ABSTRACT
Pilonidal disease is a common anorectal problem that typically affects young people. Numerous surgical procedures have been described, but treatment failure and disease recurrence are frequent, leading to considerable morbidity in these otherwise healthy patients. To manage this problem successfully, surgeons must consider the pathogenesis and presentation of the disease and weigh the advantages and disadvantages of any operation. Discussed in this article are the pathogenesis of pilonidal disease and basic treatment options for acute pilonidal abscesses, sinus tracts, and chronic or recurrent pilonidal disease.
Keywords: Pilonidal, review, pathogenesis, treatment
Pilonidal disease is a common anorectal problem that typically affects young men and women. Numerous surgical procedures have been described, but treatment failure and disease recurrence are frequent, leading to considerable morbidity in these otherwise healthy patients. To manage this problem successfully, surgeons must consider the pathogenesis and presentation of the disease and weigh the advantages and disadvantages of any operation.
PATHOGENESIS
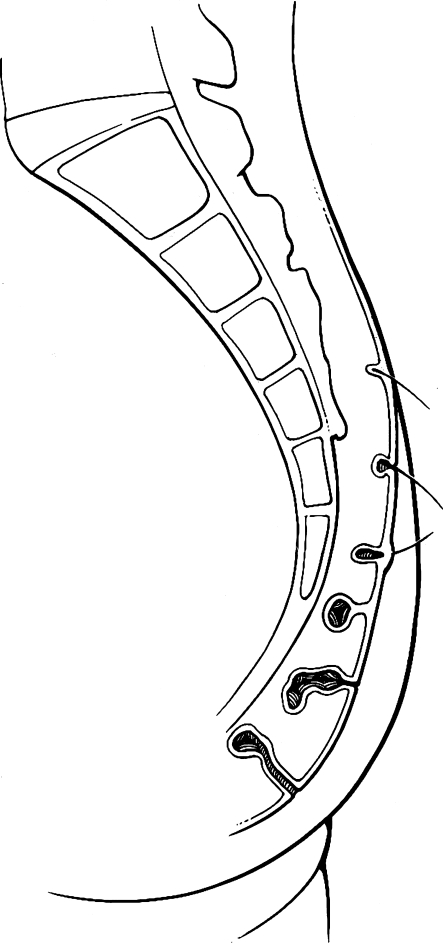
The term pilonidal is derived from the Latin words for hair (pilus) and nest (nidus).1 Pilonidal disease is caused by hair invading the skin at the natal cleft. This hair causes a foreign body reaction that commonly leads to a hair-filled abscess cavity (Fig. 1). Midline pits are the sine qua non of pilonidal disease and represent hair follicles that have become infected or inflamed.2 The resulting folliculitis produces edema that obstructs each follicle's opening. Over time, hair shafts are drawn into the pits by motion from the buttocks, which produces a vacuum effect. Expulsion in the reverse direction is prevented by barbs on the hair shafts. Keratin accumulation distends the follicle, which eventually forms an epithelialized tube. This tube may rupture into underlying subcutaneous fat, forming an abscess. When an abscess forms, it drains back to the skin through true sinus tracts. The etiology of pilonidal disease as a foreign body reaction is supported by histological examination. It demonstrates foreign body giant cells associated with hair shafts that are embedded in chronic granulation tissue lining the abscess cavity and sinus tracts.3,4
Figure 1.
Pathogenesis. Hair invading skin at the natal cleft causes a pilonidal abscess and sinus tracts. Figure used by permission of Mayo Foundation for Medical Education and Research. All rights reserved.
EPIDEMIOLOGY
An incidence of 26 cases per 100,000 people was calculated by Sondenaa and others in a study of 322 patients with pilonidal disease.5 It is, however, more common in young adult men, a population with an incidence of 1.1%.6 Men are thought to be at higher risk because of their more hirsute nature. Other associations with pilonidal disease are obesity (37%), sedentary occupation (44%), and local irritation or trauma (34%).5 Jeep drivers in World War II were subjected to this type of local irritation so frequently that Louis Buie, a Mayo Clinic proctologist, recognized the association and described it in 1944 as “jeep disease.”7 Personal hygiene does not seem to be implicated.5
PRESENTATION
Pilonidal disease is a common anorectal problem that may arise in one of three forms:
Acute abscess
Sinus tracts
Complex disease characterized by chronic or recurrent abscesses with extensive, branching sinus tracts
Symptoms related to pilonidal abscess include fever, chills, and pain, and intermittent discharge or bleeding is common from sinus tracts. Affected patients are typically in their middle to late 20s and have had symptoms for 4 to 5 years at initial presentation.5,8
The majority of patients present with an acute abscess cephalad in the natal cleft. This position distinguishes the disease from other common anorectal problems, such as perirectal abscesses and anal fistulae, which are typically found near the anus. Midline pits are the distinguishing feature, occurring in 100% of cases, and they can typically be identified 4 to 8 cm from the anus.5,9 Hair within the abscess cavity is present in approximately two thirds of cases in men and one third of those in woman.9 As the acute abscess resolves, whether spontaneously or with treatment, chronic sinus tracts develop toward the skin. Chronic or recurrent abscesses with extensive, branching sinus tracts develop in a small minority of patients. This complex variant of the disease may stem from prolonged neglect of symptoms but also occurs despite appropriate treatment.
