ABSTRACT
With the advent of restorative proctocolectomy or ileal pouch–anal anastomosis (IPAA) for ulcerative colitis (UC), not only has there been potential for cure of UC but also patients have enjoyed marked improvements in bowel function, continence, and quality of life. However, IPAA can be complicated by postoperative small bowel obstruction, disease recurrence, and pouch failure secondary to pelvic sepsis, pouch dysfunction, mucosal inflammation, and neoplastic transformation. These may necessitate emergent or expeditious elective reoperation to salvage the pouch and preserve adequate function. Local, transanal, and transabdominal approaches to IPAA salvage are described, and their indications, outcomes, and the clinical parameters that affect the need for salvage are discussed. Pouch excision for failed salvage reoperation is reviewed as well. Relaparotomy is also frequently required for recurrent Crohn's disease (CD), especially given the nature of this as yet incurable illness. Risk factors for CD recurrence are examined, and the various surgical options and margins of resection are evaluated with a focus on bowel-sparing policy. Stricturoplasty, its outcomes, and its importance in recurrent disease are discussed, and segmental resection is compared with more extensive procedures such as total colectomy with ileorectal anastomosis. Lastly, laparoscopy is addressed with respect to its long-term outcomes, effect on surgical recurrence, and its application in the management of recurrent CD.
Keywords: Restorative proctocolectomy, pouch failure, salvage/reoperation, ulcerative colitis, recurrent Crohn's disease
Although there have been great advances in the medical management of patients with inflammatory bowel disease (IBD), surgery is still frequently indicated for the complications or medically refractory symptoms of ulcerative colitis (UC) and Crohn's disease (CD). Despite the potential for cure with surgery for UC, postoperative complications, failure of the primary surgical intervention, and even initial misdiagnosis can necessitate reoperation in some cases.1 Proctocolectomy may be curative for UC, but the recurrent nature of CD has thus far rendered it incurable even by operative intervention.2 In this article, we review outcomes, complications, and postoperative recurrence in IBD patients that can lead to failure of the index surgery and require reoperation. The variety of surgical options to correct these problems and their indications and associated outcomes are also discussed. Lastly, given the increasing trend toward minimally invasive surgery, laparoscopy is addressed with respect to its impact on reoperation and its possible application in the treatment of recurrent CD.
ULCERATIVE COLITIS
Adhesive Small Bowel Obstruction
Small bowel obstruction (SBO) has been reported to be the most common complication requiring surgery following ileal pouch–anal anastomosis (IPAA), with rates ranging from 13 to 35% in various studies.3,4,5 Some of these studies, however, have been limited by small numbers, short and/or incomplete patient follow-up, and retrospective design with data not actuarially analyzed.4 In one of the largest series to date of patients diagnosed with SBO, patients were followed up for a mean of 8.7 years. There was a total of 351 episodes of SBO, and the cumulative rate for SBO was 9% at 30 days and 18%, 27%, and 31% at 1, 5, and 10 years, respectively. The corresponding reoperation rates for these obstructions were 1%, 3%, 7%, and 8%.4 This study further categorized SBO as early (delayed bowel function leading to hospital stay greater than 10 to 14 days or readmission or reoperation for SBO within 30 days of discharge) or late (readmission 30 days after discharge with diagnosis of SBO). Fifty-six percent of the observed episodes of SBO were defined as late. The need for reoperation was much more common in late SBO compared with early SBO, with 37% of late SBO patients requiring surgery compared with 5% of early SBO patients. For patients who required laparotomy and lysis of adhesions, there was a 21% risk of recurrent clinical SBO and a 5% risk that reoperation would be required.
After IPAA, adhesions are responsible for the vast majority of patients who require laparotomy for SBO. The most common sites for adhesion formation are between the small bowel and pelvis and adjacent to the prior ileostomy site.5 One of the primary strategies in adhesion prevention is to separate surfaces mechanically with biologic membranes and factors such as Silastic, Surgicel™, Interceed™, and Seprafilm™. Seprafilm™ is a sodium hyaluronate–based bioresorbable membrane that has been widely studied in several clinical applications. In a multicenter study, Becker and colleagues conducted a randomized controlled trial and found that the use of Seprafilm™ significantly decreased the frequency of anterior abdominal wall adhesions after IPAA.6 Less than half (49%) of patients in whom Seprafilm™ was used developed adhesions to the midline incision compared with almost all (94%) of the control group. Given these favorable findings and considering that the majority of post-IPAA adhesions tend to occur in the pelvis or at the ileostomy, the application of Seprafilm™ to these sites may be cost effective for adhesion prophylaxis.
Ileal Pouch–Anal Anastomosis Failure and Salvage Surgery
Although IPAA has become the operation of choice for UC patients,7,8,9 up to 17% of patients experience failure of the procedure.10,11,12,13 Failure appears to increase with duration and completeness of follow-up.10,11,12,13 In a large study reported by the Mayo Clinic, the cumulative probability for pouch failure was 2%, 5%, and 9% at 1, 5, and 10 years after IPAA, respectively.12 In a large cohort of patients, Tulchinsky and colleagues reported an overall IPAA failure rate of 9.7% after a mean follow-up period of 85 months. Three quarters of pouch failures occurred more than 1 year after IPAA, with the 3% early failure rate rising to 9% at 5 years and 13% by the tenth postoperative year.14
IPAA failure is defined as the need for excision of the pouch or its indefinite defunctioning with a proximal ileostomy. In general, failure can be attributed to sepsis, poor bowel function, mucosal inflammation, and neoplastic transformation. Septic complications leading to IPAA failure include pelvic abscess, anastomotic leak or stenosis, and pouch fistulization. Several factors may account for poor pouch function, including mechanical outlet obstruction, inadequate reservoir capacity, and anal sphincter dysfunction. Reoperative surgery is directed at resolving the first two causes of failure. Pouchitis, the most common long-term complication after IPAA,15 can be controlled medically in most cases. Fecal diversion is occasionally required to control refractory pouchitis.16 The development of adenocarcinoma within the pouch or retained rectal mucosa is very unusual and for obvious reasons not an indication for salvage of the pouch.13
The majority of late failures are caused by pelvic sepsis and poor pouch function. Despite the overwhelming impact of sepsis and pouch dysfunction, pouch failure is multifactorial in nature. In a multivariate analysis of 631 IPAA patients, 9.7% of whom were considered surgical failures, factors significantly associated with failure were not only initial pelvic sepsis but also a one-stage procedure, a final diagnosis of CD, use of a J or S pouch compared with a W reservoir, and female gender, most likely related to the relatively high incidence of pouch-vaginal fistula.14 A more recent study supported the association between higher failure rates and sepsis (especially pouch-vaginal or perineal fistula), a diagnosis of CD or indeterminate colitis, and duration of follow-up but did not show a relationship between pouch configuration and pouch failure. The strongest predictors of failure were a diagnosis of CD, pelvic sepsis, and fistula.17
PELVIC SEPSIS
Sepsis is the most common cause of pouch failure, accounting for over 50% in numerous reports, with an approximately equal contribution to early and late failure.3,10,12,14,18,19 In a series from the University of Utah, 5% of patients had J pouch–related complications, the majority of which could be attributed to pouch leaks and cuff abscesses. Over 80% of these complications required reoperative management. However, with timely therapy, including reoperation, there was an overall failure rate of less than 1%.20 When pelvic sepsis involves the anal sphincter, the failure rate is increased significantly in comparison with a more proximally located septic site (5-year failure rate of 50% versus 29%).
Early pelvic sepsis arises with fever, anal pain, tenesmus, and anal discharge of pus or blood. Delayed abdominal or pelvic sepsis, on the other hand, often manifests as an abscess, with or without a fistula. These fistulae usually originate in the ileal reservoir, most commonly from the posterior pouch wall at the ileoanal anastomosis and in rare cases from the blind end of a J-shaped pouch.21 Patients with delayed sepsis often have a history of anastomotic complications.21
One study showed that septic complications and the subsequent need for pouch excision were more frequent in patients with hand-sewn anastomoses (n = 238) than those with stapled IPAA (n = 454). However, a significant difference in sepsis-related reoperation rates between the two groups could not be demonstrated.22 Of interest, some of the authors in this group later reported that vaginal fistulae were associated with a high incidence of pelvic sepsis, particularly in patients with a hand-sewn anastomosis.23
In an effort to delineate the etiology of cuff abscesses and ileoanal anastomotic separations, Lindquist and coauthors suggested that these complications were “provoked” by kinking of the long (> 7 cm) efferent pouch limb consistently found in the complication group.24 More important, this group of patents usually had a troublesome mucosectomy secondary to severe rectal inflammation, which may have also increased the likelihood of injury during dissection, thereby predisposing to compromised tissue integrity, anastomotic disruption, and pelvic sepsis.
