach of the transverse colon to the rectum. The tumor-bearing segment of colon can now be isolated with linear staplers. Again, if the tumor is adherent to adjacent organs, these must be removed en bloc with the colon to prevent spillage of tumor cells into the peritoneum. The mesentery and marginal artery are now divided, and the specimen is removed. The colorectal anastomosis can then be constructed in a stapled or hand-sewn fashion, depending on the preference of the surgeon.
Figure 3.
Left hemicolectomy. (From Keighley MRB. Atlas of Colorectal Surgery. New York: Churchill Livingstone; 1996.) Reprinted with permission.
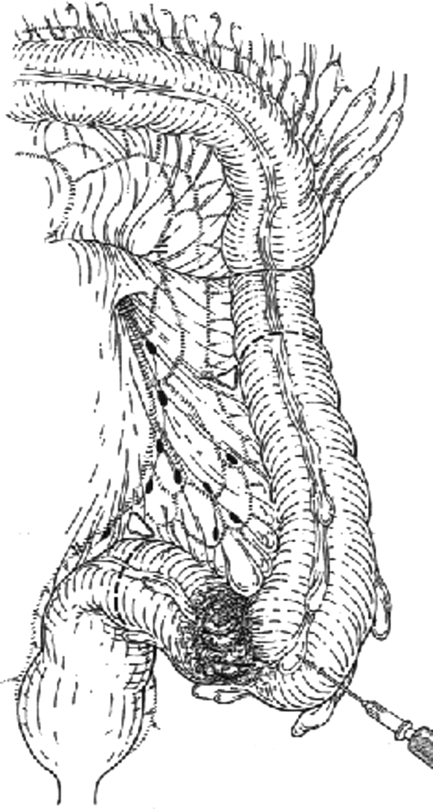
Sigmoid colectomy (Fig. 4) is not advisable for malignant disease unless it is a palliative procedure in a patient with metastatic disease. The procedure is similar to a left colectomy except that less colon is resected and the ascending and descending branches of left colic vessels are preserved, as are the superior hemorrhoidal vessels. This procedure does not allow complete clearance of the lymphatic basin draining the sigmoid colon.
Figure 4.
Sigmoid colectomy. (From Keighley MRB. Atlas of Colorectal Surgery. New York: Churchill Livingstone; 1996.) Reprinted with permission.
Subtotal colectomy may be appropriate for patients with HNPCC, FAP, synchronous lesions within the colon, or a metachronous lesion in the colon. It is also an option for patients with a distal obstructing lesion. Patients must be advised of the necessity of sigmoidoscopic surveillance. Subtotal colectomy may or may not include the sigmoid colon depending on the distribution of the tumors. The ileocolic, middle colic, and inferior mesenteric arteries are all ligated at their origins, and all lymph nodes should be taken with the specimen.
Proximal perforation from a distal obstructing colon cancer is an ominous complication. Chen and Sheen-Chen retrospectively studied 1950 patients and found a perioperative mortality of 9% for patients with perforation at the site of the tumor and 31% for patients with a proximal perforation.34 Patients with perforation secondary to colon cancer are treated similar to patients with any perforation, that is, prompt resuscitation and surgical exploration. Patients with obstruction from a right-sided colon cancer may be treated with a right hemicolectomy and primary anastomosis; patients with a distal cancer require special maneuvers. These options include (1) end ileostomy and Hartmann’s stump, (2) on-table lavage with primary anastomosis and intraoperative colonoscopy, or (3) preoperative colonic stent placement with subsequent bowel preparation and colonoscopy through the stent. Patients with perforation are best treated with subtotal colectomy with construction of an end ileostomy and oversewn rectum.
