ABSTRACT
Perineal wound complications following abdominoperineal resection (APR) is a common occurrence. Risk factors such as operative technique, preoperative radiation therapy, and indication for surgery (i.e., rectal cancer, anal cancer, or inflammatory bowel disease [IBD]) are strong predictors of these complications. Patient risk factors include diabetes, obesity, and smoking. Intraoperative perineal wound management has evolved from open wound packing to primary closure with closed suctioned transabdominal pelvic drains. Wide excision is used to gain local control in cancer patients, and coupled with the increased use of pelvic radiation therapy, we have experienced increased challenges with primary closure of the perineal wound. Tissue transfer techniques such as omental pedicle flaps, and vertical rectus abdominis and gracilis muscle or myocutaneous flaps are being used to reconstruct large perineal defects and decrease the incidence of perineal wound complications. Wound failure is frequently managed by wet to dry dressing changes, but can result in prolonged hospital stay, hospital readmission, home nursing wound care needs, and the expenditure of significant medical costs. Adjuvant therapies to conservative wound care have been suggested, but evidence is still lacking. The use of the vacuum-assisted closure device has shown promise in chronic soft tissue wounds; however, experience is lacking, and is likely due to the difficulty in application techniques.
Keywords: Abdominoperineal resection, perineal wound complication, wound management, tissue transfer, vacuum-assisted closure device
Abdominoperineal resection (APR) is performed for patients with low rectal cancer, salvage for recurrent or persistent anal cancer, and severe inflammatory bowel disease (IBD). Perineal wound complications following APR are a common and significant problem, and include wound infection, abscess, dehiscence, delayed healing, and persistent perineal sinus. These complications result in significant morbidity that requires prolonged hospital stay, hospital readmission, home-nursing wound care needs, and the expenditure of significant medical costs.1 Furthermore, patients with significant perineal wound complications after APR for cancer have an increased incidence of local recurrence adversely affecting long-term survival, which may be a direct result of delay in adjuvant therapy.2 For the patient, these wound complications are painful, malodorous, and require constant care, which adversely affects quality of life. The purpose of this article is to review the risk factors associated with perineal wound complications in APR, to discuss operative techniques such as tissue transfer to optimize perineal wound healing, and present commonly available methods to treat perineal wound failure.
RISK FACTORS
The high incidence of perineal wound complications after APR is not surprising. Resection of the rectum and anus from the pelvis creates a large cavity that is fixed by surrounding pelvic boney structures. This pelvic dead space results in accumulation of fluid and blood clot that increases the risk of developing a pelvic abscess, a wound infection, and perineal wound sinus tracts. Furthermore, the rigidity of the surrounding structures of the pelvis makes the perineum a difficult wound to close. Primary closure is frequently under tension and is a significant factor in wound breakdown. However, the fixed anatomic factors of the pelvis do not completely explain the wide variation of reported perineal wound complications (14 to 80%) following APR.3,4,5,6 Specific risk factors such as operative perineal wound management, the use of preoperative radiation therapy (XRT), and indications for surgery (e.g., rectal cancer, anal cancer, or inflammatory bowel disease) have been shown to influence perineal wound healing after APR.
Perineal wound management following APR has evolved as a direct result of the continual search for techniques to decrease wound complications, and has been extensively reviewed by Opelka.7 Historically, the perineum was left open and packed to support the perineal floor and promote hemostasis and drainage8; however, this technique resulted in significant patient discomfort with delayed wound healing, often taking 4 months or more. By the 1970s, options for perineal wound closure focused on four main issues: (1) primary wound closure, (2) closure of the peritoneum, (3) closed suction drainage of the pelvis through perineal or transabdominal drainage, and (4) pelvic wound irrigation and active closed drainage. Subsequent studies have shown that closed suction of the pelvis improves wound healing, and the addition of irrigation was not necessary.9 Furthermore, closure of the pelvic peritoneum was associated with prolonged perineal wound healing after primary closure at time of APR.10 The rationale here is that closure of the peritoneum and perineum results in a significant closed pelvic dead space that is difficult to drain, which results in fluid and hematoma accumulation that can become secondarily infected. Leaving the peritoneum open allows intraabdominal viscera to occupy the presacral space obliterating the dead space. Alternatively, investigators have advocated the use of the omentum or uterus to fill the dead space and prevent small bowel adhesions to the pelvis.11,12,13 Areas of current controversy and investigation involve the use of tissue-transfer techniques to fill the pelvic dead space and promote perineal wound healing by bringing well-vascularized tissue into the irradiated perineal wound (discussed below). Currently, it is our practice to primarily close the perineum when possible, fill the pelvic dead space with the omentum, and drain the pelvic cavity with transabdominal active closed suction drains.