In some patients, early sepsis may be self-limited and require no more than antibiotic therapy. More severe cases, however, may necessitate aggressive intervention. The initial management of sepsis is typically performed through a local perineal or transanal approach. According to some authors, this should be the starting point for all revisional or salvage surgery.25 This often entails radiologically guided percutaneous abscess drainage and the use of intravenous antibiotics, which may resolve the complication in ~20% of cases in some reports.20 However, other patients require more aggressive therapy, including operative transanal or perineal drainage, seton placement, and partial fistulotomy.26 Any abscess should be deroofed and curettage of the cavity performed through the anus to create a large communication between the pouch and abscess.27 Multiple local procedures may be necessary to treat sepsis fully,13 and control of local sepsis is requisite prior to employing more major revisional procedures.26
Anastomotic separation is often the cause of abscess and pelvic sepsis. After satisfactory treatment with drainage and curettage, the disruption may be repaired transanally. These transanal approaches require a nonfibrotic pelvis and a normal-sized pouch (at least 12 cm long). Simply resuturing the anastomotic defect with counterdrainage, a pouch mucosal advancement flap procedure, and advancement of the ileal pouch with resuturing of the IPAA are the main transanal surgical options.13,21,27 Ileal pouch advancement is performed by gaining adequate exposure of the anal canal and circumferentially incising the anastomosis at the dentate line with cephalad extension superficial to the sphincteric plane, thus avoiding injury to the sphincter mechanism. Any remnant rectal mucosa and fibrotic tissue from perianastomotic stenosis should be excised and, if present, any fistulous tracts exposed and curetted. After trimming the distal edge of the pouch, it may then be advanced distally and sutured to the dentate line, using interrupted absorbable sutures. If complications are anticipated in the patient, a temporary protective stoma should be established.
Although generally debated in the context of a primary IPAA, it is important to note the controversy surrounding the protective temporary ileostomy. Some studies have shown that a one-stage restorative proctocolectomy is safe for selected patients. However, in a study of 100 UC patients treated with IPAA, half as a one-stage IPAA procedure and the other 50 patients with temporary ileostomy, life-threatening complications (including severe acute pelvic sepsis) were more common and the requirement of emergent reoperation higher in the former group. Both groups had a similar incidence of overall postoperative complications.28 Nonetheless, a temporary ileostomy is generally recommended to prevent extensive anastomotic breakdown and serious septic sequelae.1
Acute severe sepsis with extensive anastomotic breakdown occurs in ~5 to 15% of patients and may necessitate an abdominal or abdominoperineal approach for salvage.13 Abdominal/abdominoperineal salvage is indicated when multiple attempts at repair have failed or there is insufficient transanal exposure, an inability to mobilize the pouch, a high fistula, chronic presacral sinus, long stricture, long exit conduit, and/or small pouch (< 12 cm).21,26 Preoperative work-up should include digital anal examination (under anesthesia if required) in combination with imaging, such as water-soluble contrast pouchography, computed tomography (CT), and/or magnetic resonance imaging. Mechanical bowel preparation and perioperative antibiotics and steroid protection with postoperative steroid weaning (usually after the third postoperative day) are other important considerations.1,13,21 As with other reoperative pelvic surgery, healing and outcomes may be optimized with preoperative and postoperative nutritional correction, including total parenteral nutrition. Perhaps most important, prior to an attempted reconstructive salvage surgery, active or acute sepsis should be resolved. Although a low percentage of septic complications respond to conservative measures alone, the vast majority require multiple surgical interventions, including the use of a diverting ileostomy. Heuschen and colleagues employed ileal diversion in 83% of 125 major surgical procedures for septic complications following IPAA.19 MacLean and associates used CT-guided percutaneous drainage or a defunctioning ileostomy, or both, to control active sepsis prior to reconstructive pouch surgery. The latter was also performed routinely at the time of abdominoperineal salvage, with bowel continuity restored 3 months after salvage if adequate healing could be demonstrated.29
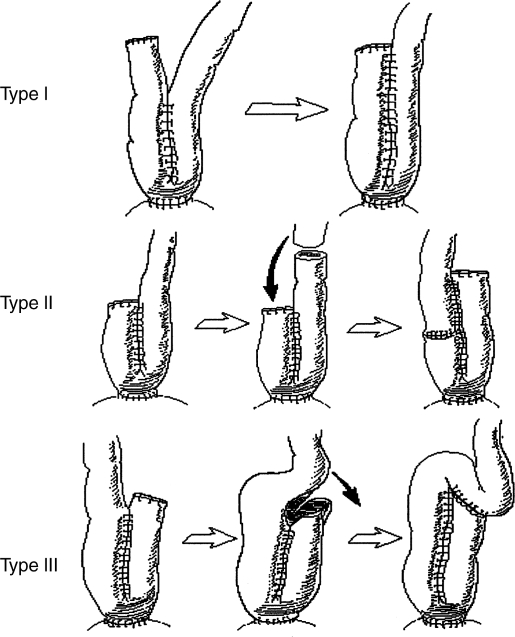
Abdominal or abdominoanal/perineal approaches involve disconnection of the ileoanal anastomosis and revision or reconstruction of the pouch with subsequent reconnection of the ileoanal anastomosis,17 in essence, a repeated IPAA (R-IPAA). After lavage and evacuation of the ileal pouch, laparotomy and lysis of any adhesions are performed. The pouch is then mobilized completely, typically starting posteriorly, and the IPAA disconnected through the abdomen or perineum. As with the transanal approach, fibrotic tissue is removed, and any infected or necrotic cavities or fistulae are opened and curetted. If needed, transperineal mucosectomy to remove any retained rectal mucosa should be done. Attention is then turned to the pouch, which may be conserved and revised, if possible. First, any defects should be repaired with interrupted absorbable sutures, and the pouch may be enlarged if small (type I, II, III pouch enlargement procedures; see Fig. 1), or it may be excised and a new pouch constructed, most often from terminal ileum and, on occasion, from the jejunum with ileal interposition30 or possibly a reversed jejunal segment.31 If possible, the first pouch revision discussed is preferable, as bowel is a precious commodity, especially in IBD. Its use or loss with the latter two options may contribute to the development of short bowel syndrome.
Figure 1.
Pouch enlargement procedures, types I–III. (Adapted from Dehni et al.21)
When a laparotomy is warranted to treat sepsis, the outcome is variable and the risk of subsequent failure of salvage has been reported to be ~60%.19,32,33,34,35 On the other hand, a success rate of 86% at 6 months was shown by Fazio and associates in 35 patients with septic complications who underwent R-IPAA.25 Moreover, similarly high success rates have been reported with R-IPAA after drainage or prolonged treatment of septic complications, or both (Table 1). Some studies, however, have revealed a worse outcome when pouch salvage was performed for septic conditions,32,35 and others even believe that sepsis contraindicates abdominal salvage.33 A study by Tekkis et al, to date the largest series of salvage surgery for IPAA, focused only on patients who had abdominal or combined abdominoanal revision. The conclusion that abdominal reoperation for salvage is contraindicated in sepsis could not be drawn, but salvage for nonseptic indications was more significantly associated with success at 5 years (85%) than for septic indications (61% success).17 MacLean and colleagues report an overall success rate of 74% with a mean follow-up of almost 6 years in patients treated with abdominoperineal salvage. Thirty-seven (65%) of these patients had septic indications for reconstruction, the majority of which were pouch-vaginal fistulae.29
Table 1.