Most surgeons approach colectomy for colon cancer by first mobilizing the colon and later dividing the blood supply to that segment. This is the so-called lateral-to-medial approach. An alternative approach was championed by Turnbull and called the “no-touch isolation” technique. This is a medial-to-lateral approach in which the blood vessels are mobilized and ligated at their origins as the initial step in the procedure. This provides preliminary lymphovascular isolation of the tumor-bearing segment prior to manipulating it and potentially shedding cells into the portal circulation. Proponents of this technique cite improved survival when it is used. However, this remains controversial, as there have not been many randomized, prospective studies to evaluate the efficacy of the no-touch technique. One randomized, prospective study of 236 patients showed no 5-year survival benefit with the no-touch technique, but this study also showed that patients in the conventional arm had a higher incidence of liver metastases and shorter time to metastasis.35 Certain technical benefits are associated with the medial-to-lateral approach. Resection of T4 lesions is often facilitated by first dividing the lymphovascular and mesenteric attachments. This allows the specimen to be elevated on a pedicle fixed at the tumor, rather than forcing the surgeon to work around a fixed lesion with the colon held flat, fixed at two points (the tumor and the mesentery). The second technical benefit is developing comfort with the medial-to-lateral approach and applying it to laparoscopic colectomy. Its use in laparoscopy allows the vessels to be divided while the colon is held out of the way by its normal attachments.
Laparoscopic surgery was first introduced for colectomy secondary to colon cancer in 1990.36 A study from 1994 showed a tumor recurrence rate of 21% at trocar sites for patients treated with laparoscopic colectomy.37 This and other studies prompted a moratorium on laparoscopic colectomies for malignancy until the matter could be resolved with a large, randomized, prospective, multicenter trial. To this end, a study at 48 different institutions was conducted that randomly assigned 872 patients with adenocarcinoma of the colon to either open or laparoscopically assisted colectomy by a credentialed surgeon.38 The mean time of follow-up was 4.4 years, and the end point was time to recurrence. At 3 years, the rates of recurrence were similar between the two groups, 16% for laparoscopically assisted colectomy and 18% for open colectomy. The recurrence rate at surgical wounds was less than 1% for both groups. The overall survival was very similar for the two groups, 86% for the laparoscopically assisted group and 85% for the open colectomy group. There was no significant difference in time to recurrence for any tumor stage between the two groups. Perioperative recovery was significantly quicker with the laparoscopically assisted group with shorter mean hospital stays, briefer use of parenteral narcotics, and less use of oral analgesics. The intraoperative complications, 30-day mortality, complications at discharge and 60 days, hospital readmission, and reoperation were similar between the two groups.
A group from Italy conducted a prospective, nonrandomized study of 197 patients undergoing elective colectomy for colon cancer.39 These patients underwent surgery by the same team with the only difference being the access, open versus laparoscopic. Follow-up ranged from 36 to 96 months with a mean of 48.9 months. This study showed local recurrence rates of 1.3% for laparoscopically assisted colectomy and 2.7% for open colectomy. Metastatic rates for the two groups were similar, 10.8% for the laparoscopically assisted group and 10.7% for the open group. A meta-analysis of 12 studies encompassing 2512 procedures showed that laparoscopic resection for colon cancer took an average of 33% longer to complete than open resection but had a lower morbidity rate, specifically wound infections.40 Laparoscopic patients passed flatus on average 33% sooner and tolerance of a solid diet 24% sooner than open patients. In addition, there were no significant differences in perioperative mortality or oncologic clearance, that is, negative margins of resection. From these data, it is evident that laparoscopic colectomy is a viable, if not preferable, alternative to open colectomy for patients with colon cancer.
The most important prognostic indicator in potentially curable colon cancer is the presence of disease in regional lymph nodes. A minimum of 14 lymph nodes should be examined to identify lymph node status accurately.41 Patients with cancer metastatic to regional lymph nodes are most likely to have recurrences and most likely to benefit from adjuvant chemotherapy. More than 25% of patients with negative lymph nodes on conventional pathologic staging develop recurrent disease within 5 years of surgery.42 There is substantial work under way to identify the individuals who have recurrences with negative lymph nodes. A more thorough investigation of regional lymph nodes with other than conventional histopathologic staining may identify tumor cells or tumor products in lymph nodes previously thought to be negative by conventional assessment. An obstacle to this more thorough investigation is the time, effort, and cost to evaluate the lymph nodes identified after fat clearance of the resected specimen.