Preoperative XRT is routinely used for low rectal and anal cancer, and significantly increases the risk for perineal wound complication after APR. Although preoperative XRT may offer benefit in terms of recurrence and local control of these cancers, there is significant postoperative morbidity associated with this therapy. In a recent retrospective review, Bullard and colleagues14 reported their experience with 160 rectal cancer patients that had APR with primary closure of the perineal wound. In this study, the overall perineal wound complication rate was 41%; however, the use of preoperative XRT increased wound complications twofold from 23 to 47%. Similar results were reported by Artioukh et al15 where preoperative XRT significantly increased the number of nonhealed perineal wounds after APR for rectal cancer (6.7% versus 39.1%). Similarly, salvage APR for epidermoid cancer of the anus after chemotherapy and XRT has been associated with a high rate of major perineal wound complications ranging from 47 to 80%.6,16,17 The adverse effects of XRT on wound healing are directly related to normal tissue injury through progressive occlusive vasculitis and fibrosis.18 In the pelvis, radiation-induced fibrosis likely limits the ability to close the perineum and pelvic sidewall increasing the risk for wound complication. Other factors may include obliteration of lymphatics and alteration of fibroblast function that is required for wound healing.
Indication for APR resection is another significant factor in the development of perineal wound complications. A recent retrospective review showed that APR after radiation for patients with epidermoid cancer of the anal canal had a much higher major wound complication than patients with rectal cancer (62% versus 11%).19 In fact, the odds of a patient with anal cancer developing a major perineal wound complication were considerably higher than those for a patient with any other indication (rectal cancer and IBD). Furthermore, minor wound complications are greatest for anal cancer (50%) and IBD (45%), with lowest rates for patients with rectal cancer (21%). Others have reported similar increased risk with IBD.20 The higher rate of perineal wound complications in patients with anal cancer is likely multifactorial and may be related to a more focused radiation field to the perineum and skin, delivery of higher radiation doses to patients with anal cancer, or the need for greater perineal resection for margins leaving a larger soft tissue defect.6 Wound complications in IBD may be a direct result of malnutrition, chronic pelvic inflammation that prohibits closure of the pelvic dead space, or preexisting drainage and sinus tracts from the rectum, which results in inoculation of the pelvic space, thus impairing wound healing.7
Other associated medical comorbidities have been studied and shown to increase the risk of perineal wound complications. Diabetes, low preoperative hematocrit, tumor size, and obesity have all been shown to be significant predictors of perineal wound complications.19 Interestingly, in obese patients this study indicated that for every point the body mass index (BMI) increased, there was a 10% increase in odds of developing wound complications.
TISSUE TRANSFER
APR, especially for oncologic reasons, often results in major tissue defects in the perineum and a large dead space in the pelvis. It has been reported that tissue transfer of well-vascularized nonirradiated tissue to the postirradiated pelvic defect results in improved perineal wound healing. Small studies have attempted to address the problem of perineal wound complications by using muscle, myocutaneous, or omental flap reconstruction.13,21,22,23 Reported wound complications rates with these techniques range from 0 to 30%. Suggested indications for tissue transfer in APR include patients with large perineal soft tissue defects, the need for posterior vaginal wall reconstruction, the need to fill a large pelvic dead space, and the reconstruction of a large perineal defect especially in the setting of preoperative radiation.22,24 If these factors can be anticipated, a multidisciplinary approach to perineal reconstruction may improve results. The most common tissue transfer techniques used in the pelvis after APR include omental pedicle flap, vertical rectus abdominis flap, and, gracilis flap. Although omental pedicle flaps are routinely performed by colon rectal surgeons, the comfort level of performing muscle and myocutaneous flaps to the perineum may be variable and require preoperative consultation with a plastic surgeon. The addition of muscle and myocutaneous flaps for closure of the perineum after APR has been reported to increase operative time by nearly 2 h, without increasing operative morbidity or prolonging hospital stay.24
Omental Pedicle Flap
The omentum possesses many physiologic properties that make it favorable for use as a flap to the pelvis after APR. The omentum plays a major role in the local immune response in bacterial peritonitis, and plays a key role in the removal of infective agents, particulate matter, and fluid from the peritoneal cavity. In addition, the angiogenic properties of the omentum create vascular adhesions that may provide an alternate blood supply to surrounding ischemic tissues.25,26,27 Furthermore, the omentum contains high concentration of tissue factor giving it significant hemostatic properties.28 These physiologic properties and its capacity to fill the pelvic dead space make the omentum an excellent candidate for tissue transfer to the pelvis.
Several studies have reported good results with the use of omental pedicle flaps to the pelvis and perineum, with a 50 to 100% primary perineal wound healing rate.11,12,13 However, a recent prospective nonrandomized multicenter trial reported that omentoplasty after APR for cancer conferred no significant advantage in perineal wound healing compared with patients without omentoplasty.29 In this study, the perineal wound healing rate at 1 month was 68% for patients with or without omentoplasty. Only the number of patients with perineal wound dehiscence was significantly lowered by omentoplasty from 16 to 5%. A recent modification of the omentoplasty to include suturing of the omentum to the perineal subcutaneous tissue before perineal skin closure has resulted in an 80% primary perineal wound healing rate.13 Postoperative perineal wound complications included perineal abscess in 6% and minor superficial wound suppuration in 4%. All wounds were healed 3 months after surgery. Collectively, these studies suggest that the omental pedicle flap is effective when sufficient in size to reach the pelvis and perineum; however, when a large perineal defect is created that is unamenable to primary closure, or the omentum is not sufficient for flap creation, other tissue transfer techniques such as pedicled myocutaneous flaps may be necessary.
