ABSTRACT
Since the early 1900s, skeletal muscle transpositions have been employed for complicated cases of fecal incontinence to augment or replace the anal sphincter. Multiple techniques have evolved that vary with the type and configuration of muscle used in the reconstruction. Transposition of the gluteus maximus muscle was popular in the early stages of development but was replaced by techniques involving transposition of the gracilis muscle. Within the past 16 years, electrical stimulators have been applied to the transposed muscle flaps to create a dynamic reconstruction improving the efficacy of these neosphincters over their static counterparts. However, the stimulated versions are technically demanding with a high rate of morbidity secondary to complications of the multiple components and variations in technique. The stimulator used in this procedure has been removed from the US market, although it is still available in other countries. Currently in the United States, gracilis transposition is still employed in the absence of an electrical stimulator as an adjunct to the artificial bowel sphincter (Acticon NeosphincterTM, American Medical Systems, Minnetonka, MN), such as in cases of severe muscle loss and congenital atresia. In European countries, the stimulated graciloplasty continues to evolve, leading to expansion of its use in total anorectal reconstruction for anal atresia and after abdominoperineal resection.
Keywords: Fecal incontinence, muscle transposition, graciloplasty, gluteoplasty, neosphincter construction
Patients with severe fecal incontinence unresponsive to conservative measures can be divided into two broad categories of surgical approach. The first group includes those patients with an identifiable anatomic sphincter defect who can expect a 40 to 60% long-term surgical success rate with overlapping sphincteroplasty.1 The absence of pudendal neuropathy yields the best outcome in these patients.2 The second group includes those patients with extensive sphincter damage, muscle loss, or pudendal neuropathy not amenable to direct sphincter repair. With the widespread prevalence of fecal incontinence and development of newer surgical techniques, the patient with severe fecal incontinence is seldom obligated to a permanent stoma. Neosphincter procedures involving transposition of autologous muscle grafts play an important role for these patients.
HISTORY OF MUSCLE TRANSPOSITION FOR FECAL INCONTINENCE
In the first half of the century, the gluteus maximus muscle was the most commonly used muscle in transpositions. Chetwood3 first described the operation in 1902 involving the gluteus maximus muscle and fascial slings to reinforce the sphincter muscles in children. In 1929, the procedure was revised such that the free ends of the fascia used to encircle the anus were anchored to the gluteus maximus muscle on each side. With this technique, the anal canal was enclosed in a fascial ring and the sphincter could be tightened by contracting the gluteus muscle.4 Since that time, multiple case reports have reinforced the gluteus maximus muscle as an effective replacement of the anal sphincter. However, enthusiasm for the gluteoplasty diminished after the introduction of the gracilis procedures.
Advantages of gracilis for transposition include its superficial location and ease of mobilization. As opposed to the fat and bulky muscle belly of the gluteus requiring bilateral transposition to encircle the anus, the gracilis is a long and flat muscle belly that allows unilateral mobilization to complete the wrap. In addition, use of the gracilis produces less of a functional movement deficit. Reports of gracilis muscle transposition were initiated by Pickrell and colleagues5 in 1952 to treat children with fecal incontinence due to neurologic and congenital anomalies. The technique involved wrapping the gracilis around the anus and attaching the free end to the contralateral ischial tuberosity. The basic concept was to create an anal encirclement repair similar to the Thiersch procedure using autologous tissue. Patients went through a period of training with exercises to learn to voluntarily contract and relax the muscle. The muscle contracted with abduction of the thigh and was made to relax by assuming the squatting position to avoid abduction of the leg. This technique met with only moderate success since these static, striated muscle flaps were prone to fatigue with chronic contraction. The transposed muscle did not have any involuntary tone at rest, and patients had to perform awkward movements to achieve imperfect continence. These problems are identical to those following the gluteus muscle transposition.
In 1981, Salmons6 reported on the transformation of skeletal muscle from fast-twitch fatigue-prone (type II) muscle fibers to slow-twitch fatigue-resistant (type I) muscle fibers by application of low frequency electrical stimulation. Expanding on this idea in 1988, Baeten and associates7 connected a pulse generator to a patient with suboptimal function of the gracilis muscle transplant. The result was a neosphincter with involuntary resting tone. Widespread adoption of this technique improved the success of gracilis transpositions. However, the procedure involves many components and requires technical expertise, predisposing it to an array of complications that proved to undermine its advantages. The increased morbidity and associated cost led to the abandonment by the manufacturer in 1999 in seeking FDA approval in the United States. Consequently, the focus shifted back to its static counterpart with modifications of the original technique to improve outcome. Graciloplasty continues to be performed in the United States to replace sphincter muscle loss and as an adjunct to the artificial bowel sphincter. However, in other parts of the world, advances in the technique of stimulated graciloplasty have led to its application to total anorectal reconstruction for anal atresia and following abdominoperineal resection (APR).
