ABSTRACT
Anal incontinence is a symptom represented by the impaired ability to control the elimination of gas and stool, with an estimated incidence of 2.2 to 7.1% of the population. These numbers likely under-represent the true prevalence because physicians and patients are reluctant to discuss this problem. Evaluation of the patient with anal incontinence requires a fundamental knowledge of the etiologic factors. Careful history and physical examination is essential in every patient and can identify the cause of most cases of incontinence. Incontinence scoring systems are tools that provide objective data regarding the severity and quality of anal incontinence. Supplemental special tests for evaluating incontinence should be aimed at achieving three goals: (1) provide additional and confirmatory information regarding the diagnosis and cause of incontinence; (2) select appropriate treatment; and (3) predict treatment outcome. Numerous studies to evaluate anal incontinence exist; however, the most useful tests to achieve these goals are anal manometry, pudendal nerve terminal motor latency, and anal endosonography, because these studies can identify physiologic, neurologic, and anatomic abnormalities of the anorectum for which there may be effective treatments.
Keywords: Anal, fecal, incontinence, evaluation
Anal incontinence is the impaired ability to control the elimination of gas and stool. This condition is a complex and challenging clinical problem that has a major impact on patients' lifestyle, ranging from mild distress to progressive social isolation, and it may have devastating psychological effects. The exact prevalence in the population is unknown; lack of knowledge of this statistic is likely due to the social “taboo” and embarrassment related to this condition that is experienced by patients and physicians. This is best demonstrated by studies showing that only 34% of incontinent patients ever discussed their problem with a physician,1 and that only 5% of patients with incontinence had their problem noted in the medical chart.2 However, recent reports of population-based surveys estimate anal incontinence of gas, liquid, or solid stool between 2.2 and 7.1%.3,4 Although any age group and gender can be affected, there is an increased incidence of anal incontinence with female gender, advancing age, and deteriorating mental and physical status and overall health. This is clearly illustrated by the fact that anal incontinence is disproportionately higher in nursing home residents and the elderly5 and is a leading cause for nursing home placement in the United States.6
Normal continence is achieved through complex interaction of the anal sphincter mechanism (internal anal sphincter, external anal sphincter, and puborectalis muscle) and sensory and motor innervation pathways. Other contributing factors include stool consistency, colonic transit time, rectal reservoir capacity, and psychological motivation of the patient. The symptom of anal incontinence occurs when one or more of these mechanisms of continence are disrupted to an extent that other mechanisms are unable to compensate. Initial evaluation of these patients is critical to identify the etiology and severity of anal incontinence, as this will help guide further work-up and appropriate therapy.
ETIOLOGY OF ANAL INCONTINENCE
The etiology of anal incontinence can be classified into four basic categories: sphincteric, neurologic, stool characteristics, and rectal compliance and sensation (Table 1). The anal sphincter mechanism is composed of the internal and external anal sphincter and the puborectalis muscle, and this mechanism is one of the most important factors in maintaining anal continence. Disruption of the anal sphincters is commonly encountered during obstetrical injuries, making it the most common cause of surgically correctible incontinence in women.7 The rate of obstetrical injuries after vaginal deliveries ranges between 0 and 24%8; however, prospective studies have reported that up to 35% of primiparous women sustain trauma to the anal sphincters that goes unrecognized at delivery.9,10 In males, sphincter injury following anorectal surgery is the most common risk factor for anal incontinence.7 Procedures associated with sphincter division include fistulotomy, internal anal sphincterotomy for fissure, hemorrhoidectomy, and anal dilation.
Table 1.
Etiology of Anal Incontinence
| Category | Etiology | |
|---|---|---|
| LAR, low anterior resection. | ||
| Sphincteric | Traumatic | Obstetric |
| Anorectal surgery | ||
| Neoplastic | Rectal or anal cancer | |
| Neurologic | Primary (idiopathic) | Pudendal neuropathy |
| Secondary | Nerve injuries | |
| Sensory | Diabetic neuropathy | |
| Stool Characteristics | Diarrheal states | Inflammatory bowel disease |
| Infectious diarrhea | ||
| Laxative abuse | ||
| Malabsorption | ||
| Radiation enteritis | ||
| Constipation | Overflow incontinence | |
| Rectum | Poor capacity and compliance | Radiation proctitis |
| Scleroderma | ||
| Rectal neoplasms | ||
| LAR/coloanal anastamosis | ||
The pudendal nerve plays a critical role in maintenance of integrity and function of the anal sphincter. Neurologic compromise of the anal sphincter complex can result in incontinence and is classified as primary (idiopathic) or secondary. Idiopathic causes are usually a result of pudendal neuropathy resulting from repeated stretch forces exerted on the terminal portion of the pudendal nerve, due to rectal prolapse, descending perineum syndrome, multiple vaginal deliveries, or habitual straining at defecation.11 Secondary causes include diabetic neuropathy and multiple sclerosis that result in loss of normal autonomic rectoanal reflex arcs and sensory and motor innervation to the pelvic floor.