Muscle and Myocutaneous Flaps
The inferiorly based rectus abdominis myocutaneous (VRAM) flap was first described in 1984,30 and provides voluminous well-vascularized tissue that can be transferred to cover large perineal skin defects, vaginal defects, and pelvic dead space created by APR (Figs. 1 and 2). This flap is based on the epigastric artery and vein and will reach any defect up to 25 cm from the groin, including the perineum, the sacrum, and the vagina. The entire rectus muscle up to the costal margin can be safely mobilized, and can support a large cutaneous island based on perforating vessels from the muscle to the subcutaneous vascular network.31 Perforator vessels are most dense around the umbilicus; for this reason, a skin paddle is most reliable if harvested from the peri-umbilical region.32 Harvesting is facilitated when a midline laparotomy is used for the initial procedure.
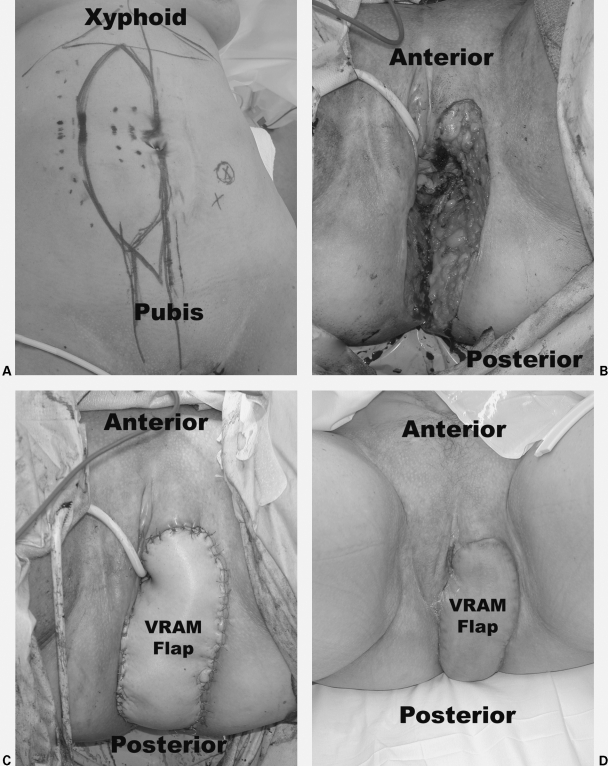
Figure 1.
Vertical rectus abdominis myocutaneous (VRAM) flap reconstruction of the left labia, posterior vagina, and perineum after abdominoperineal resection for squamous cell cancer of the left Bartholin's gland. The tumor was invading the anal sphincter complex. The patient received neoadjuvant chemotherapy and radiation therapy. (A) Pictures show preoperative markings. (B) Intraoperative perineal, vaginal, and labial defect. (C) Immediate postoperative flap reconstruction. (D) 6-week follow-up. Photographs courtesy of Susan M. Pike, M.D., Plastic and Reconstructive Surgery, Scott & White University Medical Campus, Round Rock, TX.
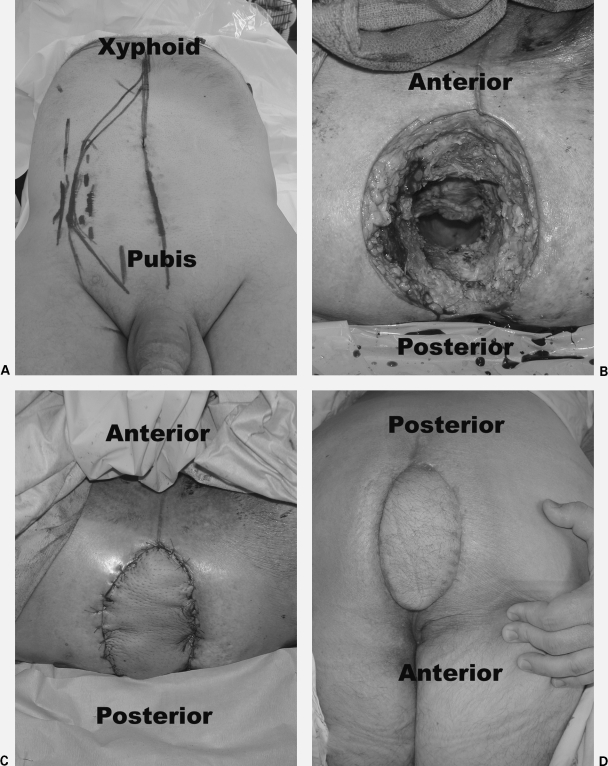
Figure 2.
Vertical rectus abdominis myocutaneous (VRAM) flap reconstruction of the perineum after abdominoperineal resection in a man with locally advanced rectal cancer that invaded through the perineal skin. The patient received neoadjuvant chemoradiation therapy. (A) Pictures show preoperative markings. (B) Intraoperative perineal defect. (C) Immediate postoperative flap reconstruction. (D) 6-week follow-up. Photographs courtesy of Susan M. Pike, M.D., Plastic and Reconstructive Surgery, Scott & White University Medical Campus, Round Rock, TX.