UNSTIMULATED GLUTEOPLASTY
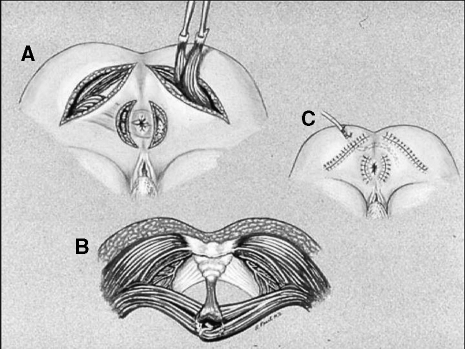
Advantages of the gluteus maximus muscle include its large muscle bulk, single proximal innervation, and proximity to the anal canal. In addition, buttock contraction is a standard response to impending incontinence. In the prone-jackknife position, the lower 10% of both of the gluteus maximus muscles and fascia are mobilized from their origins on the ileum and sacrum and distally freed in two strips. The neurovascular bundle is preserved where it arises near the ischial tuberosity. The two strips on each side are tunneled beneath the skin and secured to their contralateral counterparts through lateral incisions on the contralateral sides of the anus (Fig. 1).
Figure 1.
(A) Unstimulated gluteoplasty. The lower 10% of both the gluteus maximus muscles and fascia are mobilized from their origins on the ileum and sacrum and distally freed in two strips. (B) The two strips on each side are tunneled beneath the skin and secured to their contralateral counterparts through (C) lateral incisions on the contralateral sides of the anus.
Though not as popular as the graciloplasty, this operation has been performed with variable results. Pearl and coworkers8 performed bilateral nonstimulated gluteoplasty without diversion in seven patients with excellent results in six. In contrast, Christiansen and colleagues9 reported poor results as none of their seven patients were continent to liquid and only three were continent to solid. Most recently, Devesa and associates10 reported the largest clinical experience to date with only moderate results. Only 9 of the 17 patients who could be evaluated who underwent bilateral nonstimulated gluteoplasty achieved normal control. The morbidity of this procedure is exclusively related to wound infection of the perianal wounds.8,9,10 While unilateral gluteoplasty has been described,11 better muscle bulk and more even distribution of tensile forces are created with bilateral gluteal muscle transpositions. Despite the wide variability in results, this technique continues to be preferred to colostomy in patients with severe fecal incontinence. The results of unstimulated gluteoplasty are shown in Table 1.
Table 1.
Unstimulated Bilateral Gluteoplasty
UNSTIMULATED GRACILOPLASTY
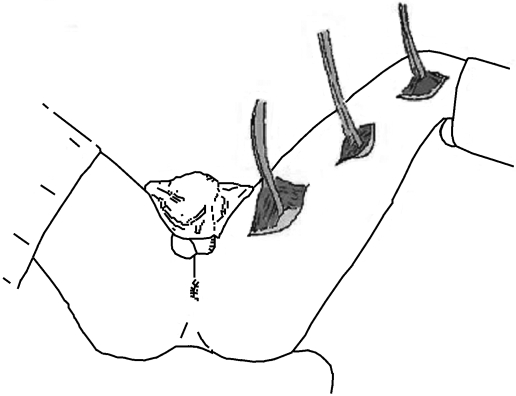
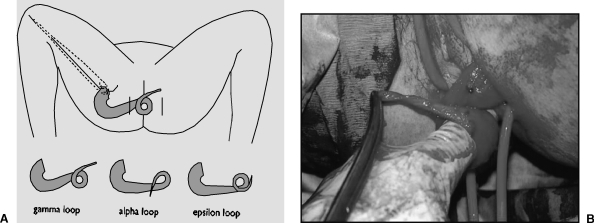
Two or three 5-cm longitudinal incisions along the length of the medial thigh allow identification and mobilization of the gracilis (Fig. 2). The neurovascular supply arises in the proximal portion of the muscle and allows division of the tendon at its attachment to the tibia without compromising viability. The muscle is then tunneled to either an anterior or posterior perianal incision. Using an additional contralateral anal incision, the muscle is wrapped around the anus using one of three configurations: α, gamma, or epsilon (Fig. 3). The tendon is anchored to the contralateral ischial tuberosity with nonabsorbable suture. However, muscle—not tendon—is meant to encircle the anus as the operation aims to achieve a dynamic result as opposed to a Thiersch-type wrap of a fibrotic band of tendon. At completion, the wrap should allow the snug insertion of one finger. The patient relaxes the sphincter by assuming a squatting position and avoiding abduction of the leg, while the sphincter is made to contract by standing and abducting the leg.
Figure 2.
Incisions for harvesting the gracilis. Two or three 5-cm longitudinal incisions along the length of the medial thigh allow identification and mobilization of the gracilis muscle.
Figure 3.
(A) Wrapping the gracilis around the anus. (B) The muscle is wrapped around the anus using one of three configurations: α, gamma, or epsilon.