Results of Abdominal Salvage Procedures for Failing IPAA
| Reference (Year) | N | Length of Follow-Up (Median or Mean) | Success (%) | Morbidity (%) | Comments on Functional Outcome/Quality of Life |
|---|---|---|---|---|---|
| IPAA, ileal pouch anal anastomosis; NR, not recorded. | |||||
| Korsgen et al (1996)34 | 11 | 31 months | 64 | NR | 27% had acceptable to excellent function |
| Sagar et al (1996)33 | 23 | 5 years | 74 | 26 | 94% had fair to excellent function |
| Cohen et al (1998)38 | 24 | NR | 75 | NR | 100% were satisfied to very satisfied with outcome |
| Fazio et al (1998)25 | 35 | ≥ 6 months | 86 | 68 | 57% had good to excellent quality of life 43% had poor to fair quality of life |
| MacLean et al (2002)29 | 57 | 69 months | 74 | 51 | > 80% had good to excellent quality of physical health |
| Baixauli et al (2004)26 | 101 | 32 months | 70 | NR | NR |
| Dehni et al (2005)21 | 45 | 31 months | 93 | 37 | 67% had good to excellent function 33% had poor to acceptable function |
| Tekkis et al (2006)17 | 112 | 46 months | 79 | NR | > 95% were continent |
POUCH-VAGINAL FISTULA
After IPAA, fistulous communications may form between the pouch and any adjacent structure, including the perineum and occasionally the bladder. However, the majority of fistulae involve the vagina, with a reported incidence ranging from 2.6 to 16% and a high rate of recurrence.3,36,37,38,39,40 Major predisposing factors previously shown to be associated with pouch-vaginal fistula (PVF) include trauma to the vagina or rectovaginal septum during rectal dissection, anastomotic disruption with subsequent pelvic sepsis, and the delayed diagnosis of CD.29,39,40,41 It has been postulated that PVFs that develop within 6 months of IPAA are due to technical error, and those that occur after 6 months are probably associated with CD.39
The delayed diagnosis of CD has implications for the challenging management of PVF that recur or persist after initial operative repair. Heriot et al studied 68 patients with PVF, 8 (12%) of whom were eventually diagnosed with CD. Compared with their UC cohorts, these CD patients had a lower incidence of successful PVF repair. All eight CD patients' PVFs persisted or recurred within 5 years of surgical treatment, leading to pouch failure in seven of these patients.39
Reported success rates of the various approaches for PVF repair are summarized in Table 2.23,36,37,38,41,42,43,44,45,46 The operative approach to repair a PVF is determined primarily by the anatomic origin of the fistula, particularly its relation to the anastomosis. It appears, however, that abdominoanal procedures for these fistulae are associated with a greater chance of success.39 When the PVF arises from an anastomosis at or above the anorectal junction, an abdominal/abdominoanal approach is appropriate. These patients have adequate distance distally to permit the advancement and resuturing of the anastomosis below the level of the fistula. A comprehensive review of six studies reported a high success rate (81%) using this technique.13
Table 2.
Successful Closure of Pouch-Vaginal Fistula after Salvage Procedure
| Reference (Year) | Patients with PVF (N) | Follow-Up Period (Mean or Median) | Endoanal Repair* | Transvaginal Repair* | Transabdominal Repair* |
|---|---|---|---|---|---|
| NR, not recorded; PVF, pouch vaginal fistula. | |||||
| Wexner et al (1989)42 | 26 | NR | 42% | 27% | 100% |
| Groom et al (1993)43 | 22 | NR | 0% | 100% | NR |
| Keighley and Grobler (1993)41 | 10 | NR | 100% | 100% | 100% |
| O'Kelly et al (1994)44 | 7 | 26 months | NR | 71% | NR |
| Paye et al (1996)36 | 21 | NR | 0% | NR | 80% |
| Lee et al (1997)23 | 25 | ≥ 9 months | 50% | 0% | 100% |
| Ozuner et al (1997)37 | 24 | 26 months | 63% | NR | 70–80% |
| Cohen et al (1998)38 | 12 | NR | NR | NR | 71% |
| Burke et al (2001)45 | 14 | 18 months | NR | 79% | NR |
| Zinicola et al (2003)46 | 38 | 42 months | NR | NR | 82% |
Values are percentages of successful procedures as a proportion of total number of procedures for each approach.
Local, endoanal, or transvaginal repairs are indicated when the fistula originates from an anastomosis within the anal canal or just above the sphincter, as anal canal length is insufficient to clear the fistula. Reportedly, fistulectomy and direct perineal repair yield poor results,42,43 and although flap procedures with the gracilis muscle and transposition of the rectus abdominis muscle have been reported with promise, long-term follow-up is lacking.13 An endoanal ileal advancement flap or transvaginal closure is most often employed for this approach.13,23,37 Some authors have reported high success rates and advocate the former technique, asserting more successful closure of the internal intestinal fistulous opening because of higher pouch versus vaginal pressures.40
The advantage of the transvaginal technique, however, is that it avoids potential dilation of or injury to the sphincter complex and is technically simple with direct access to the fistula. The patient is placed in the lithotomy position, and an inverted T incision is made in the midline posterior vaginal wall, with the horizontal limb at the junction of the perineal skin with the posterior vagina. Two full-thickness lateral flaps are then created after dissection of the vagina from the anal canal and ileal pouch on each side. This exposes the anterior wall of the ileoanal pouch and anastomosis. The internal opening in the bowel is then excised and the defect closed transversely with interrupted sutures. The vaginal flaps are replaced and reapproximated with interrupted sutures, and a vaginal pack is inserted to eliminate the dead space and prevent the formation of a hematoma. After a median follow-up of 18 months, Burke et al reported success of repair with good functional outcomes in 11 of 14 PVF patients treated transvaginally. However, 5 of these 11 patients required more than one attempt to achieve success.45
POUCH DYSFUNCTION
Pouch dysfunction is characterized by increased bowel frequency with small-volume stools, difficulty with evacuation, urgency, and incontinence. These can be a result of mechanical outflow obstruction, insufficient reservoir capacity, or anal sphincter dysfunction. There is a paucity of reports on salvage surgery for anal sphincter dysfunction, although Thompson and Quigley reported satisfactory outcomes with anal sphincteroplasty in two cases.47 Improved selection of patients with careful and appropriate pre-IPAA evaluation, including a thorough assessment of anal sphincter function, may help avert some of these failures.13
There are three main mechanical causes of outflow obstruction of the pouch: stricture of the ileoanal anastomosis, a long efferent pouch conduit, and the presence of retained rectum. Anastomotic stenosis may be treated with anal dilation. More aggressive therapy, for which there is a report of success, entails transanal excision of the stricture with distal advancement of the pouch. However, this approach is difficult and may still warrant further study.13 Repeated IPAA has been touted as the procedure of choice for patients with poor pouch function secondary to long, tortuous strictures refractory to repeated dilation and for long exit conduits.25,33,48,49 Long efferent conduits are associated most often with malposition and kinking of the distal limb of the S-shaped reservoir. An early European report described successful reconstruction by completely mobilizing the pouch and its efferent limb and shortening the conduit. The reservoir was then placed lower in the pelvis in close proximity to the anus and the ileoanal anastomosis recreated manually.50 This technique has also been used with satisfactory results in larger patient cohorts in this country.48 The S pouch configuration is rarely used currently, and consequently this complication is not seen commonly.