TREATMENT
Treatment of pilonidal disease depends on the presentation. Intervention may range from simple incision and drainage to wide excision with extensive reconstructive procedures. Ideally, the selected treatment should involve a simple procedure with minimal morbidity, a short healing time, and low recurrence rates.
Acute Pilonidal Abscess
Acute pilonidal abscesses are adequately treated with incision and drainage in the majority of cases. In a prospective study of 73 patients treated with simple incision and drainage for acute pilonidal abscess, Jensen and Harling reported 58% of patients cured within 10 weeks.10 Incision and drainage is recommended as the first treatment because it is a simple office procedure performed under local anesthesia and offers relief of symptoms and brief recovery. However, the number of patients who go on to need a more definitive operation is substantial. This group includes 21% of the patients who are initially cured but go on to develop recurrent disease as well as the 42% in whom initial treatment fails. The patients who are more likely to experience failure or recurrence are predicted by significantly more midline pits and lateral sinus tracts.10
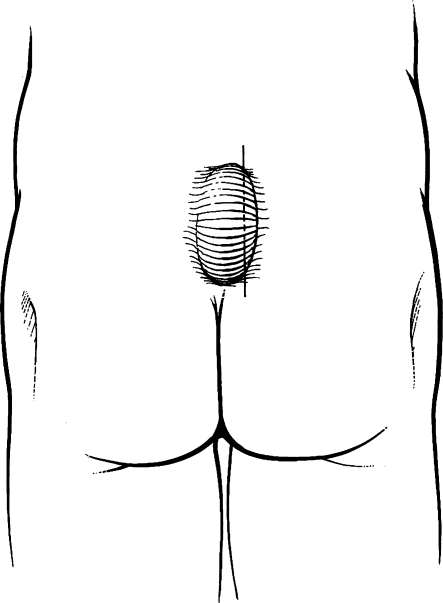
To improve the chance of cure and decrease the risk of recurrence, many authors recommend taking measures beyond simple incision and drainage. A lateral incision should be made over the cavity so that midline pits do not interfere with the healing wound (Fig. 2). The cavity should also be vigorously curetted, débriding the walls of embedded hairs, and the surrounding skin should be meticulously depilated at the time of operation and over subsequent weeks. Trimming, shaving, and plucking are typically recommended for hair removal. However, many patients describe this important phase of treatment as inconvenient and difficult. For that reason, laser depilation of the natal cleft has been suggested, and it has been shown to be effective.11,12
Figure 2.
Incision and drainage. The incision should be made lateral to midline. Figure used by permission of Mayo Foundation for Medical Education and Research. All rights reserved.
Laser depilation has also been described as definitive nonoperative treatment of recurrent pilonidal disease. In a small series of six patients with recurrent disease, Landa and others reported resolution of folliculitis and no further operations after 3 to 11 laser treatments.13 However, no randomized trials have been performed, and although laser therapy does seem to be an effective adjunct to surgery, it is not regarded as curative in and of itself.12
Pilonidal Sinus
The simplest procedure to manage pilonidal sinuses is laying open the tract. This was originally described by Buie in 1938.14 With this technique the surgeon simply unroofs the midline and lateral sinus tracts, which are allowed to heal by secondary intention. Lord and Millar proposed a closed technique in which the sinus tracts are not unroofed but instead débrided with a “bottle” brush.15 They also recognized the importance of the tiny midline pits in the pathogenesis of the disease and excised them down to the cavity, effectively unroofing them.
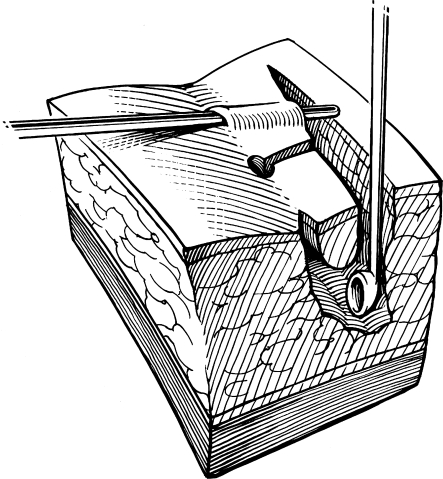
The importance of avoiding midline incisions and placing any healing wounds off midline to reduce recurrence was recognized by Bascom.2 In Bascom's procedure, a lateral incision is made over the sinus cavity, which is curetted to remove hair and granulation tissue. The incision is left open with a light dressing to heal by secondary intention. He excised the midline pits and sinus tracts rather than simply brushing the sinus tracts as in Lord's procedure. Using this technique in 218 patients, Senapati et al found that all but one wound healed after a mean of 4 weeks (range, 1 to 15 weeks). Recurrence requiring reoperation occurred in 10% of patients with a mean follow-up of 12 months.16 Another 6% had redeveloped midline pits but had not yet required surgery when the study ended. To reduce the risk of recurrence in our practice, we lay open all midline pits and sinus tracts toward the lateral incision rather than excising them (Fig. 3).17 Complications of Bascom's procedure include a 4% risk of hemorrhage and an 8% risk of abscess formation at the lateral incision.