Stool consistency is another important etiologic factor in anal incontinence that must be considered. Incontinence to diarrhea may be a result of rapid transit of liquid stool to the rectum, which overloads the normal continence mechanisms that include capacity of the rectum, pelvic floor muscles, and anal sphincter complex. The presence of loose stool may be a result of inflammatory bowel disease, infectious enteritis or colitis, dietary habits, or laxative abuse.11 In elderly institutionalized patients, fecal impaction is found in up to 42% of patients,12 which can be a risk factor for incontinence. In these patients, chronic constipation is treated with high-dose laxatives resulting in overflow incontinence, where liquid fecal material leaks around the obstructing fecalith.13
The rectum functions as a reservoir for the storage of stool. Loss of rectal compliance can lead to anal incontinence. Inflammatory changes and scarring to the rectal wall resulting from processes such as radiation proctitis and inflammatory bowel disease result in decreased rectal compliance, reduction in reservoir capacity, and alterations in anorectal sensation, which collectively lead to incontinence.14 In systemic diseases such as scleroderma, up to 38% of patients develop anal incontinence.15 The decreased rectal capacity and compliance in these patients is associated with collagenous replacement of the muscularis propria and smooth muscle of the sigmoid colon and rectum.16
A basic knowledge of the risk factors and etiology of anal incontinence is important in the initial evaluation of these patients. A complete history and physical exam directed to eliciting the causes of this symptom are important for diagnosis, further work-up, and management.
INITIAL PATIENT EVALUATION
Proper evaluation of patients with anal incontinence will provide clues to the etiology and severity of the problem. The etiology of anal incontinence may be the single most important criterion for therapeutic planning. History and physical exam with endoscopic assessment of the anorectum is essential in every patient. Physiologic, neurologic, and anatomic studies should be used selectively to confirm the diagnosis, clarify the anatomy and function of the anorectal sphincter mechanism, and help guide choice of therapy.
History
Patients with anal incontinence are reluctant to admit their symptoms and discuss their problems. Therefore, a careful and directed history with pointed questions is required. First, it is important for the physician to define the patient's incontinence and the severity (flatus or liquid or solid stool). Signs and symptoms must be elicited directly. Patients should be asked to characterize their bowel movements, which includes frequency, duration, and pattern of incontinence. True anal incontinence should be differentiated from fecal soiling, which may be a result of fistula, prolapsed hemorrhoid, or rectal prolapse.17 Associated symptoms including urgency and fecal incontinence, with or without warning, should be determined. Urgency is a symptom that can result from decreased rectal compliance. It occurs in patients with inflammatory diseases of the rectum, such as radiation proctitis, or after coloanal anastamosis. Patients with sensory loss are unaware of the urge to defecate and likely have neuropathic incontinence. Alternatively, patients who are aware of the urge to defecate but are unable to achieve control usually have pelvic floor or anal sphincter injuries. Stool consistency should be determined. Incontinence associated with diarrhea may be a result of dietary habits, medications, irritable bowel syndrome, infectious diarrhea, malabsorptive states, or inflammatory bowel disease, and appropriate questions should be asked to identify a cause.11 In these patients the volume of liquid stool enters the rectal reservoir at a rapid rate, overwhelming the normal capacity of the rectum and anal sphincter complex. A history of constipation may raise the suspicion of overflow incontinence.
Incontinence scoring systems are useful tools to obtain an objective degree of incontinence and impact on quality of life. Although there is no universally accepted incontinence scoring system, we use the one proposed by Jorge and Wexner6 that obtains basic information of five separate categories regarding type of incontinence (solid, liquid, or gas), pad usage, and lifestyle changes. Each category is assigned a numerical value that corresponds to frequency (0, never; 1, rarely; 2, sometimes; 3, usually; and 4, always). Totals for each category are added and the score may range from 0, perfect continence, to 20, complete incontinence. It is important to note that these scoring systems are not used by all physicians, and their results do not necessarily change patient management; however, these tools bring objectivity to the evaluation for individual patient outcome analysis and comparison of treatment modalities in the literature.
A list of medications and dietary habits should be obtained. The use of laxatives, recent antibiotic therapy, or pancreatic replacement enzymes may suggest overflow incontinence, Clostridium difficile colitis, or malabsorption due to exocrine pancreatic insufficiency, respectively. Diarrhea associated with excessive dairy or wheat, rye, and barley ingestion would suggest lactose intolerance or celiac disease.
A detailed review of past medical history will help identify many etiologies of anal incontinence. In female patients, a complete obstetrical history including number of full-term pregnancies, vaginal deliveries, and complications (vaginal tear and episiotomy) should be determined, since obstetrical injury is the most frequent cause of anal incontinence in women. A careful review of past surgical procedures, specifically any anorectal surgery, should be obtained to determine whether traumatic injury to the anal sphincter may have occurred. History of traumatic injuries to the head, spine, and perineum should also be obtained.