The original description by Pickrell and coworkers5 demonstrated 100% continence with his pediatric patients. Subsequently, Corman12 reported on 14 patients followed for at least 5 years, 11 of whom had excellent or fair results. He attributes his success to appropriate patient selection including only relatively young, motivated patients without functional colonic dysmotility and with disabling incontinence secondary to trauma or congenital anomaly. Christiansen et al13 were also successful with 13 patients of whom all but 3 improved. Sielezneff and associates14 were successful with 8 patients, all of whom improved using the original procedure described by Pickrell combined with postoperative biofeedback. In contrast, Yoshioka and Keighley15 reported poor results in 6 patients who underwent graciloplasty all of whom required a colostomy and 5 of whom had a septic complication. Eccersley et al16 reported that two thirds of patients had improvement in continence with half of the patients experiencing good function. They concluded that these results were comparable to the results of their stimulated counterparts. In this study, better results were obtained in younger patients and in individuals without pudendal neuropathy, suggesting that in such patients nonstimulated graciloplasty alone may be effective.
In an attempt to improve outcome, the procedure was modified by Kumar and colleagues17 to include bilateral gracilis transpositions. They performed this procedure in 10 patients with a colostomy for diversion. All of the 9 who underwent colostomy reversal were fully continent at 2 years. Though more investigation is needed, the improved results compared with unilateral graciloplasty suggests that the bilateral wrap may be a better alternative to the stimulated graciloplasty. Results of the nonstimulated graciloplasty are shown in Table 2.
Table 2.
Unstimulated Graciloplasty
| Author | Country | Year | Patients | Success |
|---|---|---|---|---|
| Pickrell et al5 | USA | 1952 | 34 | 34 |
| Corman et al12 | USA | 1985 | 14 | 11 |
| Christiansen et al13 | Denmark | 1990 | 13 | 10 |
| Sielezneff et al14 | France | 1996 | 8 | 8 |
| Yoshioka and Keighley15 | UK | 1988 | 6 | 0 |
| Kumar17* | UK | 1995 | 9 | 9 |
| Eccersley16** | UK | 1999 | 8 | 8 |
Bilateral graciloplasty as opposed to unilateral graciloplasty.
Combined with postoperative biofeedback.
DYNAMIC GRACILOPLASTY
The technique of stimulated graciloplasty generally involves two or three phases with the number of required operations dependent on the use of an optional stoma. Phase 1 consists of transposition of the gracilis muscle from the thigh to form a skeletal muscular ring around the anus. The distal severed portion is anchored to the contralateral ischial tuberosity. The stimulator (Fig. 4) is implanted in the abdominal wall while the leads are placed either on the main trunk or into the intramuscular portion the gracilis near the nerve. Phase 2 involves 8 weeks of muscle conditioning with increasing levels of low-frequency neuromuscular stimulation. During this conditioning, the muscle fibers gradually convert from rapid-twitch, easily fatiguable fibers to tonic, slow-contracting fibers. The use of diverting stoma requires additional operative intervention for creation and closure. Upon completion of phase 2, the patient is able to control continence with the use of an external magnet (Fig. 5). The patient can turn the neurostimulator on, causing the muscle to contract, and off, causing the muscle to relax.
Figure 4.
The stimulator. The stimulator is implanted in the abdominal wall while the leads are placed either on the main trunk or into the intramuscular portion of the gracilis near the nerve.
Figure 5.
The magnet. The patient is able to control continence with the use of an external magnet that turns the stimulator on and off.
Stimulated graciloplasty is currently the most studied and employed transposition procedure for fecal incontinence with most recent reports of success between 57% and 93% (see Table 3). The Dynamic Graciloplasty Therapy Study Group has provided the most prospective multicenter data with regard to outcome of this procedure. The initial report on the efficacy revealed improvement in continence and quality of life in the majority (60%) of patients.18 Long-term efficacy reported in a separate study revealed a 62% success rate and improvements in functional and quality-of-life variables that persisted during a 2-year duration.19
Table 3.
Stimulated Graciloplasty
| Author | Country | Year (n) | Patients (months) | Follow-up | Morb (%) | Comp# (%) | Reoperation(%) | Success |
|---|---|---|---|---|---|---|---|---|
| IM, intermuscular; DS, direct nerve stimulation. | ||||||||
| Mavrantonis et al22 | USA | 1999 | 21IM | 21 | 93 | |||
| 6DS | 13 | 10 | ||||||
| Mander et al18 | Multicenter | 1999 | 64 | 10 | 38 | 56 | ||
| Madoff et al25* | Multicenter | 1999 | 128 | 26 | 41 | 138 | 66 | |
| Baeten et al27 | Multicenter | 2000 | 123 | 12 | 74 | 40 | 60 | |
| Konsten et al23 | Netherlands | 2001 | 200IM | 3 | 74 | |||
| 81DS | 26 | 57 | ||||||
| Wexner et al19 | Multicenter | 2002 | 129 | 24 | 62 | |||
| Bresler et al28 | France | 2002 | 24 | 42 | 46 | 79 | ||
| Rongen et al24 | Netherlands | 2003 | 200 | 72 | 138 | 72 | ||
Study involves both stimulated graciloplasty and gluteoplasty.
