ABSTRACT
The use of sacral nerve stimulation as a treatment for fecal incontinence for intact but functionally deficient sphincter and pelvic floor musculature, as well as for some sphincter injuries, is an attractive concept that is currently undergoing clinical trials in the United States. Electrical stimulation of the peripheral nerve supply to the striated anal sphincter muscles at the level of the sacral spinal nerves exploits the accessibility of the most distal common location of the dual peripheral nerve supply to these muscles. While the mechanism of sacral nerve stimulation's salutary effect remains conjectural at present and is likely multifactorial, current experimental data point toward both an enhancement in striated muscular activity as well as neuromodulation of sacral reflexes that regulate rectal sensitivity and contractility.
Keywords: Fecal incontinence, sacral nerve stimulation, anal sphincter muscles
Fecal incontinence resulting from injury to the anal sphincter complex can be treated by surgical repair with satisfactory results in most cases. Yet surgical approaches to functional deficits without demonstrable anatomic defects remain unsatisfactory. The use of sacral nerve stimulation (SNS) as a treatment for fecal incontinence for intact but functionally deficient sphincter and pelvic floor musculature, as well as for some sphincter injuries, is therefore an attractive concept. Electrical stimulation of the peripheral nerve supply to the striated anal sphincter muscles at the level of the sacral spinal nerves exploits the accessibility of the most distal common location of the dual peripheral nerve supply to these muscles.1 Currently this is an area of ongoing investigation in the United States.
Initially used to treat urinary incontinence,2,3,4 SNS was first reported for the treatment of fecal incontinence by Matzel and colleagues.5 They described an approach of using intraoperative peripheral nerve evaluation (PNE) to identify the most efficient spinal nerve for stimulation, followed by a temporary (subchronic) stimulation period of 1 to several weeks. If fecal incontinence improves during this evaluation period, patients are then offered permanent (chronic) electrode implantation. This staged approach is widely accepted as the standard.
Subsequent investigators have attempted to evaluate the salutary effect of SNS on fecal incontinence by measuring anal physiologic parameters preoperatively, during PNE, and at intervals following permanent implantation. Validated continence scores and patient quality of life questionnaires are also generally utilized. Together, these have demonstrated both medium6 and long-term7 improvements in patients with SNS. Hence, for patients with fecal incontinence and an intact sphincter complex for which treatment options are otherwise limited, SNS appears to significantly improve measurable parameters of continence and quality of life.
EVALUATION OF PATIENTS
Suitable patients for SNS are typically referred to colon and rectal surgeons for evaluation of chronic fecal incontinence. A detailed history and review of a patient incontinence diary will often lead the clinician to a likely etiology for incontinence and will place patients into one of two groups: those with urge incontinence, where there is inability to defer defecation, and those with passive incontinence, where there is no immediate awareness of the loss of stool. Although not considered an exclusion criterion, those with the former have been shown to have better results with SNS than those with the latter.8
After medical etiologies such as infectious diarrhea and inflammatory bowel disease have been ruled out as contributing to fecal incontinence, patients are further evaluated with comprehensive anal endosonography and physiologic testing. Patients most appropriate for SNS are those in whom the external anal sphincter (EAS) is at least partially intact. Although most investigators have included only patients without demonstrable EAS defects,7,8,9,10,11,12 some have included patients treated with prior overlapping sphincteroplasty6 or those in whom demonstrable sphincter defects were not considered to be the main cause of fecal incontinence (i.e., limited defect less than or equal to 30 degrees,4 or EAS intact for at least 50% of its length13).
Anorectal physiology testing has focused on resting pressures, maximum squeeze pressures, and threshold, urge, and maximum tolerated rectal volumes to balloon distention.10 Although follow-up of these parameters beyond initial patient evaluation may not have the same clinical relevance of simpler continence and quality of life scores, physiologic testing preoperatively, during PNE, and after permanent implantation has provided investigators with valuable insights as to possible mechanisms by which SNS may exert its therapeutic effect.6,7,9,10,11,12,14
A normal pudendal nerve terminal motor latency at least unilaterally is considered by most investigators to be a positive predictor of response to SNS.6,7,8,15 Ganio and colleagues noted that in two patients lacking pudendal nerve conduction bilaterally, no response to stimulation during PNE was obtained.8 Hence, it seems appropriate that the presence of at least a unilateral normal pudendal nerve terminal motor latency be confirmed before a patient is considered for SNS.