Physical Examination
After the complete history is obtained, there should follow a physical examination with detailed focus on the perineum and digital rectal exam. Abdominal exam is performed to rule out any masses, and neurologic exam should include extremity reflexes and sensory and muscle strength. The patient is then placed in the left lateral decubitus, prone-jackknife, or lithotomy position with proper illumination to facilitate the exam. First, inspection of the perineum and external anus is performed, and any dermatitis, scarring, or deformity of the anus and surrounding skin is documented. Scarring is most frequently found in patients with history of fistulas, anorectal surgery, or obstetrical injury. The presence of a patulous anus signifies major sphincter compromise. Sensation is assessed by touching or stroking the perianal skin with a finger or cotton-tipped applicator stick. The presence of the anocutaneous reflex (anal “wink”) suggests an intact sacral reflex arc and pudendal nerve innervation of the external anal sphincter.18 During observation the patient is asked to strain to evaluate the presence of perineal descent, rectocele, cystocele, or rectal prolapse. A digital rectal exam is performed to evaluate the anal sphincter resting tone and squeeze pressure, which represent internal and external sphincter function, respectively. Proximal posterior palpation of the top of the anal sphincter complex during voluntary squeeze will assess puborectalis function. Defects of the anal sphincter may be palpated as ridges in the region of perianal scars. Rectal masses or impacted stool may be identified. In females, bimanual exam should be performed to assess the rectovaginal septum to identify the presence of a rectocele and assess the thickness of the perineal body.
Anorectal Endoscopy and Other Preliminary Studies
Anorectal endoscopy is a simple and effective means of examining the anorectum in an office setting. Patients are given a tap-water or saline enema to clear the rectal vault. Anoscopy is useful to exclude disease processes such as hemorrhoids, fissure, fistula, or abscess that may manifest as fecal soiling or passage of mucus from the anus. Rigid or flexible proctosigmoidoscopy should be performed to exclude malignancy, distal villous adenoma, inflammatory bowel disease, proctitis, or proximal fecal impaction as a cause of incontinence.
Before proceeding with special anorectal studies, all intestinal and systematic disorders must first be excluded as a causative factor. Urine and blood glucose levels should be obtained to rule out the presence of diabetes. Diarrhea should be evaluated with specific stool studies to determine whether it is osmotic or secretory in nature. Stool culture, microscopic examination for ova and parasites, and identification of Clostridium difficile may also be necessary on an individual basis.
SPECIAL PHYSIOLOGY STUDIES
The majority of patients with anal incontinence can be diagnosed with history and physical exam alone, and the use of additional testing may be limited because of physician bias and availability of certain tests.19 However, specialized anorectal physiology studies are useful to complete the clinical evaluation to further define anal incontinence and to obtain specific objective data on factors important in maintaining continence. It must be noted that no single test can be considered as the definitive test for anorectal function.20 Specific studies are obtained to complement information from the initial history and physical exam to help guide therapeutic decisions and predict outcome from medical and surgical treatment of anal incontinence.21,22,23,24 Therefore, anorectal physiology testing permits clinician-patient communication on specific treatment recommendation and realistic expectations of outcomes. Common studies used to evaluate anorectal physiology include anorectal manometry, pudendal nerve terminal motor latency (PNTML), anal endosonography, and endoanal magnetic resonance imaging (MRI).
Anorectal Manometry
Anorectal manometry is a basic test of anorectal function and is widely used as an initial study for patients with anal incontinence.25 This study provides a profile of anal canal pressures during rest and voluntary squeeze, evaluation of the rectal-anal inhibitory reflex (RAIR), and values for rectal sensation, compliance, and capacity. Normal values are shown in Table 2. Manometry can corroborate physical exam findings by providing an objective value to anal pressures at rest and during voluntary squeeze. These measurements can be used for comparison after treatment; however, they cannot determine the etiology of incontinence or differentiate between an anal sphincter defect and other causes of decreased pressure such as denervation of the pelvic floor and external sphincter muscles.26 It is well established that the internal anal sphincter is tonically contracted and generates 80% of the anal resting pressure, and the external anal sphincter is striated muscle under voluntary control.27,28 Therefore, in the presence of a known anal sphincter defect, a normal resting tone with decreased squeeze pressure may indicate an isolated external sphincter injury; decreased resting pressure and normal squeeze pressure would suggest an isolated internal sphincter injury. Anal endosonography is a more accurate and preferred method to identify anal sphincter defects.
Table 2.