While many studies have proven the efficacy of the stimulated graciloplasty, concern arose about the high rates of complications and need for reoperation. In the original study by the Dynamic Graciloplasty Therapy Study Group,18 the complication and reoperative rates were 74% and 40%, respectively. Other studies also revealed high rates of infection, hardware failure, and postoperative evacuatory dysfunction. In a single institution study performed at Cleveland Clinic Florida, complications included lead fibrosis, seroma of the thigh incision, excoriation of the skin above the stimulator, fecal impaction, anal fissure, parastomal hernia, rotation of the stimulator, premature battery discharge, fracture of the lead, perineal skin irritation, perineal sepsis, rupture of the tendon, tendon erosion, muscle fatigue during programming sessions, electrode displacement from the nerve, and fibrosis around the nerve.20 Complications reported in other studies included hardware infections, muscle detachment, device malfunction and migration, pulmonary embolism, and severe unresolved pain that resulted in hospitalization and reoperation in numerous cases. Some of these complications led to stoma creation or death. Consequently, the Dynamic Graciloplasty Therapy Study Group investigated the etiology and impact of these complications.21 In this report, 211 complications occurred in 93 cases of dynamic graciloplasty. Forty-two percent had severe complications, though recovery was achieved in 92%. Fortunately, only major infections adversely affected outcome, leading the authors to conclude that although the complication rate was high, most of the complications could be treated and did not adversely affect outcome.
Modifications of certain aspects of the procedure have decreased some of the morbidity. Placing the leads along the intramuscular portion of the nerve rather than directly on the exposed portion of the nerve trunk has virtually eliminated problems with nerve fibrosis, lead displacement, and high impedance.22,23 Improved infection control measures have decreased the rate of infectious complications.24 Aside from the critical influence of the method of stimulation (intramuscular versus directly on the nerve trunk), no other parameters have been identified to be predictive of successful outcome.22 However, outcome strongly correlates with surgical experience.25
Several studies have found evacuatory dysfunction to be a common problem in up to 25% of patients after stimulated graciloplasty, particularly in patients with altered preoperative rectal sensitivity and compliance.21,22,24 It is suggested that severe urgency secondary to rectal hypersensitivity and low rectal compliance predisposes to poor outcome. Williams and associates26 reported a procedure involving rectal augmentation with a segment of distal ileum in combination with the dynamic graciloplasty. Urgency was abolished and continence restored in all three individuals in whom this procedure was employed. However, long-term follow-up of patients with stimulated graciloplasty revealed that the postoperative evacuatory dysfunction resolves spontaneously with time in most cases, making the augmentation procedure unnecessary.24,27,28
Despite the positive effects of cost, quality of life, and durability, Medtronics Inc. (Minneapolis, MN) discontinued seeking FDA approval in the United States of the neurostimulator in 1999. Currently, this operation remains a viable option throughout the rest of the world.
TOTAL ANORECTAL RECONSTRUCTION
In 1976, Simonsen and coworkers29 were the first authors to use the gracilis muscle to restore continence in a perineal colostomy after APR. In the mid 1980s, the procedure was performed worldwide and included transposition of both gracilis muscles to replace the sphincter and puborectalis mechanism followed by placement of an external neurostimulator. The results of recent studies are shown in Table 4. Cavina et al30 reported on the largest series of 98 patients with the longest follow-up of 55 months with an 87% success rate and 37% morbidity. Rouanet and colleagues31 reported good results with success in 5 of 7 patients who could be evaluated. In a study by Rullier and colleagues,32 in 12 of 15 patients with a 28-month follow-up, 7 were continent and 12 complications occurred. In this study, neosphincter stenosis was common and resulted in poor functional results, leading the authors to suggest using a single gracilis. Violi et al33 described a different approach in which the procedure was followed by biofeedback training and only delayed, selective use of implantable pulse generators to minimize complications and conserve resources. Seven of the 8 patients who had their colostomy reversed had full or partial continence. Though these studies suggest satisfactory continence and avoidance of a colostomy in up to 50% with this procedure, the rate of morbidity from complications is high. The most dramatic complication is significant tissue necrosis of the anus or neoanus that occurs more frequently when this procedure is performed after APR than for incontinence alone. Consequently, careful analysis of the risk-benefit ratio should be taken into consideration and discussed with the patient before embarking on this procedure.
Table 4.