With the increasing use of stapling in IPAA, the complication of a retained rectal stump occurs when distal transection of the rectum during IPAA is too high. This essentially results in persistent symptoms of proctitis, primarily bleeding, burning, and urgency with evacuation difficulty. Given that the goal of restorative proctocolectomy is to excise all tissue at risk for disease, a retained rectal stump basically represents an inadequate resection—an errant, inadvertent ileorectal anastomosis (IRA). Although local therapy such as steroids may provide relief, long-term resolution of this complication necessitates disconnection of the IRA, excision of retained rectal tissue and mucosectomy, and manual creation of an IPAA at the dentate line.33,51,52 Although this can occasionally be performed transanally, most patients require the abdominoanal approach as described earlier.13 In a study by Tulchinsky et al, 22 of 25 patients with a retained rectal stump underwent abdominoanal salvage with success in 15 patients (68%). Although these patients benefited with a marked improvement in pouch function and quality of life, the overall success rate of this corrective surgery was notably lower than the general reported success rate for first-time IPAA in the literature,52 which can be as high as ~95%. Moreover, the patients were subjected to another major operation, not to mention a risk of malignant transformation in the remnant rectal tissue.53 This underscores the importance of creating a true ileoanal anastomosis at the index IPAA, whether by a stapled or hand-sewn technique. In spite of the popularity of the stapled anastomosis, proficiency at both methods is paramount, especially as in some patients, particularly those with a narrow pelvis, the conditions or anatomy may not permit accurate dissection at the anorectal junction and correct application of a transverse stapler at this distal level.52
INSUFFICIENT POUCH VOLUME
Adequate reservoir compliance and volume are major determinants of postoperative bowel function. An inverse relationship between pouch compliance or volume and stool frequency has been well established. Accordingly, high stool frequency, with or without urge incontinence, may result when pouch capacity or compliance or both are insufficient. These reservoir parameters should be evaluated with contrast imaging and balloon volumetry to gauge urge and the maximum tolerable pouch volume.1,54
Should medical measures fail to provide a tenable level of bowel function, the pouch may need surgical augmentation using the abdominal salvage approaches described previously (see Fig. 1). If not technically prohibitive, a more conservative approach to pouch remodeling than the aforementioned types of reservoir enlargement would simply involve the addition of an immediately proximal loop of ileum to the superior portion of the pouch. Because J reservoirs are currently more commonly used, the pouch may be converted to the triple-limbed W pouch for increased capacity, which has, in some studies, been shown to improve 24-hour evacuations and decrease nocturnal stool frequency.1,55
EXCISION FOR POUCH FAILURE
When repeated local surgery or an abdominal procedure or both do not provide clinical improvement, the only remaining surgical options are pouch excision with end ileostomy or conversion to continent ileostomy56,57 or a proximal defunctioning stoma without pouch excision. The prospect of continence with a Kock ileostomy is attractive. However, before embarking on such an endeavor, this alternative should be carefully considered and the patient adequately counseled, given its potential to compound the morbidity of the pouch excision procedure itself, which is no small undertaking and has its own complications. Karoui and colleagues studied 68 patients who underwent pouch excision after failed IPAA.58 The mortality rate was 1.4%, and overall morbidity rate was 62%. Long-term complications were significantly more common than short-term problems. Hospital readmission at 1 year was 38% and increased by 20% at 5 years after surgery. Almost three quarters of all patients readmitted for a late complication required reoperation. Interestingly, over half of the reoperations after pouch excision were related to delayed healing of the perineal wound, that is, a persistent perineal sinus. Unlike perineal wound healing after total proctocolectomy without IPAA, delayed pouch excision healing was not associated with age, gender, indication for excision, or final histologic diagnosis.
Many perineal wounds can be treated locally with procedures such as curettage alone; however, some patients require more involved surgery, including muscle flaps. In a study already referenced,58 some nonhealing perineal wounds were treated using a gracilis muscle flap with a reported success rate of 74%. Similarly, in a study of 15 patients with IBD and delayed postoperative perineal wound healing, Ryan reported healing in 80% after gracilis muscle transposition.59 Rius et al have likewise enjoyed success with the gracilis flap.60 The use of the rectus abdominis flap or omentoplasty in this clinical situation have been promising, but larger studies are needed to draw valid conclusions.
CROHN'S DISEASE
Even though surgery is not curative for CD, it is often emergently necessary for complications of the disease, such as perforation or bowel obstruction, and electively for management of medically refractory symptoms or complications of the disease. More than 75% and up to 90% of patients with CD require surgery in their lifetime. After first resection, the reoperation rate is ~40 to 50% at 10 to 15 years, with some reporting repeated surgery in as many as 76% of patients at 10 years after the primary surgery.61,62,63
Postoperative Recurrence
When CD recurs or relapses postoperatively and frequently requires reoperation, the interventions generally consist of additional bowel resections, stricturoplasty, or both, similar to that performed in the index surgery. Synchronous procedures, such as abscess drainage, may be required in more complicated cases. CD patients undergoing their first and second reoperations have similar outcomes to those undergoing their primary resection. However, it has been reported that patients requiring multiple reoperations are more likely to need a permanent ileostomy eventually and have a greater tendency to experience early clinical recurrence.64 Given that 33 to 90% of CD patients will need an operation and that 22 to 33% will require more than two resections, the development of short bowel syndrome presents a major challenge.65,66 A bowel-sparing policy has thus been advocated in the surgical management of CD. In addition, the liberal use of aggressive immune modifying drugs such as 6-mercaptopurine and infliximab should be considered in these patients to induce and maintain clinical remission of CD and minimize the risk of another surgical procedure.2,67
Several factors have been examined to assess their role in predicting postoperative CD recurrence. Recurrence rates have proved to be unaffected by increased length of resection68 or by the presence of microscopic disease in the resection margins.69,70 Pennington and coauthors demonstrated no statistically significant difference in clinical or suture line recurrence or the need for reoperation between patients with microscopic CD in the resection margins and those whose margins were microscopically normal.69 A large, controlled trial of patients undergoing ileocolic resection for CD confirmed these findings.70
The association between CD recurrence and the technique used in constructing the surgical anastomosis after resection has also been studied. The literature appears to favor functional end-to-end stapled anastomosis over its hand-sewn counterpart, with trends of lower recurrence and complication rates in the former.71,72,73 Munoz-Juarez and associates, in their case-control comparison of wide-lumen (functional end-to-end) stapled versus sutured anastomosis, demonstrated a lower reoperation rate in the stapled anastomosis group.73 Interestingly, a more recent report suggested a trend toward lower clinical and surgical recurrence rates with a side-to-side rather than end-to-side anastomosis configuration, regardless of stapled or hand-sewn technique.74
CD patients have been classified in the literature as aggressive and perforating (or fistulizing) versus more indolent and nonperforating (or fibrostenotic) phenotypes. The effect of both CD phenotypes on postoperative recurrence has been investigated. Although some studies have shown a significantly decreased time to recurrence and increased risk for reoperation, especially in the first 2 years after surgery, in patients with perforating as opposed to nonperforating disease,75 other studies have not confirmed these observations.76,77,78
Smoking has been well documented in the literature to be a risk factor for postoperative recurrence of CD.62 Furthermore, a group at Stanford University showed that current smokers were more likely to have undergone up to three reoperations for CD at any site and one reoperation for ileocecal CD than patients who quit smoking.79 In contrast, a study of 91 patients who had undergone segmental colonic resection, a third of whom also underwent reoperation, showed a longer reresection-free interval for smokers in the first 4 postoperative years. This advantage, however, did not carry over after 5 years.65
Several other clinical parameters have been associated with aggressive CD after the primary surgery. One study identified female gender and a history of perianal disease as being independently associated with an increased risk for reresection.65 Perianal disease was also cited as an independent risk factor for postoperative recurrence, along with IRA and segmental resection, in a comparison study of IRA, segmental resection, and colectomy or proctocolectomy with ileostomy.80 Borley et al demonstrated that, relative to ileocolic or colic CD, ileal disease was an independent risk factor for recurrence and reoperation.81 Another study revealed an independent association of ileocecal resection with decreased risk for reoperation. Four other parameters were associated with an increased risk for relaparotomy: young age at onset of disease, prior stricturoplasty, enterocutaneous fistula, and jejunal involvement.82 Keh et al also demonstrated that jejunal CD is associated with an increased early risk of reoperation (relative to ileocecal disease case controls). This observation, however, was not borne out for patients who developed long-term recurrences, as the difference in reoperation rate for jejunal and ileocecal CD at 10 years was not as notable as it was 3 and 5 years postoperatively.63
Surgery for Recurrent Small Bowel Crohn's Disease
Although recurrent CD of the small bowel can be treated with resection, stricturoplasty is preferable when the postoperative recurrence is fibrostenotic. As with primary surgery, both operations may be needed in the same patient at the same operation. Given the tendency to practice “bowel economy,” stricturoplasty has a particularly important application in recurrent disease, especially in patients who have already had multiple or significant prior resections or have diffuse recurrent disease, or both.