Normal Values for Anal Manometry Testing
| Anal Manometry Test | Normal Values |
|---|---|
| mm Hg, millimeters of mercury; RAIR, rectoanal inhibitory reflex. | |
| Resting pressure | 40–70 mm Hg |
| Squeeze pressure | 100–180 mm Hg |
| RAIR | Present |
| Sensory threshold | 10–30 mL |
| Rectal capacity | 100–250 mL |
| Rectal compliance | 3–15 mL/mm Hg |
The RAIR is the relaxation of the proximal internal anal sphincter in response to rectal distention. It is measured by inflating a balloon within the rectal lumen and observing for a decrease in the resting anal pressure. A drop of at least 50% of the resting pressure after distention in at least one channel is considered a positive reflex. The RAIR is typically absent in anal incontinent patients with rectal prolapse and can be abnormal in patients with scleroderma, dermatomyositis, and other connective tissue disorders.29,30,31
Rectal sensation measurements provide data on capacity, which determines frequency of defecation and rectal compliance. This is performed with a balloon attachment on the manometry catheter that is distended with water at a steady rate. Rectal capacity is the volume defined by the maximal volume needed to elicit an urgent desire to defecate minus the volume needed to first appreciate rectal filling (sensory threshold). The pressure change obtained between sensory threshold volume and maximal volume is recorded and used to determine rectal compliance (rectal capacity/change in pressure), which is responsible for the degree of urgency for evacuation. Rectal capacity and rectal compliance are reduced in conditions associated with rectal fibrosis or inflammation, including scleroderma, radiation proctitis, and inflammatory bowel disease. Sensory threshold and compliance values are important and may help determine whether biofeedback training can improve fecal incontinence.32
Pudendal Nerve Terminal Motor Latency
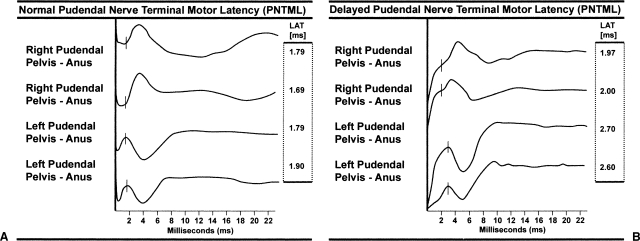
The pudendal nerve innervates the external anal sphincter and the puborectalis muscle. Damage to this nerve is an important factor in anal incontinence. This test measures the length of time required for a fixed electrical stimulus to travel along the pudendal nerve to the sphincter complex. The pudendal nerves are stimulated transanally through the rectal wall as they traverse the ischial spine using an electrode at the tip of the examining finger. The electromechanical response of the external anal sphincter is received by an electrode at the base of the examining finger and is registered by an oscilloscope. Normal value for pudendal nerve latency is 2.0 ± 0.2 milliseconds with a longer delay being indicative of damage to the pudendal nerve.33 Figure 1 shows characteristic tracings of a normal and delayed PNTML (Fig. 1A and 1B, respectively).
Figure 1.
Pudendal nerve terminal motor latency (PNTML). Tracing (A) shows a normal right and left PNTML with values < 2.0 milliseconds. Tracing (B) shows a delayed PNTML on the left with values of 2.6 and 2.7 milliseconds.
Interpretation of PNTML results is challenging in the context of patients with incontinence. A recent retrospective review compared results of PNTML with anal manometry in 2067 patients who received anorectal physiologic testing.34 Up to 31% of patients with bilateral neuropathy had mean squeeze pressures in the normal range, and 49% of patients with normal PNTML and an intact sphincter had squeeze pressures below the normal range. The authors indicate that these data suggest that either the test for pudendal neuropathy is not sensitive, or that neuropathy is relatively unimportant in fecal incontinence. Therefore, interpretation of PNTML should be performed in the context of data obtained from the history and physical exam as well as from anal manometry.
It has been suggested that PNTML has prognostic value when considering surgical treatment of fecal incontinence. This is another area of significant controversy, especially in surgical outcomes of overlapping sphincteroplasty.35 The relationship of sphincter injury, pudendal nerve function, sphincter muscle innervation, and outcome of surgery seems obvious; however, published series have reported conflicting results (Table 3)23,36,37,38,39,40,41 and it is unclear whether these discrepancies are due to patient selection bias or other factors. There is general agreement that overlapping sphincteroplasty is an appropriate first-line therapy for incontinent individuals with significant sphincter defects.35 Results of these outcome studies in the context of PNTML provide the physician and patient with realistic expectations of success after overlapping sphincteroplasty. Patients with normal pudendal nerve latency can expect a 70 to 80% success rate, while those with evidence of pudendal neuropathy can expect a 40 to 50% success rate.
Table 3.