Total Anorectal Reconstruction
| Author | Year | Country | Patients | Follow-up | Success | Morb |
|---|---|---|---|---|---|---|
| Cavina et al30 | 1998 | Italy | 98 | 55 | 85 (87%) | 37% |
| Violi et al33 | 1999 | Italy | 8 | 6 | 7 (88%) | |
| Rouanet et al31 | 1999 | France | 7 | 32 | 5 (71%) | 57% |
| Rullier et al32 | 2000 | France | 12 | 28 | 7 (58%) | 73% |
| Saunders34 | 2004* | UK | 14 | 53 | 8 (57%) | 71% |
Includes the continent colonic conduit or antegrade continent enema as an adjunct to the procedure.
Because of severe evacuatory dysfunction, patients undergoing anorectal reconstruction with electronically stimulated gracilis neoanal sphincter for atresia or after APR do not fare as well as patients with incontinence alone.34 Consequently, those individuals with severe evacuatory problems unresponsive to conservative treatment showed significant improvement and avoidance of a colostomy in 50% when a continent colonic conduit or antegrade colonic enema procedure was performed subsequent to the total anorectal reconstruction.34
MYOPLASTY FOR CONGENITAL ATRESIA
In 1952, Pickrell and coworkers5 used the gracilis in the management of children with fecal incontinence secondary to congenital malformation (spina bifida, meningocele). In 1988, Baeten et al7 implanted the first neurostimulator in a patient with anal atresia who had been treated with conventional graciloplasty 10 years earlier, resulting in complete continence. In 1995, Baeten et al35 published their expanded experience in a series of 9 patients with anal atresia, achieving continence in 55%. Recent results from a multicenter prospective trial revealed a 50% success rate for congenital fecal incontinence, though these results were not as good as the 71% success reported for acquired fecal incontinence and 66% success noted after APR for rectal cancer.25 In a recent study comparing the Acticon NeosphincterTM and stimulated graciloplasty for congenital atresia, the overall improvement of continence was 60% for Acticon NeosphincterTM, American Medical Systems, Minnetonka, MN, and 50% for stimulated graciloplasty, with a 50% morbidity rate for both procedures.36 The stimulated gracilis neosphincter is an efficient method of treating patients with congenital fecal incontinence.
Alternatively, augmented unilateral gluteoplasty with fascia lata graft was used to treat 11 young patients between 5 and 19 years of age with fecal incontinence due to congenital or neurologic disorders.37 The use of the facia lata tendon yielded a lower incidence of morbidity related to the tension on either the muscle flap or its neurovascular bundle. Most (72%) of the patients were clinically improved as demonstrated by incontinence scores and anorectal physiologic studies. This procedure may be a new option for patients with congenital atresia.
SUMMARY
Myoplasties have acquired an important role in anal sphincter repair for those patients with end-stage fecal incontinence who wish to avoid a colostomy. The stimulated graciloplasty is currently the most popular and widely performed myoplasty procedure and is an effective alternative in restoring continence. A recent systematic review concluded that stimulated graciloplasty was clearly superior and highly effective with 42 to 85% success in comparison to colostomy whereby colostomy is, by design, incapable of restoring continence.38 In addition, the stimulated graciloplasty is cost-effective. In a prospective longitudinal clinical trial involving patients with intractable fecal incontinence, cost was compared between stimulated graciloplasty, conventional treatment, and stoma.39 The cost of treatment for a lifelong dynamic graciloplasty ($31,733) was more expensive than lifelong conventional therapy ($12,180) but resulted in better quality of life. Stoma treatment was the most expensive (life-long stoma care $71,576) and by nature does not attempt to restore continence. In another study of 200 patients with the longest follow-up to date, the durability of good results over a 5-year period led the authors to conclude that the dynamic graciloplasty was a cost-effective treatment for fecal incontinence.24
With improved techniques and increasing experience, the adynamic versions of myoplasties are playing an increasing role in the initial approach to sphincter reconstruction. In some centers, elective use of the stimulators serves to conserve resources and minimize morbidity and need for reoperation resources while maintaining the same efficacy and outcome as their stimulated counterparts. In addition, the adynamic myoplasty remains an effective option in health-care settings where the stimulator is not available. Anecdotal experience at our institution with adynamic graciloplasty includes use for sphincter augmentation in cases of severe traumatic muscle loss prior to the placement of an artificial bowel sphincter (Acticon NeosphincterTM, American Medical Systems, Minnetonka, MN).
Stimulated myoplasty procedures are particularly complex procedures prone to complications during the steep learning curve. Consequently, any type of stimulated muscle transposition necessitates careful patient selection, properative planning, and greater experience with the specific technique to minimize patient morbidity and justify this approach economically. Currently, an accurate predictor of successful outcome does not exist. No preoperative investigation has provided a clear perspective on outcome. Patients must have the psychological strength, emotional commitment, and financial resources that may be necessary for multiple revisional surgeries or ultimate device failure. Consequently, these procedures are more likely to benefit the young healthy patient with intractable fecal incontinence than the elderly frail patient with minimally symptomatic fecal soilage. In addition, increased experience is a critical factor in avoiding complications and improving successful outcome and therefore these procedures should be performed at institutions with the necessary volume of patients to maintain expertise.