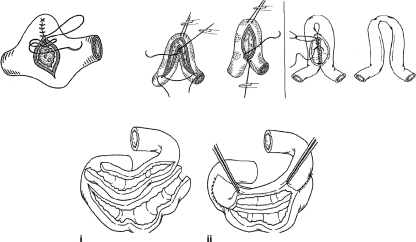
Most commonly, a Heineke-Mikulicz (HM) type of stricturoplasty is performed for short (≤ 6 cm) strictures and a Finney-type stricturoplasty used for longer strictures (see Fig. 2).83,84 The Jaboulay procedure can also be considered. Although HM stricturoplasty is more frequently used in CD patients, a meta-analysis of stricturoplasty for CD reported a lower rate of reoperative surgery when a Finney procedure was used.84 Five-year cumulative reoperative rates after stricturoplasty for CD have ranged from 22 to 45%.85,86,87 Moreover, several studies have revealed a rate of restricture at prior stricturoplasty sites of 5 to 9%, increasing to as high as 14% in series with significantly longer follow-up.85,86,87,88,89 Long stricturoplasty (> 20 cm) is an alternative to resection and has been reported to have postoperative complication and disease-free rates comparable to those of short stricturoplasty.90 Side-to-side isoperistaltic stricturoplasty has been shown to be feasible for long strictures in the setting of CD.91 Some studies report success with stricturoplasty to treat stenotic recurrence at the ileocolonic anastomosis.92,93 It appears, however, that the incidence of recurrence at an ileocolonic stricturoplasty site is relatively high, especially in studies with longer follow-up.84,93
Figure 2.
Types of stricturoplasty. (A) Heineke-Mikulicz. (B) Finney. (C) Jaboulay. (D) Side-to-side isoperistaltic stricturoplasty. (i) Diseased bowel looped together isoperistaltically. (ii) Completed enteroenterostomy. (Adapted from Kumar and Alexander-Williams83 and Tichansky et al.84)
Surgery for Recurrent Colorectal Crohn's Disease
Up until the past 15 years, the traditional surgical approach for a patient with segmental colonic CD had been full abdominal colectomy with IRA. Segmental resection has assumed an ever increasing role in the management of these patients. A study comparing segmental resection and subtotal colectomy with anastomosis for Crohn's colitis demonstrated no difference in reresection rate between the two procedures. However, patients with segmental resection had better symptomatic outcomes and anorectal function.94 Surgical treatment of colonic recurrence of CD often entails further resection in patients who have had a prior segmental resection or colectomy with IRA. In some cases, especially when the rectum develops severe disease, a permanent stoma may be needed, and the rectum itself may require resection.
Cattan and colleagues reported an 86% probability of rectal preservation at 5 and 10 years after IRA, in spite of a high clinical recurrence rate of 58% and 83% at 5 and 10 years, respectively. Crohn's patients with extraintestinal manifestations had an increased risk of recurrence as well as a lower chance of rectal preservation. Both recurrence and rectal preservation failure rates were shown to be decreased by the administration of 5-aminosalicylic acid products after ileorectal anastomosis.95 Surprisingly, several patients in this study who had to undergo proctectomy eventually had an IPAA, despite the fact that CD is a relative contraindication to IPAA.2,96 Unfortunately, the outcome of IPAA in this subgroup of patients was not reported. Some authors, however, believe that IPAA may be considered in selected CD patients with no ileal and no anal or perianal involvement.97 Even in these patients, the pouch complication rate is nearly three times that of patients who have IPAA for UC,98 and at least half of the pouches fail at 10 years.96,98 The pouch failure and excision rate was higher for patients whose CD was diagnosed after IPAA as a result of postoperative complications.98 However, the functional outcomes of the patients whose pouches survived were similar to those of patients with successful IRA.98 The exact role that IPAA plays in the surgical treatment of patients with CD remains undefined.
Laparoscopy in Crohn's Disease
The use of laparoscopy in CD has become increasingly popular. Long-term outcome after laparoscopic ileocolic resection for CD has not been shown to be statistically different from that after open resection.99 Although some studies suggest that there may be a lower incidence of SBO and even surgical recurrence rates in patients who have undergone laparoscopy,100,101 other series have not confirmed these trends.99 A meta-analysis of trials comparing laparoscopic and open resection in CD has supported a decreased rate of SBO and need for reoperation after laparoscopy.101 Although these data are favorable, caution must be used in their interpretation, as there is probably some degree of bias due to selection of patients for laparoscopic surgery.
The decreased rates of postlaparoscopy SBO have implications for the technical difficulty of reoperation. Laparoscopy has been shown to result in fewer adhesions than open surgery in some experimental animal models and clinical studies.102 Some groups have found laparoscopy to be a feasible alternative in the elective surgical management of selected patients (no complex fistula or multiple lesions and no history of more than two operations) with recurrent CD.103 Although the role of laparoscopy in CD is still evolving, the literature thus far has shown much promise.
REFERENCES
- 1.Corman M. Colon and Rectal Surgery. 5th ed. Philadelphia: Lippincott, Williams, & Wilkins; 2004. pp. 1319–1535.
- 2.Penner R, Madsen K, Fedorak R. Postoperative Crohn's disease. Inflamm Bowel Dis. 2005;11:765–777. doi: 10.1097/01.mib.0000171273.09757.f2. [DOI] [PubMed] [Google Scholar]
- 3.Fazio V W, Ziv Y, Church J M, et al. Ileal pouch anal anastomosis complications and function in 1005 patients. Ann Surg. 1995;222:120–127. doi: 10.1097/00000658-199508000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacLean A, Cohen Z, MacRae H, et al. Risk of small bowel obstruction after the ileal pouch-anal anastomosis. Ann Surg. 2002;235:200–206. doi: 10.1097/00000658-200202000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francois Y, Dozois R R, Kelly K A, et al. Small intestinal obstruction complicating ileal pouch-anal anastomosis. Ann Surg. 1989;209:46–50. doi: 10.1097/00000658-198901000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker J M, Dayton M T, Fazio V W, et al. Prevention of post-operative abdominal adhesions by a sodium hyaluronate-based bioresorbable membrane: a prospective, randomized, double-blind multicenter study. J Am Coll Surg. 1996;183:297–306. [PubMed] [Google Scholar]
- 7.Kelly K A. Anal sphincter-saving operations for chronic ulcerative colitis. Am J Surg. 1992;163:5–11. doi: 10.1016/0002-9610(92)90244-l. [DOI] [PubMed] [Google Scholar]
- 8.Becker J M. Ileal pouch-anal anastomosis: current status and controversies. Surgery. 1993;113:599–602. [PubMed] [Google Scholar]
- 9.Gemlo B T, Wong W D, Rothenberger D A, et al. Ileal pouch-anal anastomosis—patterns of failure. Arch Surg. 1992;127:784–787. doi: 10.1001/archsurg.1992.01420070036009. [DOI] [PubMed] [Google Scholar]
- 10.Korsgen S, Keighley M R. Causes of failure and life expectancy of the ileoanal pouch. Int J Colorectal Dis. 1997;12:4–8. doi: 10.1007/s003840050069. [DOI] [PubMed] [Google Scholar]
- 11.Bullard K M, Madoff R D, Gemlo B T. Is ileoanal pouch function stable with time? Results of a prospective audit. Dis Colon Rectum. 2002;45:299–304. doi: 10.1007/s10350-004-6171-7. [DOI] [PubMed] [Google Scholar]
- 12.Meagher A P, Farouk R, Dozois R R, Kelly K A, Pemberton J H. J ileal pouch-anal anastomosis for chronic ulcerative colitis: complications and outcome in 1310 patients. Br J Surg. 1998;85:800–803. doi: 10.1046/j.1365-2168.1998.00689.x. [DOI] [PubMed] [Google Scholar]
- 13.Tulchinsky H, Cohen C RG, Nicholls R J. Salvage surgery after restorative proctocolectomy. Br J Surg. 2003;90:909–921. doi: 10.1002/bjs.4278. [DOI] [PubMed] [Google Scholar]