Figure 3.
Incision and drainage. Midline pits and sinus tracts are laid open toward the lateral incision, and the cavity is curetted. Figure used by permission of Mayo Foundation for Medical Education and Research. All rights reserved.
Injecting phenol as a sclerosing agent into pilonidal sinuses promotes healing by débriding the tract. It was first proposed by Maurice and Greenwood in 1964 as an alternative to more extensive surgical procedures. Its efficacy has since been demonstrated in several studies, although overall results are mixed.18 After one to two injections over 2 months, successful healing rates of nearly 70% have been reported.19,20 It is a simple operation that can be done under local anesthesia as an outpatient procedure. Reported recurrence rates, however, vary widely from 6.5% to 40.5%.20,21
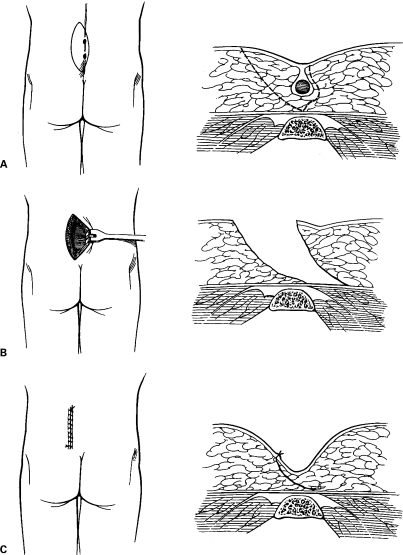
For chronic or recurrent pilonidal sinuses, excision of all involved skin and subcutaneous tissue may be necessary for definitive treatment. These wounds may then be managed open, with healing by secondary intention, or closed by primary suture. The open approach has the advantage of brief hospitalization, but substantial morbidity results from prolonged healing and frequent, uncomfortable dressing changes. For these patients, who are typically young, otherwise healthy, and active, these wounds cause considerable social and economic disability. Marsupialization (partial closure) reduces the size of pilonidal wounds and has been shown to decrease healing time.22,23 This approach is preferred over standard open management but still requires multiple dressing changes each day over a prolonged period of time (Fig. 4).
Figure 4.
Marsupialization. The skin edge is sutured to the base of the wound. Figure used by permission of Mayo Foundation for Medical Education and Research. All rights reserved.
More recently, negative pressure dressings, which promote granulation, have been suggested as an alternative to open packing. Primary VAC therapy (V.A.C. Therapy®, Kinetic Concepts, San Antonio, TX) following excision of pilonidal disease has been reported with favorable results.24,25,26 It may be performed as an outpatient procedure with low risk of primary failure or infection while accelerating healing and reducing the frequency of dressing changes. Duxbury and others first described VAC therapy in one patient following excision of a chronic pilonidal sinus.24 They applied the device for 6 weeks, and the wound was completely healed by 8 weeks. McGuinness and others reported applying the device to one patient for 3 weeks, achieving complete wound healing in 7 weeks.25 In randomized controlled trials compared with wet-to-dry dressing changes, VAC therapy has demonstrated significantly faster healing of chronic wounds.27,28 Negative pressure exerted on wounds has been shown to increase local blood flow, upregulate cell proliferation, decrease bacterial counts, and facilitate wound granulation.29,30 The benefits of negative pressure wound therapy should be applicable to pilonidal disease and early reports are encouraging; however, follow-up for recurrence has not been reported.
Compared with open management, primary closure of pilonidal wounds following excision requires longer hospitalization but significantly reduces healing time. Reviewing 22 reports of primary closure between 1967 and 1987, Allen-Mersh found that 90% of wounds were healed within 2 weeks.31 Primary dehiscence and infection following closure are potential early complications, but these wounds can then be managed open. At least two randomized trials have suggested that primary closure is preferable to open management with healing by secondary intention. Al-Hassan and others randomly allocated 200 patients and found a significantly shorter mean healing time in the primary closure group (10.3 days) compared with the open management group (13 weeks). The cure rates were the same, although, with 29 months mean follow-up, recurrence was significantly greater in the primary closure group (20%) compared with the open group (12%).32 Another randomized study of 120 patients undergoing treatment for recurrent pilonidal disease again found a shorter mean healing time in the primary closure group (3.2 weeks) compared with the open group (12.2 weeks). Recurrence, however, was also more frequent in the primary closure group (10%) compared with the open (5%).33
Karydakis was the first to advocate asymmetric closure of pilonidal wounds to decrease recurrence. This technique avoids placing a wound in the midline at the depth of the natal cleft. It also flattens the cleft, reducing hair accumulation and mechanical irritation. In his advancing flap operation, an asymmetric elliptical incision is made around all involved tissue down to the postsacral fascia and the wound is undermined, creating a thick flap that is closed off midline (Fig. 5).34 Karydakis reviewed his 35-year experience with this operation, which he performed on 6545 patients, and found a 1% recurrence rate.35 In another single-surgeon experience, a 4% recurrence rate using the Karydakis procedure in 114 patients was reported. Kitchen attributed most recurrences to part of the wound encroaching on the midline.36
Figure 5.