Recent reports have studied the results of pelvic reconstruction with VRAM flaps after APR. Perineal wound complications ranged from 0 to 30%. Higher rates of perineal wound complications were seen for salvage APR in patients with persistent or locally recurrent anal cancer,22 while lower rates were seen for rectal cancer.23 Nearly all patients in these studies received preoperative XRT. These results suggest that VRAM flaps may reduce perineal wound complications in APR, especially in the setting of preoperative XRT; however, these studies are limited because no comparison was made to primary closure. Prospective studies are necessary to better evaluate these findings.
In pelvic and vaginal reconstruction, the VRAM flap offers several advantages including an excellent and safe pedicle, a large arc of rotation, provides bulky well-vascularized tissue, acceptable donor-site morbidity, no interference with primary colostomy site, and ease of access in relation to the APR procedure.22 Disadvantages include lack of sensation to the cutaneous portion of the flap (vagina and perineum), abdominal weakness, and a risk of fascial dehiscence and hernia formation. Furthermore, use of the VRAM flap limits colostomy placement or re-siting in the future should the primary site suffer from a significant complication. Overall patient satisfaction has been high, with primary complaints most commonly found in women due to vaginal stenosis and dyspareunia.
The gracilis muscle flap is based on the major pedicle of the medial circumflex femoral artery that is located ~10 cm to 14 cm from the pubic tubercle, and enters the deep surface of the gracilis along its anterior boarder. Use of this flap has shown promising results in delayed reconstruction of persistent perineal sinus tracts after APR for inflammatory bowel disease.1,33,34 Recent investigators have reported their results of gracilis muscle flaps following APR and intraoperative radiation therapy in patients with recurrent carcinoma of the rectum.21 This retrospective review showed that the use of gracilis muscle flap to the pelvis decreased the incidence of major pelvic abscess from 46 to 12%. Furthermore, primary wound healing was significantly improved from 33 to 63%.
Advantages of the gracilis flap in the setting of APR are primarily related to its avoidance of interfering with the creation of a colostomy site. The flap is particularly useful in small defects that are relatively narrow and distal in the pelvis. Disadvantages include a high incidence of precarious vascularity, smaller muscle mass with decreased effectiveness in large perineal defects and pelvic dead space, and high susceptibility to vascular spasm and cutaneous skin paddle ischemia. Given the advantages and disadvantages of both types of flaps, it is our preference to use the VRAM flap for perineal reconstruction after APR; however, the gracilis flap is our second choice if the VRAM flap is unavailable, the patient has a scaphoid abdomen that may limit closure of the VRAM donor site, or the defect to the perineum and pelvis is small (Fig. 3).
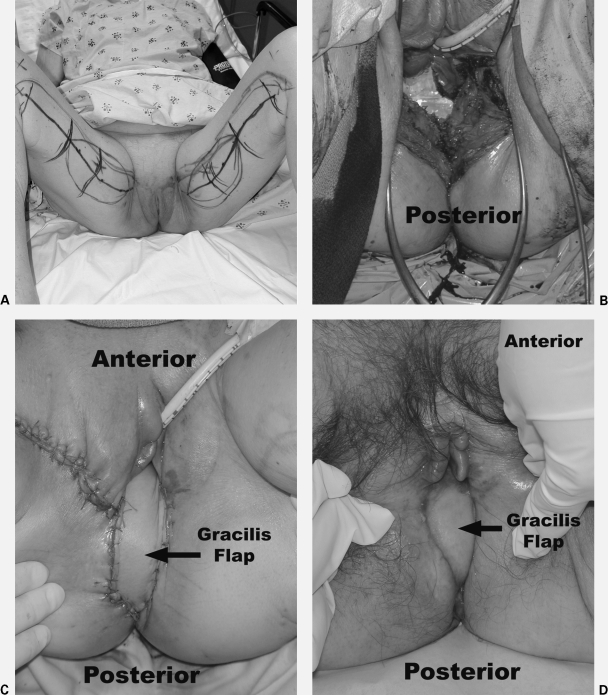
Figure 3.
Gracilis myocutaneous flap reconstruction of the perineum and posterior wall of the vagina in a woman with locally advanced rectal cancer that invaded into the vagina. The patient received neoadjuvant chemoradiation therapy. (A) Pictures show preoperative markings. (B) Intraoperative perineal and vaginal defect. (C) Immediate postoperative flap reconstruction. (D) 3-month follow-up. Photographs courtesy of Susan M. Pike, M.D., Plastic and Reconstructive Surgery, Scott & White University Medical Campus, Round Rock, TX.
