ABSTRACT
Ostomy creation is a common surgical procedure performed by a variety of surgical specialties. Complications associated with stomas are frequent and run the gamut from technical, mechanical, physiologic, and psychologic. The impact of these complications ranges from simple inconvenience to life threatening. The majority of these complications may not occur for years following creation of the stoma. In this article, the author reviews many of the late complications associated with stomas and options regarding their management.
Keywords: Ostomy, colostomy, ileostomy, urostomy, complication, stricture, prolapse, parastomal hernia
Ostomy creation is a frequently performed surgical procedure. The number of patients living with stomas in the United States is unknown, but estimates range up to 450,000 people, with 120,000 new stomas created each year. Other estimates predict the number of ostomates to increase by 3% per year.1 Average age for all stomates is 68.3 years. And the distribution of procedures is 36.1% colostomy, 32.2% ileostomy, and 31.7% urostomy.1
Indications vary from emergency procedures performed for trauma, intestinal perforation, or operative misadventure to elective permanent stoma creation as part of radical cancer surgery. Although ostomy creation is frequently meant to be temporary, up to 40 to 60% will never be reversed. Many stomas are created to improve quality of life; however, complications related to the stoma often have a significant reduction in quality of life and lead to social isolation.
In this article, we focus on complications related to stomal creation that develop late after creation of the stoma and after the initial period of patient adjustment. Several factors effect the type and frequency of complications including surgical specialty and experience, emergency versus elective creation, preoperative marking by a dedicated enterostomal nurse,2 and patient issues such as patient age, obesity, diabetes, and ability to care for the stoma.3
Late complications are defined as occurring after the period of physiologic adjustment. For most patients this is 6 to 10 weeks. One large series identified 93% of late complications occurred within the first 6 months.4 Other series identified new complications diagnosed up to 15 years after stomal creation. Rates of overall late complications vary from a low of 6%2 to highs exceeding 76% in selected series.5
PARASTOMAL HERNIA
It doesn't matter if God Himself made your ostomy. If you have it long enough you have a 100% risk of a parastomal hernia.
J Byron Gathright
Personal communication to CRS residents
1996
To bring the intestine through the abdominal wall and expect the serosa to heal circumferentially to the fascia has little other precedent in surgery. In almost all other operations, we attempt to recreate some aspect of normal functional anatomy. The job of the surgeon is to minimize the risk of a parastomal hernia, recognize the occurrence, and intervene at the appropriate time in the most effective manner.
In most series parastomal herniation is the most common late complication.2,3,4,5,6
The reported incidence of parastomal hernia varies widely based on type of stoma and time of follow-up. In a series of greater than 1600 patients over 20 years from the Cook County Hospital Stoma registry, the rate of parastomal hernia, 1.18%, was much lower than expected.4 A review of 142 ostomies created for similar indications over 5 years published the previous year found a parastomal hernia rate of 9.3%.6 However, in two long-term actuarial analyses the group at St. Mark's hospital reviewed their experience with 203 end colostomies and 150 end ileostomies over 10 years. The 10-year cumulative rate of parastomal hernia was 36.7% and 16%, respectively.5,7
Many factors are believed to increase the rate of parastomal herniation: age, obesity, perioperative steroid use, and siting of the stoma outside the rectus muscle. However, reports vary as to the exact impact of these risk factors.3,6,7,8,9 Current practice supports placement of stomas within the rectus not only to prevent herniation, but also to facilitate maintenance of the appliance.10
The best method for prevention of complication remains attention to proper surgical technique in creating a well-vascularized, untraumatized, tension-free anastomosis between the end of the intestine and the skin. The trephination in the skin and the fascia should be large enough to pass the bowel easily, without trauma and maintain adequate perfusion and venous drainage. Usually this is the size of two to three of the surgeon's fingers. In a more obese patient a larger channel is required to pass the bulkier end of the bowel and more of it's mesentery through a longer subcutaneous distance. There are many studies touting laparoscopic techniques as safe and perhaps superior to laparotomy for creation of fecal diversion. Laparoscopic techniques for creation of ostomies are not effective in prevention of hernia.11,12 Fascial fixation and creation of an extraperitoneal path for the stoma may reduce the risk of herniation, but a statistically significant advantage has not been shown.9
Prosthetic mesh has been used at the initial creation of the stoma in an attempt to prevent parastomal hernia. The safety of the use of synthetic mesh in the presence of open bowel has been demonstrated.13 Several techniques have been described including extrafascial,14 preperitoneal,15 and subfascial16 placement of the mesh. Two randomized, controlled trials have demonstrated the safety and effectiveness of prophylactic placement of mesh at the time of initial stomal creation.16,17
Repair of Parastomal Hernia
Simple presence of a parastomal hernia does not necessitate repair. In otherwise asymptomatic patients, with large fascial defects the risks of incarceration, strangulation, and obstruction are low.9,10 Patients with mild discomfort and cosmetic concerns are well served with abdominal support or custom-created girdles. Indications for repair include acute or intermittent incarceration with strangulation or obstruction, chronic pain, or difficulty maintaining a seal on the appliance.