Sentinel lymph node sampling is a technique that has been utilized in melanoma and breast cancer to identify patients who would benefit from adjuvant therapy without the need for an extensive lymph node dissection. It is thought that this technique could be applied to colon cancer specimens to identify a sentinel lymph node for a more thorough histopathologic and immunohistochemical investigation. Bertagnolli et al conducted a study by 25 surgeons at 13 institutions to determine the feasibility of sentinel lymph node sampling in colon cancer. They studied 72 (66% node-negative) patients with identification of sentinel nodes by injection of 1% isosulfan blue into the subserosa above the primary cancer. Sentinel nodes were identified in 92% of patients with an average of 2.1 sentinel nodes per patient. Sentinel nodes were negative in 58% of patients with node-positive disease, and multilevel sectioning identified disease in one sentinel lymph node. Overall, sentinel lymph node examination with multilevel sectioning failed to identify metastatic disease in 54% of patients.43 Merrie et al identified sentinel lymph nodes in 23 of 26 tumors (88%) and showed a sensitivity of 55% for prediction of lymphatic disease and a negative predictive value of 45%.44 Esser et al studied 31 patients and were able to identify sentinel lymph nodes in only 18 patients, but when identified, they found that the sentinel lymph node had a sensitivity of 67%, a specificity of 100%, positive predictive value of 100%, and negative predictive value of 94%.45 These studies certainly support the feasibility of identifying sentinel lymph nodes, but they do not, at this time, support the clinical utility of the identifying sentinel lymph nodes. Further research in this area may elaborate the role of sentinel lymph node sampling in patients at high risk for recurrence.
SURVEILLANCE
Surveillance of patients after surgery for colon cancer serves two purposes. The first is to increase lead time in the detection of recurrent disease, and the second is to identify metastatic disease early in its course. Several different surveillance strategies have been put forth that range from patients returning when they experience symptoms to frequent visits with comprehensive laboratory testing and imaging. The American Society of Clinical Oncology (ASCO) published evidence-based clinical practice guidelines in 1999 and updated these guidelines in 2000.46 The ASCO recommends testing for CEA every 2 to 3 months for at least 2 years for patients with stage II or III disease, even with a normal preoperative CEA. An elevated CEA justifies a search for metastatic disease but does not justify initiation of systemic therapy for the presumed metastases.46 Graham et al studied 421 patients and showed that CEA measurement was the most cost-effective test in detecting potentially curable recurrent disease with a cost per recurrence of $5696. Comparatively, the cost per recurrence detected with other surveillance methods was $10,078 for chest radiography and $45,810 for colonoscopic surveillance. Of note, physician examinations failed to identify a single resectable recurrence and cost a total of $418,615.47 Of the 96 patients in this study who were identified with potentially resectable recurrent disease, 30 were identified first with CEA, 12 with chest radiography, 14 with colonoscopy, 24 with symptoms, and 16 with multiple tests.
The ASCO recommends that clinical history, test coordination, and patient counseling be performed every 3 to 6 months for the first 3 years after surgical resection of colon cancer. The ASCO also recommends colonoscopic surveillance every 3 to 5 years. Tests that the ASCO recommends against for routine use include liver function tests, FOBT, and CBC. For chest radiography and CT scan, the ASCO recommends against routine use, but these are acceptable tests for the work-up of suspected metastatic disease as evidenced by an elevated CEA.46 The American Society of Colorectal Surgeons (ASCRS) makes several recommendations for the surveillance of patients who have undergone surgical resection for colon cancer.48 The ASCRS recommends that office visits be part of the follow-up program, that CEA be monitored, and that the anastomosis be periodically evaluated. The ASCRS makes no recommendations on the timing of the follow-up visits, CEA monitoring, and anastomotic surveillance but does recommend that these occur at least three times a year for the first 2 years. Colonoscopy, when practical, is recommended by the ASCRS at 3-year intervals following resection of colon cancer. No recommendations for or against routine chest radiography are given by the ASCRS. The ASCRS also recommends against routine checks of hemoglobin, liver function tests, Hemoccult testing, and routine hepatic imaging.