The above studies on muscle and myocutaneous flap reconstruction of the perineum after APR have shown impressive results; however, the benefit of flap closure has not been a universal finding. In a recent study examining the risk factors for perineal wound complications following APR, Christian et al19 showed that flap closure of the perineum was a significant predictor of major perineal wound complications (odds ratio = 5.7). However, the majority of flap closures in this study were performed in patients with anal cancer (57%), which is a group that has a higher incidence of wound complications that is likely related to preoperative radiation therapy. Another study examined the use of flap closure to the perineum in salvage therapy for persistent or recurrent anal cancer.6 In this study, primary closure of the perineum was associated with a 70% wound complication rate, and surprisingly, flap closure increased the complication rate to 100%. It is important to note that there were small numbers of patients in this series, there were no flap losses, and major wound complications in the flap group was limited to those that had extensive resection at APR that included the vagina, labia, and groin. Although not statistically significant, Kapoor et al24 reported that compared with nontissue transfer closure of the perineum, tissue transfer flap closure was associated with a higher rate of wound complications (59% versus 40%); however, they also found a lower rate of overall wound failure (9% versus 17%). In this study, patients with tissue transfer closure were more likely to have cancer (95.5% versus 77.1%), have received preoperative XRT (77% versus 36%), and recurrent disease (41% versus 34.3%), which may indicate a selection bias in those receiving tissue transfer flap closure and may account for the increased wound complication rate. With the inclusion of multiple variables such as indication for surgery, preoperative XRT, size of defect created by APR, and selection bias of current studies, it is not surprising that conflicting data exists. Further prospective randomized controlled studies are required to effectively study the use of flap closure in these patients.
MANAGEMENT OF PERINEAL WOUND COMPLICATIONS
Our efforts to understand wound physiology and tissue response after surgery has improved patient outcomes through breakthroughs in sterility, surgical techniques, anesthesia, and use of antibiotics; however, perineal wound complications and failure continue to be a challenging problem after APR. As mentioned previously, prevention of perineal wound breakdown is multifactorial and includes meticulous surgical technique, hemostasis, filling of the pelvis, and use of closed suction drainage. Even when these guidelines are followed, perineal wound complications continues to be a major cause of morbidity after APR. Minor wound complications include superficial skin separation, granulation tissue and chronic perineal sinus. Major wound complications include deep tissue and pelvic abscess and perineal wound dehiscence. The reported rates of perineal wound complication range from 14 to 80%,3,4,5,6 and is related to reported risk factors.19 A thorough evaluation to identify and define the problem is necessary to implement effective treatment for these patients.
Management of perineal wounds in the acute or chronic setting is the focus of much scrutiny and the source of debate. Patients with a perineal wound following APR require a careful history and physical exam. Most patients will complain of perineal drainage, and the character and fluid should be determined. Superficial wounds are common and are managed with routine wound care. The presence of excessive granulation tissue can hinder complete wound healing and may require application of silver nitrate. Patients presenting with fever and pelvic pain should raise the suspicion of a pelvic abscess, which usually occurs in the acute setting. Chronic draining sinus tracts are easily found in the perineum, and may represent a communication to the pelvic dead space. Laboratory values and radiographic imaging are useful and should include a white blood cell count and computed tomography (CT) scan. In the presence of a pelvic abscess, a CT scan is diagnostic and therapeutic as placement of percutaneous drainage catheters is effective. In the presence of pelvic sepsis and abscess, admission and broad spectrum antibiotics are recommended. In patients with perineal sinus tracts, a CT scan may show an incompletely drained fluid collection in the pelvis. In patients with persistent perineal sinus tracts, exam under anesthesia may provide a valuable diagnostic and therapeutic approach through the probing and unroofing of simple tracts, removal of foreign body (i.e., suture material), and the débridement and curettage of devitalized tissue. Tissue biopsy may be beneficial if there is a concern of cancer recurrence. Opening the wound enough to provide adequate drainage and access for wound packing and dressing changes is important when healing by secondary intention is anticipated.
Perineal wound dehiscence is an acute complication and is easily identified during examination. Operative intervention may be required if small bowel evisceration is found. The use of omental pedicle flaps and the uterus to fill the pelvic dead space at time of APR may help to prevent evisceration. When perineal wound dehiscence is found, wound management with wet to dry dressing changes and sharp débridement of devitalized tissue will promote wound healing. Wound failure after 6 months will likely mandate surgical intervention and may require placement of well-vascularized, nonirradiated tissue flap to a large defect, or skin grafting to clean granulating wounds.
Wound Packing
Management of chronic wounds with damp to dry dressings is a well-established and effective means of promoting wound healing. Frequent dressing changes results in serial débridement of the wound, decreases bacterial counts, and wicks excess fluid away from the wound. Normal saline is used for routine wounds; however, ¼ strength Dakin's solution may be used for wounds with excessive exudates, high bacterial counts, and low-grade infection. Over time, the wound begins to heal by secondary intention. In the absence of infection or necrotic tissue, supportive care allows for up to 89% of wounds to heal within 6 months.20
Several adjuvant therapies have been introduced to promote healing in these difficult perineal wounds. These include the addition of hydrotherapy (through pulsed lavage or immersion techniques), enzymatic débridement (papain), growth factors (tissue growth factor β and becaplermin), and subatmospheric pressure dressings. Hydrotherapy can be helpful in initial cleansing of the perineal wound and can promote débridement, but long-term use is not practical and not routinely used in the outpatient setting at our institution. Enzymatic débridement and growth factor therapies are used to promote wound healing; however, no study to date has examined the effects on management of the perineal wound. Indirect evidence may suggest a benefit. The use of recombinant human platelet-derived growth factor-BB has been reported to promote rapid healing in chronic dehisced pilonidal cystectomy wounds,35 skin ulcerations associated with perineal hemangiomas of infancy,36 and chronic neck wounds following radiation therapy.37 Enzymatic products containing papain are routinely used for the débridement of infected wounds and chronic skin ulcers, and may play a role in the management of perineal wound complications. It has been our experience that the addition of topical enzymatic preparations to perineal wounds is not effective, and therefore has been abandoned in our practice. However, we recognize that our practice is not based on experimental evidence, and feel that further prospective randomized studies are necessary to determine the effects of enzymatic débridement and growth factors as adjuvant therapy to promote healing of the perineal wound, especially in the setting of preoperative radiation therapy.