Karydakis procedure. (A) An asymmetric elliptical incision is carried down to the postsacral fascia. (B) The wound is undermined and (C) closed off midline. Figures used by permission of Mayo Foundation for Medical Education and Research. All rights reserved.
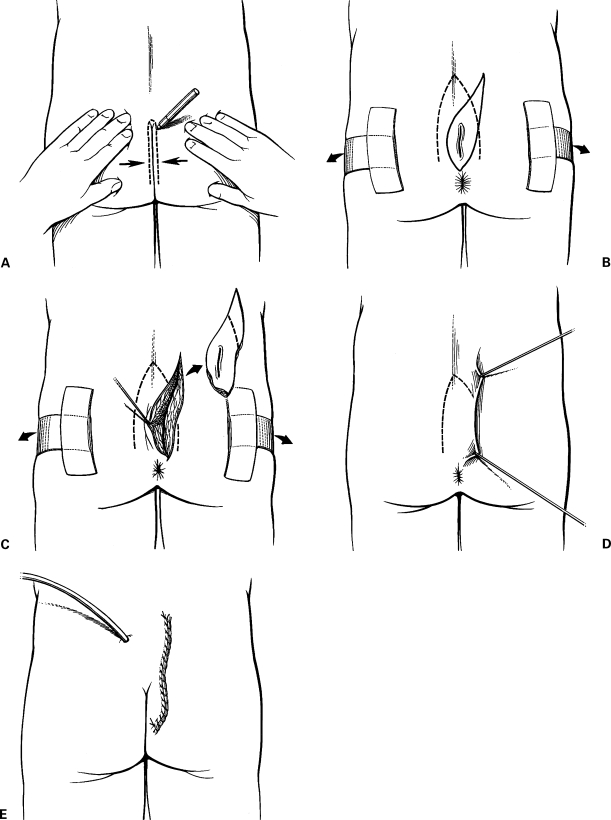
Significantly lower failure and late recurrence rates among asymmetric versus midline closure techniques were found in a pooled analysis of 74 publications including 10,090 patients treated for pilonidal disease over 35 years.37 When a midline incision is used and fails to heal, it can subsequently be placed off midline using cleft closure as described by Bascom (Fig. 6).38 This technique is performed by making an asymmetric elliptical incision around the wound defect. Unlike the Karydakis procedure, in this procedure no subcutaneous tissue is excised. Dermis is undermined and a full-thickness skin flap is raised and closed off midline. Leaving the subcutaneus tissue in place reduces the depth of the natal cleft. The advantage, according to Bascom and Bascom, is the restoration of favorable healing conditions, removing the incision from the warm, moist, bacteria-laden environment found at the depth of the natal cleft.39 They reported using the operation in 31 patients with refractory pilonidal disease and persistently open midline surgical wounds. Following cleft closure, wounds in all 31 patients healed with a median follow-up of 20 months (range, 1 month to 15 years). Twenty-two of the 31 patients healed by 1 week whereas 6 patients required segments of their incision to heal by secondary intention and three required operative revision of the incision.39
Figure 6.
Cleft closure. (A) The line of contact between the buttocks is marked. (B) The buttocks are taped apart and an asymmetric elliptical incision is drawn around the unhealed midline wound. (C) The incision is carried into the subcutaneous fat to wedge out the unhealed wound. (D) A dermal flap is raised, undermining the skin to the marked line of contact. (E) The skin flap is closed over a suction drain. Figures used by permission of Mayo Foundation for Medical Education and Research. All rights reserved.
Surgical principles important for successful management of pilonidal sinus disease include unroofing or excising all midline pits and sinus tracts as well as placing any surgical incision off midline. When excision of all involved tissue is undertaken, primary closure is preferred over open management, although primary wound VAC therapy seems to be a feasible alternative.
Complex Pilonidal Disease
Infrequently, patients develop complex pilonidal disease, which is characterized by chronic or recurrent abscesses and extensive, branching sinus tracts. This manifestation of the disease demands a more aggressive surgical approach, and definitive treatment requires wide excision of all involved tissue. Primary closure of these wounds is not a viable option given a dehiscence rate of up to 37%, and open management with healing by secondary intention is not suitable because of the length of time that would be required for healing.31 Reconstructive procedures are typically required in these cases. These procedures not only cover the wound but also, in theory, flatten the natal cleft, reducing hair accumulation, mechanical irritation, and risk of recurrence. Each offers relatively short healing time but requires an extensive operation under general anesthesia and lengthy hospitalization. Complications, including flap necrosis, wound dehiscence, and infection, are a considerable risk.