Effects of Pudendal Neuropathy on Successful Outcome after Sphincteroplasty
| Author, Year | n | Normal PNTML | Abnormal PNTML | p value |
|---|---|---|---|---|
| n, number; PNTML, pudendal nerve terminal motor latency; NS, not significant. | ||||
| Wexner et al, 199136 | 16 | 92% | 50 | NS |
| Londono-Schimmer et al, 199437 | 94 | 55 | 30 | < 0.001 |
| Simmang et al, 199438 | 14 | 100 | 67 | NS |
| Nikiteas et al, 199639 | 26 | 67 | 53 | NS |
| Sangwan et al, 199623 | 15 | 100 | 14 | < 0.005 |
| Gilliland et al, 199840 | 100 | 63 | 10 | < 0.01 |
| Buie et al, 200141 | 89 | 61 | 71 | NS |
Anal Endosonography
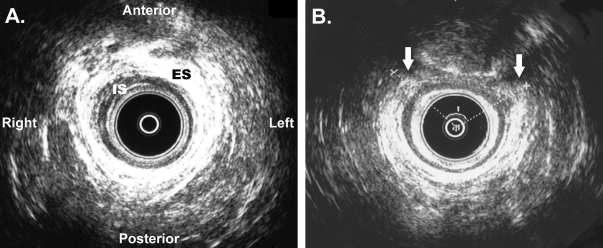
Anal endosonography (AES) has assumed an increasingly important role in evaluating patients with anal incontinence. High-resolution images and 360-degree evaluation of the anal canal is obtained using a rotating endoprobe with a 10-mm MHz transducer. This procedure can be performed in the office setting since it requires little preparation and is well tolerated by patients. Anatomic and morphometric assessment of the internal and external sphincters, puborectalis muscle, and rectovaginal septum are obtained. Normal internal (hypoechoic) and external (hyperechoic) sphincter muscles are best seen as two intact rings in the mid anal canal (Fig. 2A). Images from the top of the anal canal will show the horseshoe-shaped puborectalis muscle which can be mistaken for an anterior sphincter defect. Measurement of the distance between the probe and examining finger inserted into the vagina determines thickness of the perineal body (normal value 1.0 to 1.5 cm). Interruption of the hyperechoic external sphincter ring signifies a defect in the muscle (Fig. 2B).
Figure 2.
Anal endosonography images showing (A) an intact anal sphincter complex, and (B) an anterior external sphincter defect. ES represents the hyperechoic (white) external anal asphincter. IS represents the hypoechoic (dark) internal anal sphincter. White arrows represent the edges of the anterior external sphincter defect.
AES is the diagnostic imaging technique of choice for providing information on the integrity of the internal and external anal sphincters and detecting sphincteric defects with a reported accuracy of 90 to 100%.42,43 In diagnosing sphincter defects in the setting of anal incontinence, AES has been shown to be superior to clinical exam, anal manometry, and electromyography.44 In fact, AES has replaced the use of electromyography sphincter mapping because of the discomfort associated with the latter.45 In comparing ultrasound and the manometric findings in incontinent patients, sphincter disruptions, not sphincter thickness, correlate with manometric findings. When a sphincter defect is present in an incontinent patient, AES provides anatomic detail to help plan surgical approach for sphincter repair. It is an effective technique for assessing results after sphincter repair.46 When a sphincter defect is not present, other tests are necessary to complement AES results to guide therapeutic management.
Endoluminal MRI
Endoluminal MRI provides visualization of the normal anatomy and pathologic conditions of the anal canal. Lesions of the external sphincter, such as sphincter defects and scar tissue, are accurately identified in 90 to 95% of patients.47,48,49,50,51,52,53 Compared with AES, endoluminal MRI is superior because of its multiplanar capability, higher inherent contrast resolution, and the ability to identify atrophy of the external anal sphincter. Atrophy of the external anal sphincter has been proposed as a predictor of negative outcomes of anterior anal repair.50,53 However, AES still provides much better definition of the internal anal sphincter. Major limitations of endoluminal MRI include its increased cost, longer length of time to schedule and perform the test, and poor availability outside specialized centers. Therefore, AES should remain the initial imaging modality for fecal incontinence.
CONCLUSIONS
Anal incontinence is a severe disability with an underestimated incidence due to patient and physician reluctance to discuss this problem. Evaluation of patients with anal incontinence is aimed at determining the most appropriate treatment modality. Treatment of this condition can be divided into surgical and nonsurgical approaches and are detailed elsewhere in this journal. The choice of treatment depends on multiple factors that include the etiology of incontinence, anatomy of the anal sphincters, age and physical condition of the patient, and the impact of incontinence on quality of life. There are many etiologic factors of anal incontinence, which can be divided into four major categories: sphincteric, neurologic, stool characteristics, and rectal capacity and compliance. Careful history and physical exam as well as the use of a continence scoring system will provide information regarding the etiology and severity of incontinence and its impact on patient quality of life. Anoscopy and rigid or flexible sigmoidoscopy are essential to evaluate the anus and rectum for conditions that cause fecal soilage, which is frequently misinterpreted as incontinence. Associated systemic diseases, diarrhea, and overflow incontinence are treated. If anal incontinence persists, selection of special tests should be aimed to achieve three goals: (1) provide additional and confirmatory information regarding the diagnosis and cause of incontinence; (2) select appropriate treatment; and (3) predict treatment outcome. The most useful tests are anorectal manometry, PNTML, and AES, because they can determine anatomic structure and physiologic data that will guide management and provide objective information for follow-up assessment of outcomes in the treatment of anal incontinence. The choice of tests is dependent on the individual physician's judgment and test availability, and results should always be correlated to history and physical exam.