Because of the widespread prevalence of fecal incontinence and the recent development of new surgical techniques, other options exist for patients with fecal incontinence. The Acticon NeosphincterTM received FDA approval in 1999. A recent multicenter, nonrandomized trial revealed the device to have significant rate of clinical success (85%), enhancement in quality of life, and a high degree of safety.40 However, like the stimulated graciloplasty, limitations in use of this technique are related to the high rate of complications (most of which are related to infections of the foreign material with subsequent need for surgical revision) and an explant rate of 36%.40 According to this study, the success rate, cost involved, and the morbidity from this and the stimulated graciloplasty are approximately the same. In a recent prospective comparison of 8 cases of dynamic graciloplasty and 8 implantations of the antificial bowel sphincter (ABBS) followed over 3 years, there was no difference in complications, wound healing problems, or explantation rates. Though the ABS was found to be more effective in improving continence scores.41 However, larger long-term studies are needed.
Sacral nerve stimulation is a promising new therapy for fecal incontinence. However, large sphincter defects or patients with rectoanal atresia are not likely to benefit from this treatment and will continue to depend on the creation of sphincters. In addition, those patients who do not respond to this neuromodulation will be selected out for a neosphincter procedure. This group might be better candidates for success with a graciloplasty procedure.
The ideal muscle transposition has not yet been elucidated. Considerable controversy exists as to the best type of muscle (gracilis versus gluteus), the best configuration (α, gamma, or epsilon and whether unilateral is better than bilateral), whether postoperative biofeedback improves outcome, and whether the advantages of external stimulation outweigh the risk and cost. Important fundamental research must be performed to improve the outcome for myoplasties and their application for fecal incontinence and anorectal reconstruction. No individual center has gleaned enough experience with the various techniques; therefore, significant shortcomings exist because of this nonrandomized approach. Consensus and standardization are needed with respect to data collection, scoring systems, and quality of life assessment. There is a desperate need for properly controlled and adequately powered randomized studies to address issues. Longitudinal studies are needed to assess long-term results. Centers of expertise need to develop additional novel surgical techniques. Future efforts should be directed toward optimizing patient selection, surgeon training, and surgical techniques to minimize morbidity and maximize satisfactory outcomes. As the technology and the techniques evolve and cost-containment is realized, the role of myoplasty will become more universal and include a broader spectrum of indications.
REFERENCES
- 1.Bravo Guierrez A, Madoff R D, Lowry A C, Parker S C, Buie W D, Baxter N N. Long-term results of anterior sphincteroplasty. Dis Colon Rectum. 2004;47:727–731. discussion 731–732. doi: 10.1007/s10350-003-0114-6. [DOI] [PubMed] [Google Scholar]
- 2.Wexner S D, Marchetti F, Jagelman D. The role of sphincteroplasty for fecal incontinence reevaluated: a prospective physiologic and functional review. Dis Colon Rectum. 1991;34:22–30. doi: 10.1007/BF02050202. [DOI] [PubMed] [Google Scholar]
- 3.Chetwood C H. Plastic operation of the sphincter ani with report of a case. Med Rec. 1902;61:529. [PMC free article] [PubMed] [Google Scholar]
- 4.Wreden R R. A method of constructing a voluntary sphincter ani. Arch Surg. 1929;18:841. [Google Scholar]
- 5.Pickrell K I, Broadbent T R, Masters F W, et al. Construction of rectal sphincter and restoration of anal continence by transplanting the gracilis muscle. Report of 4 cases in children. Ann Surg. 1952;135:853–862. doi: 10.1097/00000658-195206000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salmons S, Henriksson J. The adaptive response of skeletal muscle to increased use. Muscle Nerve. 1981;4:94–105. doi: 10.1002/mus.880040204. [DOI] [PubMed] [Google Scholar]