Z-plasty closure following excision of pilonidal sinuses was first described in the 1960s.40 In a series of 120 patients treated with Z-plasty, Mansoory and Dickson reported only two recurrences with 1 to 9 years follow-up.41 These excellent results were repeated by Toubanakis, who found no recurrences in 110 patients observed for 1 to 10 years.42 The procedure has been criticized, however, for a 20% incidence of tip necrosis. A series of 21 patients followed up for an average of 28 months showed considerable morbidity, including hypesthesia in nearly every patient, and only 67% overall satisfaction.43,44 Patients frequently complained of discomfort, pruritus, and poor cosmesis.44 As with other primary closure techniques, healing can be achieved in 7 to 10 days.17
In 1984 Azab and others described the use of a rhomboid excision and Limberg transposition flap in complex pilonidal disease.45 They demonstrated 29 well-healed wounds in 30 patients after a mean hospitalization of less than 10 days. There were no recurrences with 3 years follow-up. Complications included one major and five minor infections. Since then, numerous studies have confirmed the procedure's effectiveness. The largest series reviewed 238 patients at a single center who underwent Limberg flap reconstruction. It demonstrated a mean hospitalization of 2.1 days and mean healing time of 8.0 days. Recurrence occurred in 1.26% of patients with mean follow-up of 29 months.46 These recurrences occurred early in the series, and no recurrences were found after the authors modified the procedure, placing the rhomboid excision lateral to the natal cleft, avoiding the midline. They reported a 0.8% infection rate and no occurrences of flap necrosis, attributing these results to the tension-free suture line, which is easily achieved by mobilizing the relatively soft, loose tissue at the donor site. The donor tissue is also well vascularized by musculocutaneous perforators from the superior and inferior gluteal arteries, offering a reliable fasciocutaneous flap.
V-to-Y advancement flaps likewise have been shown in several series to be reliable and effective in covering large pilonidal wounds. Schoeller and others demonstrated a mean hospitalization of 7.3 days and mean healing time of 15.3 days using this procedure.47 In their series of 24 patients, there were no recurrences with a mean follow-up of 4.5 years. To prevent recurrences in this series, the authors attempted to flatten the natal cleft by deepithelializing the medial end of the flap and folding it under itself to fill in the wound defect. Recognizing the importance of closing the wound off midline, Berkem and others performed V-Y advancement flaps in 34 patients, randomly allocating them into two groups: vertical suture line unrelated to midline and vertical suture line related to midline.48 Two recurrences in the group with the vertical suture line related to midline occurred at 15 and 22 months, whereas there were no recurrences when the suture line was placed off midline.
For patients in whom repeated interventions fail, gluteus maximus myocutaneous flap reconstruction seems to be effective with acceptable morbidity.49,50 The procedure has been criticized for being too extensive because it sacrifices a deep functional muscle, but Rosen and Davidson performed the procedure in five young men (mean age, 34 years) whose pilonidal disease significantly affected their lives. They found no loss of strength or range of motion in any of the patients and felt that in well-motivated patients plagued with chronic, recurrent, or recalcitrant disease this extensive reconstructive procedure offers a good chance of cure and acceptable morbidity.50
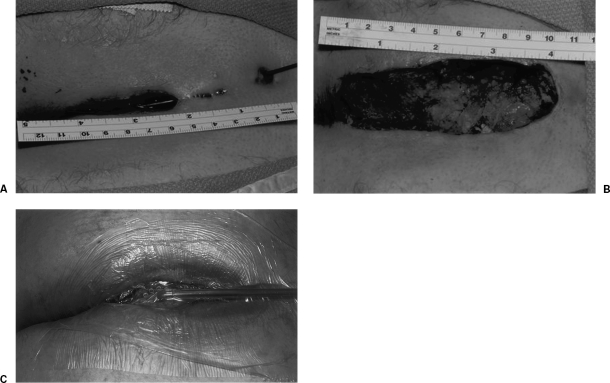
As previously discussed, negative pressure wound therapy has recently been described in managing pilonidal disease. We treated three men and two women with complex disease with resection and primary application of a portable VAC device. The wound defects were large, averaging 11 × 4 × 5 cm, and the mean duration of VAC therapy was comparably long, averaging 6 weeks (range, 4 to 9 weeks) (Fig. 7). These were simple outpatient procedures, and the duration of therapy was thought to be markedly less than would have been required for wet-to-dry dressing changes. Each wound healed well, although one required operative débridement and replacement of the VAC device. Long-term follow-up is not yet available, and risk of recurrence is not known.
Figure 7.
Negative pressure wound therapy. (A) Complex pilonidal disease. (B) Excision of all involved tissue. (C) Primary V.A.C. device placement. Figures used by permission of Mayo Foundation for Medical Education and Research. All rights reserved.
Numerous reconstructive procedures following excision of complex pilonidal disease have been described. Each is an extensive operation that carries considerable risk of complications, but any well-vascularized flap should provide adequate coverage, quickly returning the patient to normal activities after hospitalization. Primary VAC therapy may be a simpler alternative to these procedures, but risk of recurrence is unknown for this newer treatment modality.