Vacuum-Assisted Closure
The vacuum-assisted closure (VAC) device was introduced in 1995, and has been used to accelerate the wound-healing process by secondary intention.38,39 The VAC consists of a medical grade, Federal Drug Administration approved, polyurethane ether foam that is applied to the wound. A noncollapsible, fenestrated evacuation tube is embedded in the foam and exits the wound site parallel to the skin. The foam is cut to fit into the wound and is covered with several layers of transparent adhesive film to create a closed system. Suction is then applied using a vacuum canister to provide subatmospheric wound pressure of 125 mm Hg. The VAC is changed every 48 to 72 h depending upon need. Pain with dressing changes is mild to moderate and tends to subside within 30 min of replacing the device. The portability of the VAC system allows for outpatient management of chronic wounds. Multiple clinical studies on chronic wounds have shown that VAC therapy increases rate of granulation tissue formation, decreases wound volume, and results in a significant cost savings over standard therapy.39,40
The mechanisms by which VAC therapy improves wound healing has been an area of active investigation. Recently, it has been shown that the VAC device stimulates angiogenesis41 and collagen deposition,42 two important events in wound healing. Increased vascularity promotes cell migration, proliferation and collagen deposition, and is a critical component of granulation tissue formation. Fluid obtained from chronic wounds contains a high amount of inflammatory promoters, matrix metalloproteinases, and protease inhibitors that have a negative effect on wound healing. The VAC effectively removes excess wound fluid and may reduce levels of these inflammatory mediators improving wound healing. Other benefits of the VAC include effective reduction of wound bacterial counts, increasing oxygen tension in healing wound, and assistance in mechanical approximation of the wound edges.
The use of VAC therapy on perineal wounds following APR has been proposed.6,7 At our institution, we have used VAC therapy for perineal wound breakdown with good results, and anecdotal successful use has been reported elsewhere7; however, there are no studies formally investigating its use or success rates in the setting of APR. This may be a direct result of the anatomic challenges. A tight seal is required to maintain negative pressure in the system. The location of the perineal wound following APR makes it difficult to maintain an adequate seal due to the irregular surfaces surrounding the gluteal folds and perineum. Techniques for a VAC application for sacral decubitus ulcers have been described, and modifications to this technique may improve wound seal and use of the VAC system in the perineum. Effective application of the VAC to the perineum may offer alternative management to the complex perineal wound after APR. Further studies are necessary to establish its use in this setting.
CONCLUSIONS
The perineal wound continues to be a significant challenge after APR. Although we have identified specific risk factors that increase the incidence of perineal wound failure, current strategies to prevent these complications are not perfect. Most patients benefit from leaving the pelvic peritoneum open, primary closure of the perineal wound, and use of closed suction pelvic drains; however, in cases of large perineal wound defects especially in the setting of radiation therapy, primary closure may not be possible. Tissue transfer techniques such as omental pedicle flaps, and VRAM and gracilis flaps can be effective, especially in the setting of preoperative radiation therapy, large perineal defects, and posterior vaginal wall reconstruction. When perineal wound complications occur, local wound management through careful débridement of devitalized tissue, effective drainage of pelvic fluid collections, and wound packing is successful in healing the majority of cases. Alternative measures to local wound care include the use of adjuvant therapies such as hydrotherapy, enzymatic débridement, growth factors, and subatmospheric pressure dressings. Many of these therapies have not been studied in the context of the perineal wound following APR, and further studies are necessary to determine efficacy. Persistent wound failure likely mandates surgical intervention and may require placement of a well-vascularized, nonirradiated tissue flap to a large defect, or skin grafting to clean granulating wounds.