- 14.Tulchinsky H, Hawley P, Nicholls J. Long-term failure after restorative proctocolectomy for ulcerative colitis. Ann Surg. 2003;238:229–234. doi: 10.1097/01.sla.0000082121.84763.4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker J M, Raymond J L. Ileal pouch-anal anastomosis: a single surgeon's experience with 100 consecutive cases. Ann Surg. 1986;204:375–383. doi: 10.1097/00000658-198610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandborn W J. Pouchitis following ileal pouch-anal anastomosis: definition, pathogenesis, and treatment. Gastroenterology. 1994;107:1856–1860. doi: 10.1016/0016-5085(94)90832-x. [DOI] [PubMed] [Google Scholar]
- 17.Tekkis P P, Heriot A G, Smith J J, Das P, Canero A, Nicholls R J. Long-term results of abdominal salvage surgery following restorative proctocolectomy. Br J Surg. 2006;93:231–237. doi: 10.1002/bjs.5242. [DOI] [PubMed] [Google Scholar]
- 18.Belliveau P, Trudel J, Vasilevsky C A, et al. Ileoanal anastomosis with reservoirs: complications and long-term results. Can J Surg. 1999;42:345–352. [PMC free article] [PubMed] [Google Scholar]
- 19.Heuschen U A, Allenmeyer E H, Hinz U, Lucas M, Herfarth C, Heuschen G. Outcome after septic complications in J pouch procedures. Br J Surg. 2002;89:194–200. doi: 10.1046/j.0007-1323.2001.01983.x. [DOI] [PubMed] [Google Scholar]
- 20.Dayton M T, Larsen K P. Outcome of pouch-related complications after ileal pouch-anal anastomosis. Am J Surg. 1997;174:728–732. doi: 10.1016/s0002-9610(97)00188-8. [DOI] [PubMed] [Google Scholar]
- 21.Dehni N, Remacle G, Dozois R, Banchini F, Tiret E, Parc R. Salvage reoperation for complications after ileal pouch-anal anastomosis. Br J Surg. 2005;92:748–753. doi: 10.1002/bjs.4973. [DOI] [PubMed] [Google Scholar]
- 22.Ziv Y, Fazio V W, Church J M, Lavery I C, King T M, Ambrosetti P. Stapled ileal pouch anal anastomoses are safer than hand-sewn anastomoses in patients with ulcerative colitis. Am J Surg. 1996;171:320–323. doi: 10.1016/S0002-9610(97)89634-1. [DOI] [PubMed] [Google Scholar]
- 23.Lee P Y, Fazio V W, Church J M, Hull T L, Eu K W, Lavery I C. Vaginal fistula following restorative proctocolectomy. Dis Colon Rectum. 1997;40:752–759. doi: 10.1007/BF02055426. [DOI] [PubMed] [Google Scholar]
- 24.Lindquist K, Nilsell K, Liljeqvist L. Cuff abscesses and ileoanal anastomotic separations in pelvic pouch surgery—an analysis of possible etiologic factors. Dis Colon Rectum. 1987;30:355–359. doi: 10.1007/BF02555454. [DOI] [PubMed] [Google Scholar]
- 25.Fazio V W, Wu J S, Lavery I C. Repeat ileal pouch-anal anastomosis to salvage septic complications of pelvic pouches: clinical outcome and quality of life assessment. Ann Surg. 1998;228:588–597. doi: 10.1097/00000658-199810000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baixauli J, Delaney C, Wu J S, et al. Functional outcome and quality of life after repeat ileal pouch-anal anastomosis for complications of ileoanal surgery. Dis Colon Rectum. 2004;47:2–11. doi: 10.1007/s10350-003-0003-z. [DOI] [PubMed] [Google Scholar]
- 27.Whitlow C B, Opelka F G, Gathright J B, Jr, Beck D E. Treatment of colorectal and ileoanal anastomotic sinuses. Dis Colon Rectum. 1997;40:760–763. doi: 10.1007/BF02055427. [DOI] [PubMed] [Google Scholar]
- 28.Williamson M E, Lewis W G, Sagar P M, Hodlsworth P J, Johnston D. One stage restorative proctocolectomy without temporary ileostomy for ulcerative colitis: a note of caution. Dis Colon Rectum. 1997;40:1019–1022. doi: 10.1007/BF02050922. [DOI] [PubMed] [Google Scholar]
- 29.MacLean A R, O'Connor B, Parkes R, Cohen Z, McLeod R S. Reconstructive surgery for failed ileal pouch-anal anastomosis: a viable surgical option with acceptable results. Dis Colon Rectum. 2002;45:880–886. doi: 10.1007/s10350-004-6321-y. [DOI] [PubMed] [Google Scholar]
- 30.Dehni N, Cunningham C, Parc R. Use of a jejunal pouch with ileal interposition in salvage surgery after restorative proctocolectomy. Dis Colon Rectum. 1998;41:1587–1589. doi: 10.1007/BF02237313. [DOI] [PubMed] [Google Scholar]
- 31.Loriau J, Benoist A, Panis Y, Joly F, Messing B, Valleur P. Salvage of ileal pouch-anal anastomosis by a reversed jejunal segment. Surgery. 2005;137:111–113. doi: 10.1016/j.surg.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 32.Galandiuk S, Scott N A, Dozois R R, et al. Ileal pouch-anal anastomosis. Reoperation for pouch-related complications. Ann Surg. 1990;212:446–454. doi: 10.1097/00000658-199010000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sagar P M, Dozois R R, Wolff B G, Kelly K A. Disconnection, pouch revision, and reconnection of the ileal pouch-anal anastomosis. Br J Surg. 1996;83:1401–1405. doi: 10.1002/bjs.1800831025. [DOI] [PubMed] [Google Scholar]
- 34.Korsgen S, Nikteas N, Ogunbiyi O A, Keighley M R. Results from pouch salvage. Br J Surg. 1996;83:372–374. doi: 10.1002/bjs.1800830325. [DOI] [PubMed] [Google Scholar]
- 35.Ogunbiyi O A, Korsgen S, Keighley M R. Pouch salvage. Long-term outcome. Dis Colon Rectum. 1997;40:548–552. doi: 10.1007/BF02055376. [DOI] [PubMed] [Google Scholar]
- 36.Paye F, Penna C, Chiche L, Tiret A, Frileux P, Parc R. Pouch-related fistula following restorative proctocolectomy. Br J Surg. 1996;83:1574–1577. doi: 10.1002/bjs.1800831127. [DOI] [PubMed] [Google Scholar]
- 37.Ozuner G, Hull T, Lee P, Fazio V W. What happens to a pelvic pouch when a fistula develops? Dis Colon Rectum. 1997;40:543–547. doi: 10.1007/BF02055375. [DOI] [PubMed] [Google Scholar]
- 38.Cohen Z, Smith D, McLeod R. Reconstructive surgery for pelvic pouches. World J Surg. 1998;22:342–346. [PubMed] [Google Scholar]
- 39.Heriot A, Tekkis P, Smith J, Bona R, Cohen R, Nicholls R. Management and outcome of pouch-vaginal fistulas following restorative proctocolectomy. Dis Colon Rectum. 2005;48:451–458. doi: 10.1007/s10350-004-0902-7. [DOI] [PubMed] [Google Scholar]
- 40.Shah N, Remzi F, Massmann A, Baixauli J, Fazio V. Management and treatment outcome of pouch-vaginal fistulas following restorative proctocolectomy. Dis Colon Rectum. 2003;46:911–917. doi: 10.1007/s10350-004-6684-0. [DOI] [PubMed] [Google Scholar]
- 41.Keighley M, Grobler S. Fistula complicating restorative proctocolectomy. Br J Surg. 1993;80:1065–1067. doi: 10.1002/bjs.1800800849. [DOI] [PubMed] [Google Scholar]
- 42.Wexner S D, Rothenberger D A, Jensen L, et al. Ileal pouch vaginal fistulas: incidence, etiology, and management. Dis Colon Rectum. 1989;32:460–465. doi: 10.1007/BF02554497. [DOI] [PubMed] [Google Scholar]
- 43.Groom J S, Nicholls R J, Hawley P R, Phillips R K. Pouch-vaginal fistula. Br J Surg. 1993;80:936–940. doi: 10.1002/bjs.1800800750. [DOI] [PubMed] [Google Scholar]
- 44.O'Kelly T J, Merrett M, Mortensen N J, Dehn T C, Kettlewell M. Pouch–vaginal fistulas after restorative proctocolectomy: aetiology and management. Br J Surg. 1994;81:1374–1375. doi: 10.1002/bjs.1800810943. [DOI] [PubMed] [Google Scholar]
- 45.Burke D, VanLaarhoven C J, Herbst F, Nicholls R J. Transvaginal repair of pouch vaginal fistula. Br J Surg. 2001;88:241–245. doi: 10.1046/j.1365-2168.2001.01663.x. [DOI] [PubMed] [Google Scholar]
- 46.Zinicola R, Wilkinson K H, Nicholls R J. Ileal pouch-vaginal fistula treated by abdominoanal advancement of the ileal pouch. Br J Surg. 2003;90:1434–1435. doi: 10.1002/bjs.4314. [DOI] [PubMed] [Google Scholar]
- 47.Thompson J S, Quigley E M. Anal sphincteroplasty for incontinence after ileal pouch-anal anastomosis. Report of two cases. Dis Colon Rectum. 1995;38:215–218. doi: 10.1007/BF02052456. [DOI] [PubMed] [Google Scholar]
- 48.Fonkalsrud E W, Bustorff-Silva J. Reconstruction for chronic dysfunction of ileoanal pouches. Ann Surg. 1999;229:197–204. doi: 10.1097/00000658-199902000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicholls R J, Gilbert J M. Surgical correction of the efferent ileal limb for disordered defaecation following restorative proctocolectomy with the S ileal reservoir. Br J Surg. 1990;77:152–154. doi: 10.1002/bjs.1800770212. [DOI] [PubMed] [Google Scholar]