ACKNOWLEDGMENTS
The author would like to thank Sharon G. Gregorcyk, M.D., for providing the anal endosonographic pictures.
REFERENCES
- 1.Johanson J F, Lafferty J. Epidemiology of fecal incontinence: the silent affliction. Am J Gastroenterol. 1996;91:33–36. [PubMed] [Google Scholar]
- 2.Enck P, Bielefeldt K, Rathmann W, Purrmann J, Tschope D, Erckenbrecht J F. Epidemiology of faecal incontinence in selected patient groups. Int J Colorectal Dis. 1991;6:143–146. doi: 10.1007/BF00341234. [DOI] [PubMed] [Google Scholar]
- 3.Nelson R, Norton N, Cautley E, Furner S. Community-based prevalence of anal incontinence. JAMA. 1995;274:559–561. [PubMed] [Google Scholar]
- 4.Drossman D A, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 5.Nelson R, Furner S, Jesudason V. Fecal incontinence in Wisconsin nursing homes: prevalence and associations. Dis Colon Rectum. 1998;41:1226–1229. doi: 10.1007/BF02258218. [DOI] [PubMed] [Google Scholar]
- 6.Jorge J M, Wexner S D. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77–97. doi: 10.1007/BF02050307. [DOI] [PubMed] [Google Scholar]
- 7.Lunniss P J, Gladman M A, Hetzer F H, Williams N S, Scott S M. Risk factors in acquired faecal incontinence. J R Soc Med. 2004;97:111–116. doi: 10.1258/jrsm.97.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thacker S B, Banta H D. Benefits and risks of episiotomy: an interpretative review of the English language literature, 1860–1980. Obstet Gynecol Surv. 1983;38:322–338. [PubMed] [Google Scholar]
- 9.Donnelly V, Fynes M, Campbell D, Johnson H, O'Connell P R, O'Herlihy C. Obstetric events leading to anal sphincter damage. Obstet Gynecol. 1998;92:955–961. doi: 10.1016/s0029-7844(98)00255-5. [DOI] [PubMed] [Google Scholar]
- 10.Sultan A H, Kamm M A, Hudson C N, Thomas J M, Bartram C I. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329:1905–1911. doi: 10.1056/NEJM199312233292601. [DOI] [PubMed] [Google Scholar]
- 11.Mavrantonis C, Wexner S D. A clinical approach to fecal incontinence. J Clin Gastroenterol. 1998;27:108–121. doi: 10.1097/00004836-199809000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Tobin G W, Brocklehurst J C. Faecal incontinence in residential homes for the elderly: prevalence, aetiology and management. Age Ageing. 1986;15:41–46. doi: 10.1093/ageing/15.1.41. [DOI] [PubMed] [Google Scholar]
- 13.Gurll N, Steer M. Diagnostic and therapeutic considerations for fecal impaction. Dis Colon Rectum. 1975;18:507–511. doi: 10.1007/BF02587220. [DOI] [PubMed] [Google Scholar]
- 14.Iwamoto T, Nakahara S, Mibu R, Hotokezaka M, Nakano H, Tanaka M. Effect of radiotherapy on anorectal function in patients with cervical cancer. Dis Colon Rectum. 1997;40:693–697. doi: 10.1007/BF02140899. [DOI] [PubMed] [Google Scholar]
- 15.Trezza M, Krogh K, Egekvist H, Bjerring P, Laurberg S. Bowel problems in patients with systemic sclerosis. Scand J Gastroenterol. 1999;34:409–413. doi: 10.1080/003655299750026434. [DOI] [PubMed] [Google Scholar]
- 16.Leighton J A, Valdovinos M A, Pemberton J H, Rath D M, Camilleri M. Anorectal dysfunction and rectal prolapse in progressive systemic sclerosis. Dis Colon Rectum. 1993;36:182–185. doi: 10.1007/BF02051176. [DOI] [PubMed] [Google Scholar]
- 17.Felt-Bersma R J, Janssen J J, Klinkenberg-Knol E C, Hoitsma H F, Meuwissen S G. Soiling: anorectal function and results of treatment. Int J Colorectal Dis. 1989;4:37–40. doi: 10.1007/BF01648548. [DOI] [PubMed] [Google Scholar]
- 18.Rao S S, Sun W M. Current techniques of assessing defecation dynamics. Dig Dis. 1997;15:64–77. doi: 10.1159/000171622. [DOI] [PubMed] [Google Scholar]
- 19.Keating J P, Stewart P J, Eyers A A, Warner D, Bokey E L. Are special investigations of value in the management of patients with fecal incontinence? Dis Colon Rectum. 1997;40:896–901. doi: 10.1007/BF02051195. [DOI] [PubMed] [Google Scholar]