REFERENCES
- 1.American Cancer Society Cancer Facts and Figures 2003. Available at http://www.cancer.org/docroot/STT/stt_0_2003.asp?sitearea=STT&level=1. Accessed December 6, 2004. Available at http://www.cancer.org/docroot/STT/stt_0_2003.asp?sitearea=STT&level=1
- 2.American Cancer Society Cancer Statistics 2004. Available at http://www.cancer.org/docroot/STT/stt_0.asp. Accessed December 7, 2004. Available at http://www.cancer.org/docroot/STT/stt_0.asp
- 3.Carstensen B, Soll-Johanning H, Villadsen E, Sondergaard J O, Lynge E. Familial aggregation of colorectal cancer in the general population. Int J Cancer. 1996;68:428–435. doi: 10.1002/(SICI)1097-0215(19961115)68:4<428::AID-IJC5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Samowitz W S, Curtin K, Lin H H, et al. The colon cancer burden of genetically defined hereditary nonpolyposis colon cancer. Gastroenterology. 2001;121:830–838. doi: 10.1053/gast.2001.27996. [DOI] [PubMed] [Google Scholar]
- 5.Aaltonen L A, Salovaara R, Kristo P, et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med. 1998;338:1481–1487. doi: 10.1056/NEJM199805213382101. [DOI] [PubMed] [Google Scholar]
- 6.Vasen H F, Mecklin J P, Khan P M, Lynch H T. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC) Dis Colon Rectum. 1991;34:424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 7.Vasen H F, Wijnen J T, Menko F H, et al. Cancer risk in families with hereditary nonpolyposis colorectal cancer diagnosed by mutation analysis. Gastroenterology. 1996;110:1020–1027. doi: 10.1053/gast.1996.v110.pm8612988. [DOI] [PubMed] [Google Scholar]
- 8.Eaden J A, Abrams K R, Mayberry J F. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillen C D, Walmsley R S, Prior P, Andrews H A, Allan R N. Ulcerative colitis and Crohn's disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590–1592. doi: 10.1136/gut.35.11.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Connell J B, Maggard M A, Ko C Y. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–1425. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 11.Kronborg O, Fenger C, Olsen J, Jorgensen O D, Sondergaard O. Randomised study of screening for colorectal cancer with fecal-occult-blood test. Lancet. 1996;348:1467–1471. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 12.Church T R, Ederer F, Mandel J S. Fecal occult blood screening in the Minnesota study: sensitivity of the screening test. J Natl Cancer Inst. 1997;89:1440–1448. doi: 10.1093/jnci/89.19.1440. [DOI] [PubMed] [Google Scholar]
- 13.Ransohoff D F, Lang C A. Screening for colorectal cancer with the fecal occult blood test: a background paper. American College of Physicians. Ann Intern Med. 1997;126:811–822. doi: 10.7326/0003-4819-126-10-199705150-00014. [DOI] [PubMed] [Google Scholar]
- 14.Trowbridge B, Burt R W. Colorectal cancer screening. Surg Clin North Am. 2002;82:943–957. doi: 10.1016/s0039-6109(02)00045-2. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman D A, Smith F W. Screening for colon malignancy with colonoscopy. Am J Gastroenterol. 1991;86:946–951. [PubMed] [Google Scholar]
- 16.U.S. Preventive Services Task Force Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 2002;137:129–131. doi: 10.7326/0003-4819-137-2-200207160-00014. [DOI] [PubMed] [Google Scholar]
- 17.Rex D K, Johnson D A, Lieberman D A, Burt R W, Sonnenberg A. Colorectal cancer prevention 2000: screening recommendations of the American College of Gastroenterology. American College of Gastroenterology. Am J Gastroenterol. 2000;95:868–877. doi: 10.1111/j.1572-0241.2000.02059.x. [DOI] [PubMed] [Google Scholar]