- 50.Liljeqvist L, Lindquist K. A reconstructive operation on malfunctioning S-shaped pelvic reservoirs. Dis Colon Rectum. 1985;28:506–511. doi: 10.1007/BF02554098. [DOI] [PubMed] [Google Scholar]
- 51.Fazio V W, Tjandra J J. Transanal mucosectomy. Ileal pouch advancement for anorectal dysplasia or inflammation after restorative proctocolectomy. Dis Colon Rectum. 1994;37:1008–1011. doi: 10.1007/BF02049314. [DOI] [PubMed] [Google Scholar]
- 52.Tulchinsky H, McCourtney J, Subba Rao K, et al. Salvage abdominal surgery in patients with a retained rectal stump after restorative proctocolectomy and stapled anastomosis. Br J Surg. 2001;88:1602–1606. doi: 10.1046/j.0007-1323.2001.01931.x. [DOI] [PubMed] [Google Scholar]
- 53.Sequens R. Cancer in the anal canal (transitional zone) after restorative proctocolectomy with stapled ileal pouch-anal anastomosis. Int J Colorectal Dis. 1997;12:254–255. doi: 10.1007/s003840050100. [DOI] [PubMed] [Google Scholar]
- 54.Oresland T, Fasth S, Nordgren S, Akervall S, Hulten L. Pouch size, the important functional determinant after restorative proctocolectomy. Br J Surg. 1990;77:265–269. doi: 10.1002/bjs.1800770310. [DOI] [PubMed] [Google Scholar]
- 55.Klas J, Myers G A, Starling J R, Harms B A. Physiologic evaluation and surgical management of failed ileoanal pouch. Dis Colon Rectum. 1998;41:854–861. doi: 10.1007/BF02235365. [DOI] [PubMed] [Google Scholar]
- 56.Hulten L, Fasth S, Hallgren T, Oresland T. The failing pelvic conversion to continent ileostomy. Int J Colorectal Dis. 1992;7:119–121. doi: 10.1007/BF00360349. [DOI] [PubMed] [Google Scholar]
- 57.Ecker K W, Haberer M, Feifel G. Conversion of the failing ileoanal pouch to reservoir-ileostomy rather than to ileostomy alone. Dis Colon Rectum. 1996;39:977–980. doi: 10.1007/BF02054684. [DOI] [PubMed] [Google Scholar]
- 58.Karoui M, Cohen R, Nicholls J. Results of surgical removal of the pouch after failed restorative proctocolectomy. Dis Colon Rectum. 2004;47:869–875. doi: 10.1007/s10350-004-0536-9. [DOI] [PubMed] [Google Scholar]
- 59.Ryan J A. Gracilis muscle flap for persistent perineal sinus of inflammatory bowel disease. Am J Surg. 1984;148:64–70. doi: 10.1016/0002-9610(84)90290-3. [DOI] [PubMed] [Google Scholar]
- 60.Rius J, Nessim A, Nogueras J J, Wexner S D. Gracilis transposition in complicated perineal fistula and unhealed perineal wounds in Crohn's disease. Eur J Surg. 2000;166:218–222. doi: 10.1080/110241500750009311. [DOI] [PubMed] [Google Scholar]
- 61.Bernell O, Lapidus A, Hellers G. Risk factors for surgery and recurrence in 907 patients with primary ileocecal Crohn's disease. Br J Surg. 2000;87:1697–1701. doi: 10.1046/j.1365-2168.2000.01589.x. [DOI] [PubMed] [Google Scholar]
- 62.Borley N R, Mortensen N J, Jewell D P. Preventing post-operative recurrence of Crohn's disease. Br J Surg. 1997;84:1493–1502. [PubMed] [Google Scholar]
- 63.Keh C, Shatari T, Yamamoto T, Menon A, Clark M A, Keighley M R. Jejunal Crohn's disease is associated with a higher postoperative recurrence rate than ileocaecal Crohn's disease. Colorectal Dis. 2005;7:366–368. doi: 10.1111/j.1463-1318.2005.00766.x. [DOI] [PubMed] [Google Scholar]
- 64.Heimann T M, Grenstein A J, Lewis B, Kaufman D, Heimann D M, Aufses A H., Jr Comparison of primary and reoperative surgery in patients with Crohn's disease. Ann Surg. 1998;227:492–495. doi: 10.1097/00000658-199804000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Polle S W, Slors J FM, Weverling G J, Gouma D J, Hommes D W. Recurrence after segmental resection for colonic Crohn's disease. Br J Surg. 2005;92:1143–1149. doi: 10.1002/bjs.5050. [DOI] [PubMed] [Google Scholar]
- 66.Krupnick A S, Morris J B. The long-term results of resection and multiple resections in Crohn's disease. Semin Gastrointest Dis. 2000;11:41–51. [PubMed] [Google Scholar]
- 67.Steinhart H. Maintenance therapy in Crohn's disease. Can J Gastroenterol. 2000;14(Suppl C):23C–28C. doi: 10.1155/2000/480782. [DOI] [PubMed] [Google Scholar]
- 68.Ellis L, Calhoun P, Kaiser D L. Post-operative recurrence in Crohn's disease. The effect of the initial length of bowel resection and operative procedure. Ann Surg. 1984;199:340–347. doi: 10.1097/00000658-198403000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pennington L, Hamilton S R, Bayless T M, Cameron J L. Surgical management of Crohn's disease. Influence of disease at margin of resection. Ann Surg. 1980;192:311–318. doi: 10.1097/00000658-198009000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fazio V W, Marchetti F, Church M, et al. Effect of resection margin on the recurrence of Crohn's disease in the small bowel. A randomized controlled trial. Ann Surg. 1996;224:563–573. doi: 10.1097/00000658-199610000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tersigni R, Alessandroni L, Barreca M, Piovanello P, Prantera C. Does stapled functional end-to-end anastomosis affect recurrence of Crohn's disease after ileocolonic resection? Hepatogastroenterology. 2003;50:1422–1425. [PubMed] [Google Scholar]
- 72.Hashemi M, Novell J R, Lewis A A. Side-to-side stapled anastomosis may delay recurrence in Crohn's disease. Dis Colon Rectum. 1998;41:1293–1296. doi: 10.1007/BF02258231. [DOI] [PubMed] [Google Scholar]
- 73.Munoz-Juarez M, Yamamoto T, Wolff B G, Keighley M R. Wide-lumen stapled anastomosis vs conventional end-to-end anastomosis in the treatment of Crohn's disease. Dis Colon Rectum. 2001;44:20–26. doi: 10.1007/BF02234814. [DOI] [PubMed] [Google Scholar]
- 74.Scarpa M, Angriman I, Barollo M, et al. Role of stapled and hand-sewn anastomosis in recurrence of Crohn's disease. Hepatogastroenterology. 2004;51:1053–1057. [PubMed] [Google Scholar]
- 75.Aeberhard P, Berchtold W, Riedtmann H J, Stadelmann G. Surgical recurrence of perforating and non-perforating Crohn's disease. A study of 101 surgically treated patients. Dis Colon Rectum. 1996;39:80–87. doi: 10.1007/BF02048274. [DOI] [PubMed] [Google Scholar]
- 76.Sachar D B, Subramani K, Mauer K, et al. Patterns of post-operative recurrence in fistulizing and stenotic Crohn's disease, a retrospective cohort of 71 patients. J Clin Gastroenterol. 1996;22:114–116. doi: 10.1097/00004836-199603000-00008. [DOI] [PubMed] [Google Scholar]
- 77.Yamamoto T, Allan R N, Keighley M R. Perforating ileocecal Crohn's disease does not carry a high risk of recurrence but usually re-presents as perforating disease. Dis Colon Rectum. 1999;42:519–524. doi: 10.1007/BF02234180. [DOI] [PubMed] [Google Scholar]
- 78.McDonald P J, Fazio V W, Farmer R G, et al. Perforating and non-perforating Crohn's disease. An unpredictable guide to recurrence after surgery. Dis Colon Rectum. 1989;32:117–120. doi: 10.1007/BF02553823. [DOI] [PubMed] [Google Scholar]
- 79.Ryan W R, Yamamoto T, Keighley M R. Crohn's disease patients who quit smoking have a reduced risk of operation for recurrence. Am J Surg. 2004;187:219–225. doi: 10.1016/j.amjsurg.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 80.Bernell O, Lapidus A, Hellers G. Recurrence after colectomy in Crohn's colitis. Dis Colon Rectum. 2001;44:647–654. doi: 10.1007/BF02234559. [DOI] [PubMed] [Google Scholar]
- 81.Borley N R, Mortensen N J, Chaudry M A, et al. Recurrence after abdominal surgery for Crohn's disease: relationship to disease site and surgical procedure. Dis Colon Rectum. 2002;45:377–383. doi: 10.1007/s10350-004-6186-0. [DOI] [PubMed] [Google Scholar]
- 82.Post S, Herfarth C, Bohm E, et al. The impact of disease pattern, surgical management, and individual surgeons on the risk for relaparotomy for recurrent Crohn's disease. Ann Surg. 1996;223:253–260. doi: 10.1097/00000658-199603000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kumar D, Alexander-Williams J. Crohn's Disease and Ulcerative Colitis: Surgical Management. London: Springer-Verlag; 1993. pp. 89–101.