Patients with a variety of prior surgical histories, systemic diseases, and injuries to the central nervous system have benefited from SNS. These include, but are not limited to, obstetric injuries; prior anorectal procedures for fistula, fissure, hemorrhoids, rectal prolapse, and EAS defects; scleroderma16; multiple sclerosis11; meningomyelocele11; and miscellaneous spinal cord injuries. Yet in some experimental protocols, patients with rectal prolapse, cauda equina lesions, sacral agenesis, inflammatory bowel disease, or prior pelvic floor irradiation were excluded.12 Whether some of these patients would benefit from SNS may be a topic for future investigation.
TECHNIQUE
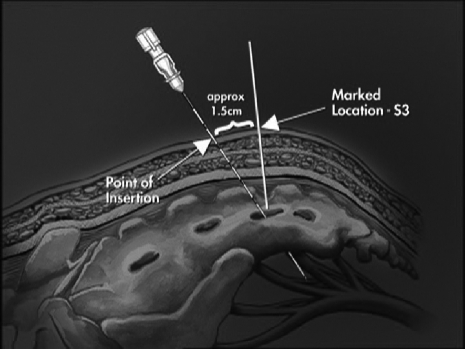
Initial acute percutaneous nerve evaluation assesses the functional relevance of each sacral spinal nerve to striated anal sphincter function. This can be noted manometrically by elevations in resting anal canal pressure, or more commonly, by characteristic movement of the perineum. Specifically, S2 stimulation results in some movement of the perineum and an inward rotation of the heel or lateral rotation of the entire leg along with contraction of the toe and foot; S3 stimulation in a clamp-like movement of the levator ani and plantar flexion of the great toe; S4 stimulation in contraction of the levator ani with a visible bellows-like perineal movement and no leg or foot activity.5 If demonstrable, S3 is the preferred foramen for stimulation. S4 is acceptable in cases where S3 cannot be located.
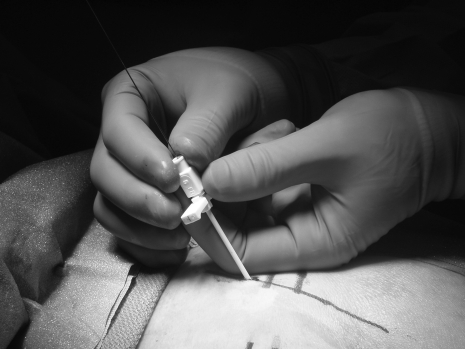
With the patient in the prone position, sheathed needle electrodes are inserted under general anesthesia without the use of muscle relaxant into the sacral foramina of S2, S3, and S4 bilaterally (Figs. 1, 2). With current applied in a graduated fashion, the evoked motor response of the pelvic floor muscles is monitored.
Figure 1.
Proper placement of sheathed electrode in the sacrum. Reprinted with permission of Medtronic, Inc., © 2004.
Figure 2.
Proper placement of sheathed electrode. Reprinted with permission of Medtronic, Inc., © 2004.
The needle electrode is next replaced by a wire electrode tunneled percutaneously to the site of that sacral nerve determined to be most effective, and then connected to an external neurostimulator. This nerve is then stimulated continuously for 1 week using monopolar stimulation with a pulse width of 210 seconds, a frequency of 15 Hz, and amplitude adaptable by the patient within a range of 1 to 10 V according to the patient's perception of muscle contraction.7
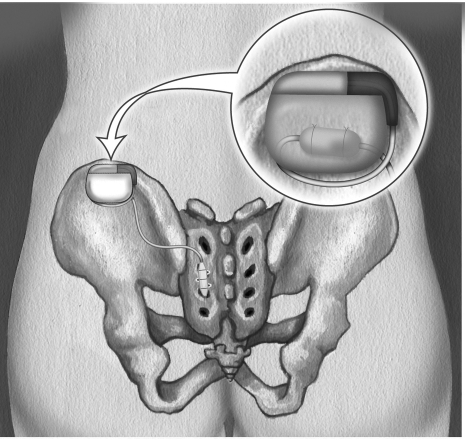
If a suitable improvement in fecal continence is noted with subchronic SNS (< 50% compared with baseline), conversion to chronic SNS with permanent implantation is performed. In the operating room, the temporary external extension electrode is removed, and the previously placed electrode is connected to the permanent stimulator. This in turn is implanted in a subcutaneous pocket in the flank lateral to the sacral electrode insertion site (Fig. 3).