- 7.Baeten C, Spaans F, Fluks A. An implanted neuromuscular stimulator for fecal continence. Dis Colon Rectum. 1988;31:134. doi: 10.1007/BF02562646. [DOI] [PubMed] [Google Scholar]
- 8.Pearl R K, Prasad M L, Nelson R L, Orsay C P, Abcarian H. Bilateral gluteus maximus transposition for anal incontinence. Dis Colon Rectum. 1991;34:478–481. doi: 10.1007/BF02049933. [DOI] [PubMed] [Google Scholar]
- 9.Christiansen J, Hansen C R, Rasmussen O. Bilateral gluteus maximus transposition for anal incontinence. Br J Surg. 1995;82:903–905. doi: 10.1002/bjs.1800820715. [DOI] [PubMed] [Google Scholar]
- 10.Devesa J M, Madrid J M, Gallego B R, Vicente E, Nuno J, Enriquez J M. Bilateral gluteoplasty for fecal incontinence. Dis Colon Rectum. 1997;40:883–888. doi: 10.1007/BF02051193. [DOI] [PubMed] [Google Scholar]
- 11.Devesa J M, Vicente E, Enriquez J M, et al. Total fecal incontinence—a new method of gluteus maximus transposition. Preliminary results and report of previous experience with similar procedures. Dis Colon Rectum. 1992;35:339–349. doi: 10.1007/BF02048111. [DOI] [PubMed] [Google Scholar]
- 12.Corman M L. Gracilis muscle transposition for anal incontinence: late results. Br J Surg. 1985;72:S21. doi: 10.1002/bjs.1800721314. [DOI] [PubMed] [Google Scholar]
- 13.Christiansen J, Sorensen M, Rasmussen O. Gracilis muscle transposition for fecal incontinence. Br J Surg. 1990;77:1039–1040. doi: 10.1002/bjs.1800770928. [DOI] [PubMed] [Google Scholar]
- 14.Sielezneff I, Bauer S, Bulgare J C, Sarles J C. Gracilis muscle transposition in the treatment of faecal incontinence. Int J Colorectal Dis. 1996;11:15–18. doi: 10.1007/BF00418849. [DOI] [PubMed] [Google Scholar]
- 15.Yoshioka K, Keighley M R. Clinical and manometric assessment of gracilis muscle transplant for fecal incontinence. Dis Colon Rectum. 1988;31:767–769. doi: 10.1007/BF02560102. [DOI] [PubMed] [Google Scholar]
- 16.Eccersley A J, Lunniss P J, Williams N S. Unstimulated graciloplasty in traumatic faecal incontinence. Br J Surg. 1999;86:1071–1072. doi: 10.1046/j.1365-2168.1999.01159.x. [DOI] [PubMed] [Google Scholar]
- 17.Kumar D, Hutchinson R, Grant E. Bilateral gracilis neosphincter construction for treatment of faecal incontinence. Br J Surg. 1995;82:1645–1647. doi: 10.1002/bjs.1800821219. [DOI] [PubMed] [Google Scholar]
- 18.Mander B J, Wexner S D, Williams N S, et al. Preliminary results of a multicenter trial of the electrically stimulated gracilis neoanal sphincter. Br J Surg. 1999;86:1543–1548. doi: 10.1046/j.1365-2168.1999.01285.x. [DOI] [PubMed] [Google Scholar]
- 19.Wexner S D, Baeten C, Bailey R, et al. Long-term efficacy of dynamic graciloplasty for fecal incontinence. Dis Colon Rectum. 2002;45:809–818. doi: 10.1007/s10350-004-6302-1. [DOI] [PubMed] [Google Scholar]
- 20.Wexner S D, Gonzalez-Padron A, Rius J, et al. Stimulated gracilis neosphincter operation. Initial experience, pitfalls, and complications. Dis Colon Rectum. 1996;39:957–964. doi: 10.1007/BF02054681. [DOI] [PubMed] [Google Scholar]
- 21.Matzel K E, Madoff R D, LaFontaine L J, et al. Dynamic Graciloplasty Therapy Study Group. Complications of dynamic graciloplasty: incidence, management, and impact on outcome. Dis Colon Rectum. 2001;44:1427–1435. doi: 10.1007/BF02234593. [DOI] [PubMed] [Google Scholar]
- 22.Mavrantonis C, Billotti V L, Wexner S D. Stimulated graciloplasty for treatment of intractable fecal incontinence: critical influence of the method of stimulation. Dis Colon Rectum. 1999;42:497–504. doi: 10.1007/BF02234176. [DOI] [PubMed] [Google Scholar]
- 23.Konsten J, Rongen M J, Ogunbiyi O A, Darakhshan A, Baeten C G, Williams N S. Comparison of epineural or intramuscular nerve electrodes for stimulated graciloplasty. Dis Colon Rectum. 2001;44:581–586. doi: 10.1007/BF02234333. [DOI] [PubMed] [Google Scholar]
- 24.Rongen M J, Uludag O, El Naggar K, Geerdes B P, Konsten J, Baeten C G. Long-term follow-up of dynamic graciloplasty for fecal incontinence. Dis Colon Rectum. 2003;46:716–721. doi: 10.1007/s10350-004-6645-7. [DOI] [PubMed] [Google Scholar]
- 25.Madoff R D, Rosen H R, Baeten C G, et al. Safety and efficacy of dynamic muscle plasty for anal incontinence: lessons from a prospective, multicenter trial. Gastroenterology. 1999;116:549–556. doi: 10.1016/s0016-5085(99)70176-9. [DOI] [PubMed] [Google Scholar]