- 18.Eaden J A, Mayberry J F. Guidelines for screening and surveillance of asymptomatic colorectal cancer in patients with inflammatory bowel disease. Gut. 2002;51:V10–V12. doi: 10.1136/gut.51.suppl_5.v10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anonymous (ASCO Subcommittee on genetic testing for cancer susceptibility) Statement of the American Society of Clinical Oncology: genetic testing for cancer susceptibility. J Clin Oncol. 1996;14:1730–1736. doi: 10.1200/JCO.1996.14.5.1730. [DOI] [PubMed] [Google Scholar]
- 20.Giardiello F M. Genetic testing in hereditary colorectal cancer. JAMA. 1997;278:1278–1281. [PubMed] [Google Scholar]
- 21.Burke W, Petersen G, Lynch P, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. I. Hereditary nonpolyposis colon cancer. Cancer Genetics Studies Consortium. JAMA. 1997;277:915–919. [PubMed] [Google Scholar]
- 22.Harrison L E, Guillem J G, Paty P, Cohen A M. Preoperative carcinoembryonic antigen predicts outcomes in node-negative colon cancer patients: a multivariate analysis of 572 patients. J Am Coll Surg. 1997;185:55–59. doi: 10.1016/s1072-7515(97)00012-4. [DOI] [PubMed] [Google Scholar]
- 23.Neri E, Giusti P, Battolla L, et al. Colorectal cancer: role of CT colonography in preoperative evaluation after incomplete colonoscopy. Radiology. 2002;223:615–619. doi: 10.1148/radiol.2233010928. [DOI] [PubMed] [Google Scholar]
- 24.McAndrew M R, Saba A K. Efficacy of routine computed tomography scans in colon cancer. Am Surg. 1999;65:205–208. [PubMed] [Google Scholar]
- 25.Barton J B, Langdale L A, Cummins J S, et al. The utility of routine computed tomography scanning in the management of veterans with colon cancer. Am J Surg. 2002;183:499–503. doi: 10.1016/s0002-9610(02)00841-3. [DOI] [PubMed] [Google Scholar]
- 26.Chung H W, Chung J B, Park S W, Song S Y, Kang J K, Park C I. Comparison of hydrocolonic sonography accuracy in preoperative staging between colon and rectal cancer. World J Gastroenterol. 2004;10:1157–1161. doi: 10.3748/wjg.v10.i8.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliveira L, Wexner S D, Daniel N, et al. Mechanical bowel preparation for elective colorectal surgery. A prospective, randomized, surgeon-blinded trial comparing sodium phosphate and polyethylene glycol-based oral lavage solutions. Dis Colon Rectum. 1997;40:585–591. doi: 10.1007/BF02055384. [DOI] [PubMed] [Google Scholar]
- 28.Slim K, Vicaut E, Panis Y, Chipponi J. Meta-analysis of randomized clinical trials of colorectal surgery with or without mechanical bowel preparation. Br J Surg. 2004;91:1125–1130. doi: 10.1002/bjs.4651. [DOI] [PubMed] [Google Scholar]
- 29.Nichols R L, Smith J W, Garcia R Y, Waterman R S, Holmes J W. Current practices of preoperative bowel preparation among North American colorectal surgeons. Clin Infect Dis. 1997;24:609–619. doi: 10.1093/clind/24.4.609. [DOI] [PubMed] [Google Scholar]
- 30.McLeod R S, Geerts W H, Sniderman K W, et al. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian colorectal DVT prophylaxis trial: a randomized, double-blind trial. Ann Surg. 2001;233:438–444. doi: 10.1097/00000658-200103000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer A J, Schramm W, Kirchhof B, Bergemann R. Low molecular weight heparin and unfractionated heparin for prevention of thrombo-embolism in general surgery: a meta-analysis of randomised clinical trials. Haemostasis. 1997;27:65–74. doi: 10.1159/000217436. [DOI] [PubMed] [Google Scholar]