Antibiotics
Bacterial colonization of pilonidal sinuses has historically ranged from 50 to 70%, typical isolates including Staphylococcus aureus and anaerobes such as Bacteroides.31,51,52 Miocinovic and others reported bacterial colonization in 78% of pilonidal sinuses at initial presentation and 88% of recurrent sinuses. Anaerobes were found in 52% at initial presentation and 64% if recurrent.53 Still, two randomized trials have failed to demonstrate any significant improvement in wound healing with an empirical course of antibiotics. Having previously shown an association between anaerobic infection and delay in healing of granulating wounds,54 Marks and others randomly assigned 40 patients with pilonidal disease to excision and healing by secondary intention with and without a 2-week course of metronidazole. They found a trend toward shorter healing time in the treated group (17.7 ± 21.9 days) compared with the untreated group (38.5 ± 43.6 days), but no statistical difference (p = 0.067) was demonstrated because of the large standard deviations.52 The authors then compared the treated group with a group treated on the basis of wound appearance. Again, no statistical difference was found in healing time between the group treated empirically and that treated if the wound appeared “unhealthy,” and 48% of the patients in the group treated on the basis of appearance were spared antibiotics. Kronborg and others randomly assigned patients with chronic discharging pilonidal sinuses to excision and primary suture with and without a course of clindamycin. Healing time with clindamycin was 11 days compared with 14 days without treatment, but this trend was not statistically significant. There was no difference in recurrence rates with 3 years follow-up.51 These studies suggest that there is no role for empirical antibiotics in the management of surgically excised pilonidal disease and that antibiotics should be reserved for patients with clinical evidence of infection.
SUMMARY
Pilonidal disease is a common anorectal problem and a surgical challenge. Treatment failure and disease recurrence are prevalent. Successful management depends on adherence to well-described surgical principles based on knowledge of pathogenesis and the patient's presentation. Abscess cavities must be vigorously débrided to remove all embedded hairs, and depilation of surrounding skin should continue for several weeks. Midline pits in all cases must be meticulously sought out and laid open or excised. Sinus tracts, when present, should be treated similarly. Surgical incisions—whether for simple incision and drainage or more extensive reconstructive procedures—should be asymmetric and closed off midline. Considerable morbidity from prolonged wound care can be avoided in these typically healthy patients when their disease is cured at initial presentation.
REFERENCES
- 1.da Silva J H. Pilonidal cyst. Cause and treatment. Dis Colon Rectum. 2000;43:1146–1156. doi: 10.1007/BF02236564. [DOI] [PubMed] [Google Scholar]
- 2.Bascom J. Pilonidal disease: origin from follicles of hairs and results of follicle removal as treatment. Surgery. 1980;87:567–572. [PubMed] [Google Scholar]
- 3.Franckowiak J J, Jackman R J. The etiology of pilonidal sinus. Dis Colon Rectum. 1962;5:28–36. doi: 10.1007/BF02616408. [DOI] [PubMed] [Google Scholar]
- 4.Hardaway R M. Pilonidal cyst—neither pilonidal nor cyst. Arch Surg. 1958;76:143–147. doi: 10.1001/archsurg.1958.01280190145027. [DOI] [PubMed] [Google Scholar]
- 5.Sondenaa K, Nesvik I, Anderson E, Natas O, Soreide J A. Patient characteristics and symptoms in chronic pilonidal sinus disease. Int J Colorectal Dis. 1995;10:39–42. doi: 10.1007/BF00337585. [DOI] [PubMed] [Google Scholar]
- 6.Dwight R W, Maloy J K. Pilonidal sinus: experience with 449 cases. N Engl J Med. 1953;249:926–930. doi: 10.1056/NEJM195312032492303. [DOI] [PubMed] [Google Scholar]
- 7.Buie L A. Jeep disease (pilonidal disease of mechanized warfare) Dis Colon Rectum. 1982;25:384–390. [PubMed] [Google Scholar]
- 8.Kooistra H P. Pilonidal sinuses. Review of the literature and report of three hundred and fifty cases. Am J Surg. 1942;55:3–17. [Google Scholar]
- 9.Notaras M J. A review of three popular methods of treatment of postanal (pilonidal) sinus disease. Br J Surg. 1970;57:886–890. doi: 10.1002/bjs.1800571204. [DOI] [PubMed] [Google Scholar]
- 10.Jensen S L, Harling H. Prognosis after simple incision and drainage for a first-episode acute pilonidal abscess. Br J Surg. 1988;75:60–61. doi: 10.1002/bjs.1800750122. [DOI] [PubMed] [Google Scholar]
- 11.Odili J, Gault D. Laser depilation of the natal cleft—an aid to healing the pilonidal sinus. Ann R Coll Surg Engl. 2002;84:29–32. [PMC free article] [PubMed] [Google Scholar]
- 12.Schulze S M, Patel Y, Hertzog D, Fares L G., 2nd Treatment of pilonidal disease with laser epilation. Am Surg. 2006;72:534–537. [PubMed] [Google Scholar]
- 13.Landa N, Aller O, Landa-Gundin N, Torrontequi J, Azpizu J L. Successful treatment of recurrent pilonidal sinus with laser epilation. Dermatol Surg. 2005;31:726–728. doi: 10.1111/j.1524-4725.2005.31601. [DOI] [PubMed] [Google Scholar]
- 14.Buie L A. Practical Proctology. Philadelphia: WB Saunders; 1938.