- 20.Coller J A. Clinical application of anorectal manometry. Gastroenterol Clin North Am. 1987;16:17–33. [PubMed] [Google Scholar]
- 21.Chen H, Humphreys M S, Kettlewell M G, Bulkley G B, George B D. Anal ultrasound predicts the response to nonoperative treatment of fecal incontinence in men. Ann Surg. 1999;229:739–743. doi: 10.1097/00000658-199905000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ternent C A, Shashidharan M, Blatchford G J, Christensen M A, Thorson A G, Sentovich S M. Transanal ultrasound and anorectal physiology findings affecting continence after sphincteroplasty. Dis Colon Rectum. 1997;40:462–467. doi: 10.1007/BF02258393. [DOI] [PubMed] [Google Scholar]
- 23.Sangwan Y P, Coller J A, Barrett R C, et al. Unilateral pudendal neuropathy. Impact on outcome of anal sphincter repair. Dis Colon Rectum. 1996;39:686–689. doi: 10.1007/BF02056951. [DOI] [PubMed] [Google Scholar]
- 24.Chen A S, Luchtefeld M A, Senagore A J, Mackeigan J M, Hoyt C. Pudendal nerve latency. Does it predict outcome of anal sphincter repair? Dis Colon Rectum. 1998;41:1005–1009. doi: 10.1007/BF02237391. [DOI] [PubMed] [Google Scholar]
- 25.Sun W M, Rao S S. Manometric assessment of anorectal function. Gastroenterol Clin North Am. 2001;30:15–32. doi: 10.1016/s0889-8553(05)70165-5. [DOI] [PubMed] [Google Scholar]
- 26.Parks T G. The usefulness of tests in anorectal disease. World J Surg. 1992;16:804–810. doi: 10.1007/BF02066974. [DOI] [PubMed] [Google Scholar]
- 27.Schweiger M. Method for determining individual contributions of voluntary and involuntary anal sphincters to resting tone. Dis Colon Rectum. 1979;22:415–416. doi: 10.1007/BF02586913. [DOI] [PubMed] [Google Scholar]
- 28.Duthie H L, Watts J M. Contribution of the external anal sphincter to the pressure zone in the anal canal. Gut. 1965;28:64–68. doi: 10.1136/gut.6.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lubowski D Z, Nicholls R J, Swash M, Jordan M J. Neural control of internal anal sphincter function. Br J Surg. 1987;74:668–670. doi: 10.1002/bjs.1800740804. [DOI] [PubMed] [Google Scholar]
- 30.Jorge J M, Wexner S D. Anorectal manometry: techniques and clinical applications. South Med J. 1993;86:924–931. doi: 10.1097/00007611-199308000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Karulf R E, Madoff R D, Goldberg S M. Rectal prolapse. Curr Probl Surg. 2001;38:771–832. [PubMed] [Google Scholar]
- 32.Fernandez-Fraga X, Azpiroz F, Aparici A, Casaus M, Malagelada J R. Predictors of response to biofeedback treatment in anal incontinence. Dis Colon Rectum. 2003;46:1218–1225. doi: 10.1007/s10350-004-6718-7. [DOI] [PubMed] [Google Scholar]
- 33.Wexner S D, Marchetti F, Salanga V D, Corredor C, Jagelman D G. Neurophysiologic assessment of the anal sphincters. Dis Colon Rectum. 1991;34:606–612. doi: 10.1007/BF02049902. [DOI] [PubMed] [Google Scholar]
- 34.Hill J, Hosker G, Kiff E S. Pudendal nerve terminal motor latency measurements: what they do and do not tell us. Br J Surg. 2002;89:1268–1269. doi: 10.1046/j.1365-2168.2002.02209.x. [DOI] [PubMed] [Google Scholar]
- 35.Madoff R D. Surgical treatment options for fecal incontinence. Gastroenterology. 2004;126:S48–S54. doi: 10.1053/j.gastro.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 36.Wexner S D, Marchetti F, Jagelman D G. The role of sphincteroplasty for fecal incontinence reevaluated: a prospective physiologic and functional review. Dis Colon Rectum. 1991;34:22–30. doi: 10.1007/BF02050202. [DOI] [PubMed] [Google Scholar]
- 37.Londono-Schimmer E E, Garcia-Duperly R, Nicholls R J, Ritchie J K, Hawley P R, Thomson J P. Overlapping anal sphincter repair for faecal incontinence due to sphincter trauma: five year follow-up functional results. Int J Colorectal Dis. 1994;9:110–113. doi: 10.1007/BF00699424. [DOI] [PubMed] [Google Scholar]