Figure 3.
Location of permanent nerve stimulator. Reprinted with permission of Medtronic, Inc., © 2004.
RESULTS
Data on the results of SNS for the treatment of fecal incontinence are relatively few. Most published reports have included small numbers of patients and only short-term follow-up. Matzel and colleagues5 published the first report of three patients followed for 6 months. All three had favorable results. Malouf and associates10 later reported on five patients with a median follow-up of 16 months, all of whom benefited from SNS. They later reported a double-blinded crossover trial with and without stimulation in two patients, which suggested that the benefit these patients received was not due to placebo.17
Matzel and colleagues next reported on six patients who benefited with a noted increase in anal squeeze pressure.7 Leroi and colleagues reported on five patients, three of whom showed clinical benefit after a follow-up of 6 months.12 After a median follow-up of 19 months, Ganio and coworkers noted an increase in anal canal resting pressure and squeeze pressures, as well as improved rectal sensation to distention.8 A larger report by Rosen and colleagues11 included 16 patients, 4 of whom had the device removed due to infection or displacement. After a median of 15 months of follow-up, patients in this study had marked improvement in continence associated with increased resting and squeeze pressures.
Medium-range data were reported by Kenefick and associates,6 whose 15 patients all experienced improved incontinence with statistically significant improvement in resting and squeeze pressures, as well as improved rectal sensation to distention over a median follow-up of 24 months. Most recently, Matzel and colleagues13 published a multicenter study including 34 patients who underwent chronic SNS if at least a 50% reduction in number of incontinent episodes per week had been achieved during subchronic stimulation. They report that frequency of incontinent episodes fell from a mean of 16.4 per week at baseline to 3.1 at 12 months and to 2.0 at 24 months. For patients in whom 36-month data were available, this improvement was sustained.
MECHANISM
The mechanism of SNS's salutary effect on fecal incontinence is uncertain. Studies have demonstrated improvements in anal resting and squeeze pressures and changes in rectal sensitivity and motility.5,6,7,10,12 As continence results not only from satisfactory sphincter function, but also from the integrity and coordinated function of several anatomic structures, SNS may affect any of these. Indeed, its salutary effect is likely multifactorial.
The finding of an increased squeeze pressure suggests that SNS augments striated anal sphincter muscle activity. The mechanism for this effect may involve either hypertrophy of existing muscle fibers or alteration in muscle fiber type, due to a permanent training effect resulting in low-frequency stimulation-induced transformation of fast twitch fatigable muscle fibers (type II) to slow twitch fatigue-resistant fibers (type I).5,17,18 Available evidence on the effect of resting anal canal pressure, however, is less clear. Moreover, noted manometric changes with SNS are not necessarily the only, or even the relevant, mechanism by which continence is improved with SNS.6
Studies have also demonstrated significant improvement in rectal sensation to distention.6,9 SNS may also have an effect on afferent sensory fibers of the rectum. In a detailed study looking at possible modes of action of SNS, Vaizey and colleagues9 demonstrated neuromodulation of sacral reflexes that regulate rectal sensitivity and contractility as well as anal motility. Again, the clinical relevance of these observations is unknown.
It is likely that SNS involves an altered function of multiple nerve fibers within the physiologic range of the implanted sacral stimulator, including somatic fibers to the EAS and pelvic floor, autonomic fibers to the internal anal sphincter and colon, and afferent sensory fibers from the anus and rectum. At present, the relative contributions of these remain to be elucidated.6
CONCLUSION
Sacral nerve stimulation as a treatment option for fecal incontinence has shown favorable results in both medium- and long-term follow-up periods. The modality is best suited for patients with a morphologically intact sphincter apparatus, whose incontinence stems from a functional rather than an anatomic deficit. Predictably good results can be anticipated based on patient response to a subchronic stimulation trial. The mechanism of SNS's salutary effect remains conjectural at present and is likely multifactorial, with current experimental data pointing toward both an enhancement in striated muscular activity as well as neuromodulation of sacral reflexes that regulate rectal sensitivity and contractility.