- 15.Lord P H, Millar D M. Pilonidal sinus: a simple treatment. Br J Surg. 1965;52:298–300. doi: 10.1002/bjs.1800520413. [DOI] [PubMed] [Google Scholar]
- 16.Senapati A, Cripps N PJ, Thompson M R. Bascom's operation in the day-surgical management of symptomatic pilonidal sinus. Br J Surg. 2000;87:1067–1070. doi: 10.1046/j.1365-2168.2000.01472.x. [DOI] [PubMed] [Google Scholar]
- 17.Nivatvongs S. In: Kelly KA, Sarr MG, Hinder RA, editor. Mayo Clinic Gastrointestinal Surgery. Philadelphia: WB Saunders; 2004. Common anorectal problems. pp. 589–626.
- 18.Maurice B A, Greenwood R K. Conservative treatment of pilonidal sinus. Br J Surg. 1964;51:510–512. doi: 10.1002/bjs.1800510711. [DOI] [PubMed] [Google Scholar]
- 19.Kelly S B, Graham W J. Treatment of pilonidal sinus by phenol injection. Ulster Med J. 1989;58:56–59. [PMC free article] [PubMed] [Google Scholar]
- 20.Hegge H G, Vos G A, Patka P, Hoitsma H F. Treatment of complicated or infected pilonidal sinus disease by local application of phenol. Surgery. 1987;102:52–54. [PubMed] [Google Scholar]
- 21.Scheider I HF, Thaler K, Kockerling F. Treatment of pilonidal sinuses by phenol injections. Int J Colorectal Dis. 1994;9:200–202. doi: 10.1007/BF00292250. [DOI] [PubMed] [Google Scholar]
- 22.Solla J A, Rothenberger D A. Chronic pilonidal disease: an assessment of 150 cases. Dis Colon Rectum. 1990;33:758–761. doi: 10.1007/BF02052321. [DOI] [PubMed] [Google Scholar]
- 23.Spivak H, Brooks V L, Nussbaum M, Friedman I. Treatment of chronic pilonidal disease. Dis Colon Rectum. 1996;39:1136–1139. doi: 10.1007/BF02081415. [DOI] [PubMed] [Google Scholar]
- 24.Duxbury M S, Finlay I G, Butcher M, Lambert A W. Use of a vacuum assisted closure device in pilonidal disease. J Wound Care. 2003;12:355. doi: 10.12968/jowc.2003.12.9.26538. [DOI] [PubMed] [Google Scholar]
- 25.McGuinness J G, Winter D C, O'Connell P R. Vacuum-assisted closure of a complex pilonidal sinus. Dis Colon Rectum. 2003;46:274–276. doi: 10.1007/s10350-004-6535-z. [DOI] [PubMed] [Google Scholar]
- 26.Lynch J B, Laing A J, Regan P J. Vacuum-assisted closure therapy: a new treatment option for recurrent pilonidal sinus disease: report of three cases. Dis Colon Rectum. 2004;47:929–932. doi: 10.1007/s10350-004-0522-2. [DOI] [PubMed] [Google Scholar]
- 27.Joseph E, Hamori C A, Bergman S, et al. A prospective randomized trial of vacuum assisted closure versus standard therapy of chronic nonhealing wounds. Wounds. 2000;12:60–67. [Google Scholar]
- 28.Eginton M T, Brown K R, Seabrook B R, et al. A prospective randomized evaluation of negative pressure wound dressing for diabetic foot wounds. Ann Vasc Surg. 2003;17:645–649. doi: 10.1007/s10016-003-0065-3. [DOI] [PubMed] [Google Scholar]
- 29.Morykwas M J, Argenta L C, Shelton-Brown E I, et al. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38:553–562. doi: 10.1097/00000637-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Saxena V, Hwang C W, Huang S, et al. Vacuum-assisted closure: microdeformation of wounds and cell proliferation. Plast Reconstr Surg. 2004;114:1086–1096. doi: 10.1097/01.prs.0000135330.51408.97. [DOI] [PubMed] [Google Scholar]
- 31.Allen-Mersh T G. Pilonidal sinus: finding the right track for treatment. Br J Surg. 1990;77:123–132. doi: 10.1002/bjs.1800770203. [DOI] [PubMed] [Google Scholar]
- 32.Al-Hassan H K, Francis I M, Neglen P. Primary closure or secondary granulation after excision of pilonidal sinus? Acta Chir Scand. 1990;156:695–699. [PubMed] [Google Scholar]
- 33.Sondenaa K, Nesvik I, Andersen E, Soreide J A. Recurrent pilonidal sinus after excision with closed or open treatment: final results of a randomized trial. Eur J Surg. 1996;162:237–240. [PubMed] [Google Scholar]
- 34.Karydakis G E. New approach to the problem of pilonidal sinus. Lancet. 1973;2:1414–1415. doi: 10.1016/s0140-6736(73)92803-1. [DOI] [PubMed] [Google Scholar]