REFERENCES
- 1.Woods J E, Beart R W., Jr Reconstruction of nonhealing perineal wounds with gracilis muscle flaps. Ann Plast Surg. 1983;11:513–516. doi: 10.1097/00000637-198312000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Kressner U, Graf W, Mahteme H, Pahlman L, Glimelius B. Septic complications and prognosis after surgery for rectal cancer. Dis Colon Rectum. 2002;45:316–321. doi: 10.1007/s10350-004-6174-4. [DOI] [PubMed] [Google Scholar]
- 3.Pollard C W, Nivatvongs S, Rojanasakul A, Ilstrup D M. Carcinoma of the rectum: profiles of intraoperative and early postoperative complications. Dis Colon Rectum. 1994;37:866–874. doi: 10.1007/BF02052590. [DOI] [PubMed] [Google Scholar]
- 4.Rothenberger D A, Wong W D. Abdominoperineal resection for adenocarcinoma of the low rectum. World J Surg. 1992;16:478–485. doi: 10.1007/BF02104451. [DOI] [PubMed] [Google Scholar]
- 5.Rosen L, Veiderheimer M, Coller J, Corman M. Mortality, morbidity, and patterns of recurrence after abdominoperineal resection for cancer of the rectum. Dis Colon Rectum. 1982;25:202–208. doi: 10.1007/BF02553101. [DOI] [PubMed] [Google Scholar]
- 6.Papaconstantinou H T, Bullard K M, Rothenberger D A, Madoff R D. Salvage abdominoperineal resection after failed Nigro protocol: modest success, major morbidity. Colorectal Dis. 2006;8:124–129. doi: 10.1111/j.1463-1318.2005.00911.x. [DOI] [PubMed] [Google Scholar]
- 7.Opelka F G. Unhealed perineal wound. Clin Colon Rectal Surg. 2001;14:65–68. [Google Scholar]
- 8.Miles W E. Technique of the radical operation for cancer of the rectum. Br J Surg. 1914;2:292–305. [Google Scholar]
- 9.Galandiuk S, Fazio V W. Postoperative irrigation-suction drainage after pelvic surgery. A prospective randomized trial. Dis Colon Rectum. 1991;34:223–228. doi: 10.1007/BF02090161. [DOI] [PubMed] [Google Scholar]
- 10.Robles Campos R, Garcia Ayllon J, Parrila Paricio P, et al. Management of the perineal wound following abdominoperineal resection: prospective study of three methods. Br J Surg. 1992;79:29–31. doi: 10.1002/bjs.1800790108. [DOI] [PubMed] [Google Scholar]
- 11.Moreaux J, Horiot A, Barrat F, Mabille J. Obliteration of the pelvic space with pedicled omentum after excision of the rectum for cancer. Am J Surg. 1984;148:640–644. doi: 10.1016/0002-9610(84)90342-8. [DOI] [PubMed] [Google Scholar]
- 12.John H, Buchmann P. Improved perineal wound healing with the omental pedicle graft after rectal excision. Int J Colorectal Dis. 1991;6:193–196. doi: 10.1007/BF00341389. [DOI] [PubMed] [Google Scholar]
- 13.De Broux E, Parc Y, Rondelli F, Dehni N, Tiret E, Parc R. Sutured perineal omentoplasty after abdominoperineal resection for adenocarcinoma of the lower rectum. Dis Colon Rectum. 2005;48:476–482. doi: 10.1007/s10350-004-0784-8. [DOI] [PubMed] [Google Scholar]
- 14.Bullard K M, Trudel J L, Baxter N N, Rothenberger D A. Primary perineal wound closure after preoperative radiotherapy and abdominoperineal resection has a high incidence of wound failure. Dis Colon Rectum. 2005;48:438–443. doi: 10.1007/s10350-004-0827-1. [DOI] [PubMed] [Google Scholar]
- 15.Artioukh D Y, Smith R A, Gokul K. Risk factors for impaired healing of the perineal wound after abdominoperineal resection of rectum for carcinoma. Colorectal Dis. 2006;9:362–367. doi: 10.1111/j.1463-1318.2006.01159.x. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson P J, Svensson C, Goldman S, Glimelius B. Salvage abdominoperineal resection in anal epidermoid cancer. Br J Surg. 2002;89:1425–1429. doi: 10.1046/j.1365-2168.2002.02231.x. [DOI] [PubMed] [Google Scholar]
- 17.der Wal B C van, Cleffken B I, Gulec B, Kaufman H S, Choti M A. Results of salvage abdominoperineal resection for recurrent anal carcinoma following combined chemoradiation. J Gastrointest Surg. 2001;5:383–387. doi: 10.1016/s1091-255x(01)80066-4. [DOI] [PubMed] [Google Scholar]
- 18.Stone H B, Coleman C N, Anscher M S, McBride W H. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–536. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 19.Christian C K, Kwaan M R, Betensky R A, Breen E M, Zinner M J, Bleday R. Risk factors for perineal wound complications following abdominoperineal resection. Dis Colon Rectum. 2005;48:43–48. doi: 10.1007/s10350-004-0855-x. [DOI] [PubMed] [Google Scholar]
- 20.Whitlow C B, Opelka F G, Hicks T C, Timmcke A E, Beck D E. Perineal wound complications following proctectomy. Perspect Colon Rectal Surg. 2000;13:61–68. [Google Scholar]
- 21.Shibata D, Hyland W, Busse P, et al. Immediate reconstruction of the perineal wound with gracilis muscle flaps following abdominoperineal resection and intraoperative radiation therapy for recurrent carcinoma of the rectum. Ann Surg Oncol. 1999;6:33–37. doi: 10.1007/s10434-999-0033-4. [DOI] [PubMed] [Google Scholar]