REFERENCES
- 1.Matzel K E, Schmidt R A, Tanagho E A. Neuroanatomy of the striated muscular anal continence mechanism: implications for the use of neurostimulation. Dis Colon Rectum. 1990;33:666–673. doi: 10.1007/BF02150742. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt R A, Senn E, Tanagho E A. Functional evaluation of sacral nerve root integrity—report of a technique. Urology. 1990;35:388–392. doi: 10.1016/0090-4295(90)80078-2. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt R A. Applications of neurostimulation in urology. Neurourol Urodyn. 1988;7:585–592. [Google Scholar]
- 4.Thon W F, Baskin L S, Jonas U, et al. Surgical principles of sacral foramen electrode implantation. World J Urol. 1991;9:138–141. [Google Scholar]
- 5.Matzel K E, Stadelmaier U, Hohenfellner M, Gall F P. Electrical stimulation of sacral spinal nerves for treatment of faecal incontinence. Lancet. 1995;346:1124–1127. doi: 10.1016/s0140-6736(95)91799-3. [DOI] [PubMed] [Google Scholar]
- 6.Kenefick N J, Vaizey C J, Cohen R C, Nicholls R J, Kamm M A. Medium-term results of permanent sacral nerve stimulation for faecal incontinence. Br J Surg. 2002;89:896–901. doi: 10.1046/j.1365-2168.2002.02119.x. [DOI] [PubMed] [Google Scholar]
- 7.Matzel K E, Stadelmaier U, Hohenfellner M, Hohenberger W. Chronic sacral spinal nerve stimulation for fecal incontinence: long-term results with foramen and cuff electrodes. Dis Colon Rectum. 2001;44:59–66. doi: 10.1007/BF02234822. [DOI] [PubMed] [Google Scholar]
- 8.Ganio E, Luc A R, lerico G, Trompetto M. Sacral nerve stimulation for treatment of fecal incontinence: a novel approach for intractable fecal incontinence. Dis Colon Rectum. 2001;44:619–629. doi: 10.1007/BF02234555. [DOI] [PubMed] [Google Scholar]
- 9.Vaizey C J, Kamm M A, Turner I C, Nicholls R J, Woloszko J. Effects of short-term sacral nerve stimulation on anal and rectal function in patients with anal incontinence. Gut. 1999;44:407–412. doi: 10.1136/gut.44.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malouf A J, Vaizey C J, Nicholls R J, Kamm M A. Permanent sacral nerve stimulation for fecal incontinence. Ann Surg. 2000;232:143–148. doi: 10.1097/00000658-200007000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen H R, Urbarz C, Holzer B, Novi G, Schiessel R. Sacral nerve stimulation as a treatment for fecal incontinence. Gastroenterology. 2001;121:536–541. doi: 10.1053/gast.2001.27120. [DOI] [PubMed] [Google Scholar]
- 12.Leroi A M, Michot F, Grise P, Denis P. Effect of sacral nerve stimulation in patients with fecal and urinary incontinence. Dis Colon Rectum. 2001;44:779–789. doi: 10.1007/BF02234695. [DOI] [PubMed] [Google Scholar]
- 13.Matzel K M, Kamm M A, Stösser M, et al. Sacral nerve stimulation for faecal incontinence: multicentre study. Lancet. 2004;363:1270–1276. doi: 10.1016/S0140-6736(04)15999-0. [DOI] [PubMed] [Google Scholar]
- 14.Kenefick N J, Emmanuel A, Nicholls R J, Kamm M A. Effect of sacral nerve stimulation on autonomic nerve function. Br J Surg. 2003;90:1256–1260. doi: 10.1002/bjs.4196. [DOI] [PubMed] [Google Scholar]
- 15.Kenefick N J, Vaizey C J, Nicholls R J, Cohen R, Kamm M A. Sacral nerve stimulation for faecal incontinence due to systemic sclerosis. Gut. 2002;51:881–883. doi: 10.1136/gut.51.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaizey C J, Kamm M A, Roy A J, Nicholls R J. Double-blind crossover study of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2000;43:298–302. doi: 10.1007/BF02258292. [DOI] [PubMed] [Google Scholar]
- 17.Pette D, Vrbova G. Adaptation of mammalian skeletal muscle fibers to chronic electrical stimulation. Rev Physiol Biochem Pharmacol. 1992;120:115–202. doi: 10.1007/BFb0036123. [DOI] [PubMed] [Google Scholar]
- 18.Salmons S, Hendricksson J. The adaptive response of skeletal muscle to increased use. Muscle Nerve. 1981;4:94–105. doi: 10.1002/mus.880040204. [DOI] [PubMed] [Google Scholar]