- 35.Karydakis G E. Easy and successful treatment of pilonidal sinus after explanation of its causative process. Aust N Z J Surg. 1992;62:385–389. doi: 10.1111/j.1445-2197.1992.tb07208.x. [DOI] [PubMed] [Google Scholar]
- 36.Kitchen P R. Pilonidal sinus: experience with the Karydakis flap. Br J Surg. 1996;83:1452–1455. doi: 10.1002/bjs.1800831040. [DOI] [PubMed] [Google Scholar]
- 37.Petersen S, Koch R, Selzner S, Wendlandt T P, Ludwig K. Primary closure techniques in chronic pilonidal sinus: a survey of the results of different surgical approaches. Dis Colon Rectum. 2002;45:1458–1467. doi: 10.1007/s10350-004-6451-2. [DOI] [PubMed] [Google Scholar]
- 38.Bascom J U. Repeat pilonidal operations. Am J Surg. 1987;154:118–122. doi: 10.1016/0002-9610(87)90300-x. [DOI] [PubMed] [Google Scholar]
- 39.Bascom J, Bascom T. Failed pilonidal surgery: new paradigm and new operation leading to cures. Arch Surg. 2002;137:1146–1150. doi: 10.1001/archsurg.137.10.1146. [DOI] [PubMed] [Google Scholar]
- 40.Monro R S. A consideration of some factors in the causation of pilonidal sinus and its treatment by Z-plasty. Am J Proctol. 1967;18:215–225. [PubMed] [Google Scholar]
- 41.Mansoory A, Dickson D. Z-plasty for treatment of disease of the pilonidal sinus. Surg Gynecol Obstet. 1982;155:409–411. [PubMed] [Google Scholar]
- 42.Toubanakis G. Treatment of pilonidal sinus disease with the Z-plasty procedure (modified) Am Surg. 1986;52:611–612. [PubMed] [Google Scholar]
- 43.Bose B, Candy J. Radical cure of pilonidal sinus by Z-plasty. Am J Surg. 1970;120:783–786. doi: 10.1016/s0002-9610(70)80049-6. [DOI] [PubMed] [Google Scholar]
- 44.Tschudi J, Ris H B. Morbidity of Z-plasty in the treatment of pilonidal sinus. Chirurg. 1988;59:486–490. [PubMed] [Google Scholar]
- 45.Azab A S, Kamal M S, Saad R A, Abou al Atta K A, Ali N A. Radical cure of pilonidal sinus by a transposition rhomboid flap. Br J Surg. 1984;71:154–155. doi: 10.1002/bjs.1800710227. [DOI] [PubMed] [Google Scholar]
- 46.Mentes B B, Leventoglu S, Cihan A, Tatlicioglu E, Akin M, Oguz M. Modified Limberg transposition flap for sacrococcygeal pilonidal sinus. Surg Today. 2004;34:419–423. doi: 10.1007/s00595-003-2725-x. [DOI] [PubMed] [Google Scholar]
- 47.Schoeller T, Wechselberger G, Otto A, Papp C. Definite surgical treatment of complicated recurrent pilonidal disease with a modified fasciocutaneous V-Y advancement flap. Surgery. 1997;121:258–263. doi: 10.1016/s0039-6060(97)90354-8. [DOI] [PubMed] [Google Scholar]
- 48.Berkem H, Topaloglu S, Ozel H, et al. V-Y advancement flap closures for complicated pilonidal sinus disease. Int J Colorectal Dis. 2005;20:343–348. doi: 10.1007/s00384-004-0699-9. [DOI] [PubMed] [Google Scholar]
- 49.Perez-Gurri J A, Temple W J, Ketcham A S. Gluteus maximus myocutaneous advancement flap for the treatment of recalcitrant pilonidal disease. Dis Colon Rectum. 1984;27:262–264. doi: 10.1007/BF02553803. [DOI] [PubMed] [Google Scholar]
- 50.Rosen W, Davidson J S. Gluteus maximus musculocutaneous flap treatment of recalcitrant pilonidal disease. Ann Plast Surg. 1996;37:293–297. doi: 10.1097/00000637-199609000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Kronborg O, Chriestensen K, Zimmerman-Nielsen C. Chronic pilonidal disease: a randomized trial with a complete 3-year follow-up. Br J Surg. 1985;72:303–304. doi: 10.1002/bjs.1800720418. [DOI] [PubMed] [Google Scholar]
- 52.Marks J, Harding K G, Hughes L E, Ribeiro C D. Pilonidal sinus excision—healing by open granulation. Br J Surg. 1985;72:637–640. doi: 10.1002/bjs.1800720818. [DOI] [PubMed] [Google Scholar]
- 53.Miocinovic M, Horzic M, Bunoza D. The prevalence of anaerobic infection in pilonidal sinus of the sacrococcygeal region and its effect on the complications. Acta Med Croatica. 2001;55:87–90. [PubMed] [Google Scholar]
- 54.Marks J, Hughes L E, Harding K G, Campbell H, Ribeiro C D. Prediction of healing time as an aid to the management of open granulating wounds. World J Surg. 1983;7:641–645. doi: 10.1007/BF01655345. [DOI] [PubMed] [Google Scholar]