- 38.Simmang C, Birnbaum E H, Kodner I J, Fry R D, Fleshman J W. Anal sphincter reconstruction in the elderly: does advancing age affect outcome? Dis Colon Rectum. 1994;37:1065–1069. doi: 10.1007/BF02049804. [DOI] [PubMed] [Google Scholar]
- 39.Nikiteas N, Korsgen S, Kumar D, Keighley M R. Audit of sphincter repair. Factors associated with poor outcome. Dis Colon Rectum. 1996;39:1164–1170. doi: 10.1007/BF02081420. [DOI] [PubMed] [Google Scholar]
- 40.Gilliland R, Altomare D F, Moreira H, Jr, Oliveira L, Gilliland J E, Wexner S D. Pudendal neuropathy is predictive of failure following anterior overlapping sphincteroplasty. Dis Colon Rectum. 1998;41:1516–1522. doi: 10.1007/BF02237299. [DOI] [PubMed] [Google Scholar]
- 41.Buie W D, Lowry A C, Rothenberger D A, Madoff R D. Clinical rather than laboratory assessment predicts continence after anterior sphincteroplasty. Dis Colon Rectum. 2001;44:1255–1260. doi: 10.1007/BF02234781. [DOI] [PubMed] [Google Scholar]
- 42.Enck P, von Giesen H J, Schafer A, et al. Comparison of anal sonography with conventional needle electromyography in the evaluation of anal sphincter defects. Am J Gastroenterol. 1996;91:2539–2543. [PubMed] [Google Scholar]
- 43.Sentovich S M, Wong W D, Blatchford G J. Accuracy and reliability of transanal ultrasound for anterior anal sphincter injury. Dis Colon Rectum. 1998;41:1000–1004. doi: 10.1007/BF02237390. [DOI] [PubMed] [Google Scholar]
- 44.Sultan A H, Kamm M A, Talbot I C, Nicholls R J, Bartram C I. Anal endosonography for identifying external sphincter defects confirmed histologically. Br J Surg. 1994;81:463–465. doi: 10.1002/bjs.1800810349. [DOI] [PubMed] [Google Scholar]
- 45.Saclarides T J. Endorectal ultrasound. Surg Clin North Am. 1998;78:237–249. doi: 10.1016/s0039-6109(05)70311-x. [DOI] [PubMed] [Google Scholar]
- 46.Felt-Bersma R J, Cuesta M A, Koorevaar M. Anal sphincter repair improves anorectal function and endosonographic image. A prospective clinical study. Dis Colon Rectum. 1996;39:878–885. doi: 10.1007/BF02053986. [DOI] [PubMed] [Google Scholar]
- 47.deSouza N M, Hall A S, Puni R, Gilderdale D J, Young I R, Kmiot W A. High resolution magnetic resonance imaging of the anal sphincter using a dedicated endoanal coil. Comparison of magnetic resonance imaging with surgical findings. Dis Colon Rectum. 1996;39:926–934. doi: 10.1007/BF02053993. [DOI] [PubMed] [Google Scholar]
- 48.Rociu E, Stoker J, Zwamborn A W, Lameris J S. Endoanal MR imaging of the anal sphincter in fecal incontinence. Radiographics. 1999;19:S171–S177. doi: 10.1148/radiographics.19.suppl_1.g99oc02s171. [DOI] [PubMed] [Google Scholar]
- 49.Rociu E, Stoker J, Eijkemans M J, Schouten W R, Lameris J S. Fecal incontinence: endoanal US versus endoanal MR imaging. Radiology. 1999;212:453–458. doi: 10.1148/radiology.212.2.r99au10453. [DOI] [PubMed] [Google Scholar]
- 50.Briel J W, Stoker J, Rociu E, Lameris J S, Hop W C, Schouten W R. External anal sphincter atrophy on endoanal magnetic resonance imaging adversely affects continence after sphincteroplasty. Br J Surg. 1999;86:1322–1327. doi: 10.1046/j.1365-2168.1999.01244.x. [DOI] [PubMed] [Google Scholar]
- 51.Briel J W, Zimmerman D D, Stoker J, et al. Relationship between sphincter morphology on endoanal MRI and histopathological aspects of the external anal sphincter. Int J Colorectal Dis. 2000;15:87–90. doi: 10.1007/s003840050238. [DOI] [PubMed] [Google Scholar]
- 52.Malouf A J, Williams A B, Halligan S, Bartram C I, Dhillon S, Kamm M A. Prospective assessment of accuracy of endoanal MR imaging and endosonography in patients with fecal incontinence. AJR Am J Roentgenol. 2000;175:741–745. doi: 10.2214/ajr.175.3.1750741. [DOI] [PubMed] [Google Scholar]
- 53.Williams A B, Malouf A J, Bartram C I, Halligan S, Kamm M A, Kmiot W A. Assessment of external anal sphincter morphology in idiopathic fecal incontinence with endocoil magnetic resonance imaging. Dig Dis Sci. 2001;46:1466–1471. doi: 10.1023/a:1010639920979. [DOI] [PubMed] [Google Scholar]