- 32.Kercher K W, Nguyen T H, Harold K L, et al. Plastic wound protectors do not affect wound infection rates following laparoscopic-assisted colectomy. Surg Endosc. 2004;18:148–151. doi: 10.1007/s00464-003-8137-6. [DOI] [PubMed] [Google Scholar]
- 33.Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583–596. doi: 10.1093/jnci/93.8.583. [DOI] [PubMed] [Google Scholar]
- 34.Chen H S, Sheen-Chen S M. Obstruction and perforation in colorectal adenocarcinoma: an analysis of prognosis and current trends. Surgery. 2000;127:370–376. doi: 10.1067/msy.2000.104674. [DOI] [PubMed] [Google Scholar]
- 35.Wiggers T, Jeekel J, Arends J W, et al. No-touch isolation technique in colon cancer: a controlled prospective trial. Br J Surg. 1988;75:409–415. doi: 10.1002/bjs.1800750505. [DOI] [PubMed] [Google Scholar]
- 36.Phillips E H, Franklin M, Carroll B J, Fallas M J, Ramos R, Rosenthal D. Laparoscopic colectomy. Ann Surg. 1992;216:703–707. doi: 10.1097/00000658-199212000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berends F J, Kazemier G, Bonjer H J, Lange J F. Subcutaneous metastases after laparoscopic colectomy. Lancet. 1994;344:58. doi: 10.1016/s0140-6736(94)91079-0. [DOI] [PubMed] [Google Scholar]
- 38.The Clinical Outcomes of Surgical Therapy Study Group A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 39.Feliciotti F, Paganini A M, Guerrieri M, De Sanctis A, Campagnacci R, Lezoche E. Results of laparoscopic vs open resections for colon cancer in patients with a minimum follow-up of 3 years. Surg Endosc. 2002;16:1158–1161. doi: 10.1007/s00464-001-8333-1. [DOI] [PubMed] [Google Scholar]
- 40.Abraham N S, Young J M, Solomon M J. Meta-analysis of short-term outcomes after laparoscopic resection for colorectal cancer. Br J Surg. 2004;91:1111–1124. doi: 10.1002/bjs.4640. [DOI] [PubMed] [Google Scholar]
- 41.Wong J H, Severino R, Honnebier M B, Tom P, Namiki T S. Number of nodes examined and staging accuracy in colorectal carcinoma. J Clin Oncol. 1999;17:2896–2900. doi: 10.1200/JCO.1999.17.9.2896. [DOI] [PubMed] [Google Scholar]
- 42.Jemal A, Tiwari R C, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 43.Bertagnolli M, Miedema B, Redston M, et al. Sentinel node staging of resectable colon cancer: results of a multicenter study. Ann Surg. 2004;240:624–630. doi: 10.1097/01.sla.0000140753.41357.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merrie A E, Rij A M van, Phillips L V, Rossaak J I, Yun K, McCall J L. Diagnostic use of the sentinel node in colon cancer. Dis Colon Rectum. 2001;44:410–417. doi: 10.1007/BF02234742. [DOI] [PubMed] [Google Scholar]
- 45.Esser S, Reilly W T, Riley L B, Eyvazzadeh C, Arcona S. The role of sentinel lymph node mapping in staging of colon and rectal cancer. Dis Colon Rectum. 2001;44:850–856. doi: 10.1007/BF02234707. [DOI] [PubMed] [Google Scholar]
- 46.Benson A B, Desch C E, Flynn P J, et al. 2000 update of American Society of Clinical Oncology colorectal cancer surveillance guidelines. J Clin Oncol. 2000;18:3586–3588. doi: 10.1200/JCO.2000.18.20.3586. [DOI] [PubMed] [Google Scholar]
- 47.Graham R A, Wang S, Catalano P J, Haller D G. Postsurgical surveillance of colon cancer: preliminary cost analysis of physician examination, carcinoembryonic antigen testing, chest x-ray, and colonoscopy. Ann Surg. 1998;228:59–63. doi: 10.1097/00000658-199807000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anthony T, Simmang C, Hyman N, et al. Practice parameters for the surveillance and follow-up of patients with colon and rectal cancer. Dis Colon Rectum. 2004;47:807–817. doi: 10.1007/s10350-004-0519-x. [DOI] [PubMed] [Google Scholar]