- 84.Tichansky D, Cagir B, Yoo E, Marcus S M, Fry R D. Stricturoplasty for Crohn's disease: meta-analysis. Dis Colon Rectum. 2000;43:911–919. doi: 10.1007/BF02237350. [DOI] [PubMed] [Google Scholar]
- 85.Ozuner G, Fazio V W, Lavery I C, Milsom J W, Strong S A. Reoperative rates for Crohn's disease following stricturoplasty. Long-term analysis. Dis Colon Rectum. 1996;39:1199–1203. doi: 10.1007/BF02055108. [DOI] [PubMed] [Google Scholar]
- 86.Hurst R D, Michelassi F. Stricturoplasty for Crohn's disease: techniques and long-term results. World J Surg. 1998;22:359–363. doi: 10.1007/s002689900397. [DOI] [PubMed] [Google Scholar]
- 87.Futami K, Arima S. Role of stricturoplasty in surgical treatment of Crohn's disease. J Gastroenterol. 2005;40(Suppl 16):35–39. doi: 10.1007/BF02990577. [DOI] [PubMed] [Google Scholar]
- 88.Tonelli F, Ficari F. Stricturoplasty in Crohn's disease: surgical option. Dis Colon Rectum. 2000;43:920–926. doi: 10.1007/BF02237351. [DOI] [PubMed] [Google Scholar]
- 89.Dietz D W, Laureti S, Strong S A, et al. Safety and long-term efficacy of stricturoplasty in 314 patients with obstructing small bowel Crohn's disease. J Am Coll Surg. 2001;192:330–338. doi: 10.1016/s1072-7515(01)00775-x. [DOI] [PubMed] [Google Scholar]
- 90.Shatari T, Clark M A, Yamamoto T, et al. Long stricturoplasty is as safe and effective as short stricturoplasty in small-bowel Crohn's disease. Colorectal Dis. 2004;6:438–441. doi: 10.1111/j.1463-1318.2004.00664.x. [DOI] [PubMed] [Google Scholar]
- 91.Tonelli F, Fedi M, Paroli G M, Fazi M. Indications and results of side-to-side isoperistaltic stricturoplasty in Crohn's disease. Dis Colon Rectum. 2004;47:494–501. doi: 10.1007/s10350-003-0084-8. [DOI] [PubMed] [Google Scholar]
- 92.Tjandra J J, Fazio V W. Stricturoplasty for ileocolic anastomotic strictures in Crohn's disease. Dis Colon Rectum. 1993;36:1099–1104. doi: 10.1007/BF02052256. [DOI] [PubMed] [Google Scholar]
- 93.Yamamoto T, Keighley M R. Long-term results of stricturoplasty for ileocolonic anastomotic recurrence in Crohn's disease. J Gastrointest Surg. 1999;3:555–560. doi: 10.1016/s1091-255x(99)80112-7. [DOI] [PubMed] [Google Scholar]
- 94.Andersson P, Olaison G, Hallbrook O, Sjodahl R. Segmental resection or subtotal colectomy in Crohn's colitis? Dis Colon Rectum. 2002;45:47–53. doi: 10.1007/s10350-004-6113-4. [DOI] [PubMed] [Google Scholar]
- 95.Cattan P, Bonhomme N, Panis Y, et al. Fate of the rectum in patients undergoing total colectomy for Crohn's disease. Br J Surg. 2002;89:454–459. doi: 10.1046/j.0007-1323.2001.02053.x. [DOI] [PubMed] [Google Scholar]
- 96.Brown C, MacLean A, Cohen Z, MacRae H, O'Connor B, McLeod R. Crohn's disease and indeterminate colitis and the ileal pouch-anal anastomosis: outcomes and patterns of failure. Dis Colon Rectum. 2005;48:1542–1549. doi: 10.1007/s10350-005-0059-z. [DOI] [PubMed] [Google Scholar]
- 97.Regimbeau J M, Panis Y, Pocard M, et al. Long-term results of ileal pouch-anal anastomosis for colorectal Crohn's disease. Dis Colon Rectum. 2001;44:769–778. doi: 10.1007/BF02234693. [DOI] [PubMed] [Google Scholar]
- 98.Mylonakis E, Allan R N, Keighley M R. How does pouch construction for a final diagnosis of Crohn's disease compare with ileoproctostomy for established Crohn's proctocolitis? Dis Colon Rectum. 2001;44:1137–1143. doi: 10.1007/BF02234634. [DOI] [PubMed] [Google Scholar]
- 99.Lowney J, Dietz D, Birnbaum E, Kodner I, Mutch M, Fleshmen J. Is there any difference in recurrence rates in laparoscopic ileocolic resection for Crohn's disease compared with conventional surgery? A long-term follow-up study. Dis Colon Rectum. 2006;49:58–63. doi: 10.1007/s10350-005-0214-6. [DOI] [PubMed] [Google Scholar]
- 100.Bergamaschi R, Pessaux P, Arnaud J P. Comparison of conventional and laparoscopic ileocolic resection for Crohn's disease. Dis Colon Rectum. 2003;46:1129–1133. doi: 10.1007/s10350-004-7292-8. [DOI] [PubMed] [Google Scholar]
- 101.Rosman A S, Melis M, Fichera A. Meta-analysis of trials comparing laparoscopic and open surgery for Crohn's disease. Surg Endosc. 2005;19:1549–1555. doi: 10.1007/s00464-005-0114-9. [DOI] [PubMed] [Google Scholar]
- 102.Gutt C N, Onui T, Schemmer P, Mehrabi A, Buchler M W. Fewer adhesions induced by laparoscopic surgery? Surg Endosc. 2004;18:898–906. doi: 10.1007/s00464-003-9233-3. [DOI] [PubMed] [Google Scholar]
- 103.Hasegawa H, Watanabe M, Nishibori H, Okabayashi K, Hibi T, Kitajima M. Laparoscopic surgery for recurrent Crohn's disease. Br J Surg. 2003;90:970–973. doi: 10.1002/bjs.4136. [DOI] [PubMed] [Google Scholar]