- 26.Williams N S, Ogunbiyi O A, Scott S M, Fajobi O, Lunniss P J. Rectal augmentation and stimulated gracilis anal neosphincter: a new approach in the management of fecal urgency and incontinence. Dis Colon Rectum. 2001;44:192–198. doi: 10.1007/BF02234292. [DOI] [PubMed] [Google Scholar]
- 27.Baeten C G, Bailey H R, Bakka A, et al. Safety and efficacy of dynamic graciloplasty for fecal incontinence: report of a prospective, multicenter trial. Dynamic Graciloplasty Therapy Study group. Dis Colon Rectum. 2000;43:743–751. doi: 10.1007/BF02238008. [DOI] [PubMed] [Google Scholar]
- 28.Bresler L, Reibel N, Brunaud L, et al. Dynamic graciloplasty in the treatment of severe fecal incontinence. French multicentric retrospective study [in French] Ann Chir. 2002;127:520–526. doi: 10.1016/s0003-3944(02)00828-3. [DOI] [PubMed] [Google Scholar]
- 29.Simonsen O S, Stolf N A, Aun F, Raia A, Habr-Gama A. Rectal sphincter reconstruction in perineal colostomies after abdominoperineal resection for cancer. Br J Surg. 1976;63:389–391. doi: 10.1002/bjs.1800630514. [DOI] [PubMed] [Google Scholar]
- 30.Cavina E, Seccia M, Chiarugi M. Total anorectal reconstruction supported by electrostimulated gracilis neosphincter. Recent Results Cancer Res. 1998;146:104–113. doi: 10.1007/978-3-642-71967-7_10. [DOI] [PubMed] [Google Scholar]
- 31.Rouanet P, Senesse P, Bouamrirene D, et al. Anal sphincter reconstruction by dynamic graciloplasty after abdominoperineal resection for cancer. Dis Colon Rectum. 1999;42:451–456. doi: 10.1007/BF02234165. [DOI] [PubMed] [Google Scholar]
- 32.Rullier E, Zerbib F, Laurent C, Caudry M, Saric J. Morbidity and functional outcome after double dynamic graciloplasty for anorectal reconstruction. Br J Surg. 2000;87:909–913. doi: 10.1046/j.1365-2168.2000.01447.x. [DOI] [PubMed] [Google Scholar]
- 33.Violi V, Roncoroni L, Boselli A S, De Cesare C, Livrini M, Peracchia A. Total anorectal reconstruction by double graciloplasty: experience with delayed, selective use of implantable pulse generators. Int J Colorectal Dis. 1999;14:164–171. doi: 10.1007/s003840050204. [DOI] [PubMed] [Google Scholar]
- 34.Saunders J R, Williams N S, Eccersley A J. The combination of electrically stimulated gracilis neoanal sphincter and continent colonic conduit: a step forward for total anorectal reconstruction? Dis Colon Rectum. 2004;47:354–363. doi: 10.1007/s10350-003-0061-2. [DOI] [PubMed] [Google Scholar]
- 35.Baeten C G, Konsten J, Heineman E, Soeters P B. Dynamic graciloplasty for anal atresia. J Pediatr Surg. 1994;29:922–924. discussion 925. doi: 10.1016/0022-3468(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 36.da Silva G M, Jorge J M, Belin B, et al. New surgical options for fecal incontinence in patients with imperforate anus. Dis Colon Rectum. 2004;47:204–209. doi: 10.1007/s10350-003-0039-0. [DOI] [PubMed] [Google Scholar]
- 37.Farid M, Moneim H A, Mahdy T, Omar W. Augmented unilateral gluteoplasty with fascia lata graft in fecal incontinence. Tech Coloproctol. 2003;7:23–28. doi: 10.1007/s101510300004. [DOI] [PubMed] [Google Scholar]
- 38.Chapman A E, Geerdes B, Hewett P, et al. Systematic review of dynamic graciloplasty in the treatment of faecal incontinence. Br J Surg. 2002;89:138–153. doi: 10.1046/j.0007-1323.2001.02018.x. [DOI] [PubMed] [Google Scholar]
- 39.Adang E MM, Engel G L, Rutten F H, Geerdes B P, Baeten C G. Cost-effectiveness of dynamic graciloplasty in patients with fecal incontinence. Dis Colon Rectum. 1998;41:725–733. discusssion 733–734. doi: 10.1007/BF02236259. [DOI] [PubMed] [Google Scholar]
- 40.Wong W D, Congliosi S M, Spencer M P, et al. Safety and efficacy of the artificial bowel sphincter for fecal incontinence: results from a multicenter cohort study. Dis Colon Rectum. 2002;45:1139–1153. doi: 10.1007/s10350-004-6381-z. [DOI] [PubMed] [Google Scholar]
- 41.Ortiz H, Armendariz P, DeMiguel M, Solana A, Alos R, Roig J V. Prospective study of artificial anal sphincter and dynamic graciloplasty for severe anal incontinence. Int J Colorectal Dis. 2003;18:349–354. doi: 10.1007/s00384-002-0472-x. [DOI] [PubMed] [Google Scholar]