- 22.Tei T M, Stolzeburg T, Buntzen S, Laurberg S, Kjeldsen H. Use of transpelvic rectus abdominis musculocutaneous flap for anal cancer salvage surgery. Br J Surg. 2003;90:575–580. doi: 10.1002/bjs.4073. [DOI] [PubMed] [Google Scholar]
- 23.Bakx R, Lanschot J JB Van, Zoetmulder F AN. Inferiorly based rectus abdominis myocutaneous flaps in surgical oncology: indications, technique, and experience in 37 patients. J Surg Oncol. 2004;85:93–97. doi: 10.1002/jso.20014. [DOI] [PubMed] [Google Scholar]
- 24.Kapoor V, Cole J, Isik F F, Sinanan M, Flum D. Does the use of a flap during abdominoperineal resection decrease pelvic wound morbidity? Am Surg. 2005;71:117–122. [PubMed] [Google Scholar]
- 25.Doherty N S, Griffiths R J, Hakkinen J P, Scampoli D N, Milici A J. Post-capillary venules in the “milky spots” of the greater omentum are the major site of plasma protein and leukocyte extravasation in rodent models of peritonitis. Inflamm Res. 1995;44:169–177. doi: 10.1007/BF01782815. [DOI] [PubMed] [Google Scholar]
- 26.Goldsmith H S, Griffith A L, Kupferman A, Catsimpoolas N. Lipid angiogenic factor from omentum. JAMA. 1984;252:2034–2036. [PubMed] [Google Scholar]
- 27.Ellis H. The aetiology of post-operative abdominal adhesions. An experimental study. Br J Surg. 1962;50:10–16. doi: 10.1002/bjs.18005021904. [DOI] [PubMed] [Google Scholar]
- 28.Logmans A, Schoenmakers C H, Haensel S M, et al. High tissue factor concentration in the omentum, a possible cause of its hemostatic properties. Eur J Clin Invest. 1996;26:82–83. doi: 10.1046/j.1365-2362.1996.107247.x. [DOI] [PubMed] [Google Scholar]
- 29.Hay J M, Fingerhut A, Paquet J C, Flamant Y. Management of the pelvic space with or without omentoplasty after abdominoperineal resection for carcinoma of the rectum: a prospective multicenter study. The French Association for Surgical Research. Eur J Surg. 1997;163:199–206. [PubMed] [Google Scholar]
- 30.Shukla H S, Hughes L E. The rectus abdominis flap for perineal wounds. Ann R Coll Surg Engl. 1984;66:337–339. [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor G I, Corlett R J, Boyd J B. The versatile deep inferior epigastric (inferior rectus abdominis) flap. Br J Plast Surg. 1984;37:330–350. doi: 10.1016/0007-1226(84)90076-6. [DOI] [PubMed] [Google Scholar]
- 32.Sinsel N K, Guelinckx P J. Peculiar indications for the pedicled or free rectus abdominis flap in reconstructive surgery. A review of our experience. Acta Chir Belg. 1995;95:289–296. [PubMed] [Google Scholar]
- 33.Bartholdson L, Hulten L. Repair of persistent perineal sinuses by means of a pedicle flap of musculus gracilis. Case report. Scand J Plast Reconstr Surg. 1975;9:74–76. doi: 10.3109/02844317509022861. [DOI] [PubMed] [Google Scholar]
- 34.Baek S M, Greenstein A, McElhinney A J, Aufses A H., Jr The gracilis myocutaneous flap for persistent perineal sinus after proctocolectomy. Surg Gynecol Obstet. 1981;153:713–716. [PubMed] [Google Scholar]
- 35.Serena T E, Nelson S, Corbran C. Becaplermin treatment of a dehisced pilonidal cystectomy wound results in rapid healing. Chicago, IL: Poster presented at: the American College of Surgeons 86th Annual Clinical Congress; October 22–27, 2000.
- 36.Metz B J, Rubenstein M C, Levy M L, Metry D W. Response of ulcerated perineal hemangiomas of infancy to becaplermin gel, a recombinant human platelet-derived growth factor. Arch Dermatol. 2004;140:867–870. doi: 10.1001/archderm.140.7.867. [DOI] [PubMed] [Google Scholar]
- 37.Hom D B, Manivel J C. Promoting healing with recombinant human platelet-derived growth factor–BB in a previously irradiated problem wound. Laryngoscope. 2003;113:1566–1571. doi: 10.1097/00005537-200309000-00029. [DOI] [PubMed] [Google Scholar]
- 38.Morykwas M J, Argenta L C. Nonsurgical modalities to enhance healing and care of soft tissue wounds. J South Orthop Assoc. 1997;6:279–288. [PubMed] [Google Scholar]
- 39.Argenta L C, Morykwas M J. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38:563–576. [PubMed] [Google Scholar]
- 40.Ferrell B A, Osterweil D, Christenson P. A randomized trial of low air loss beds for treatment of pressure ulcers. JAMA. 1993;269:494–497. [PubMed] [Google Scholar]
- 41.Chen S Z, Li J, Li X Y, Xu L S. Effects of vacuum-assisted closure on wound microcirculation: an experimental study. Asian J Surg. 2005;28(3):211–217. doi: 10.1016/S1015-9584(09)60346-8. [DOI] [PubMed] [Google Scholar]
- 42.Miller Q, Bird E, Bird K, Meschter C, Moulton M J. Effect of subatmospheric pressure on the acute healing wound. Curr Surg. 2004;61:205–208. doi: 10.1016/j.cursur.2003.07.015. [DOI] [PubMed] [Google Scholar]