LOCAL REPAIR
Reduction or resection of the hernia sac with local repair of the muscular defect is an option in patients with very limited reserve. However because the tissues are often very attenuated expected recurrence range from 50 to 100%.9,10 Local repair can be supported with the use of prosthetic, or biosynthetic material. Rosen described mesh-supported local repair in 197718 with subsequent modifications.19,20,21 Overall success with mesh-supported local repair is ~88%. Infectious complications are rare with 11 cases reported in 22 published series, with one case of erosion into the bowel and 4 patients requiring removal of the mesh.21
TRANSLOCATION
The most definitive solution to almost all stomal complications is to resect the ostomy and create a new one in a fresh location with native virgin tissue. Conversely, this is often the most invasive and morbid alternative as well. Success is not guaranteed. In fact, recurrent herniation in the new location has been reported as high as 68%.22 Tissue surrounding any hernia is frequently attenuated, and previously operated tissue has a higher rate of failure. Therefore, if the patient will tolerate a laparotomy the preferred procedure is to either move the stoma to the opposite side or move it cephalad. In the latter case, it is essential that an adequate distance lie between the fascial openings and blood supply to the rectus is preserved. Otherwise, a simply larger and more complex hernia is created. Translocation of a stoma is an elective stoma creation and should have appropriate preoperative assessment including evaluation and marking by an enterostomal therapist if available. Translocation without formal laparotomy has been described.23,24,25 However, these techniques offer limited visualization and may be limited due to intraabdominal adhesions and inability to achieve adequate mobilization. If chosen for a patient with limited reserve, they may still require conversion to laparotomy. Laparoscopy represents a more modern approach to less invasive intervention.
LAPAROSCOPIC REPAIR WITH PROSTHETIC MESH
Laparoscopy has enjoyed increasing popularity in the recent past and has replaced several conventional “open” surgical procedures. Several authors have investigated the role of laparoscopy for stomal hernia repair. The first report of laparoscopic repair of a parastomal hernia was published by Porcheron et al26who described a combination laparoscopic fascial closure followed by mesh placement. Since then, several techniques have been described. These include placement of mesh with a slit and a central aperture for the bowel,27,28,29 the use of double layers of overlapping meshes,29 and the “onlay” technique where mesh is placed directly over the bowel without a slit or aperture.30,31 The recurrence rates for laparoscopic repair have been reported to be between 4 and 44%.28,29,31 LeBlanc et al29 published their experience with 12 patients with mesh onlay without slit and mesh with a slit and central aperture. They opined that the onlay technique appeared to be superior to using mesh with slit and central aperture. The largest reported series of laparoscopic stomal hernia repair was a multiinstitutional review of 25 patients with a median follow-up of 19 months. The authors reported a 4% recurrence rate.31
Polytetrafluoroethylene (PTFE) appears to be the most suitable synthetic material as it is composed of a softer material and results in a less intense inflammatory reaction. These traits may result in fewer incidences of bowel obstruction and erosion into adjacent bowel. To create a more durable repair, the mesh should be of sufficient size to overlap the fascial defect by 3 to 5 cm.27,28
In light of the available literature, laparoscopic stomal hernia repair appears to be an attractive option; however, most studies report short- to intermediate-term results and long-term results with this emerging technique are still awaited.
SUBCUTANEOUS PROLAPSE (PSEUDOHERNIA)
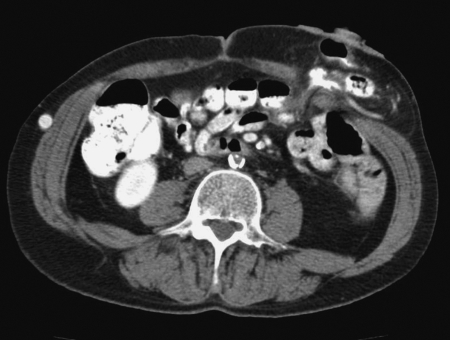
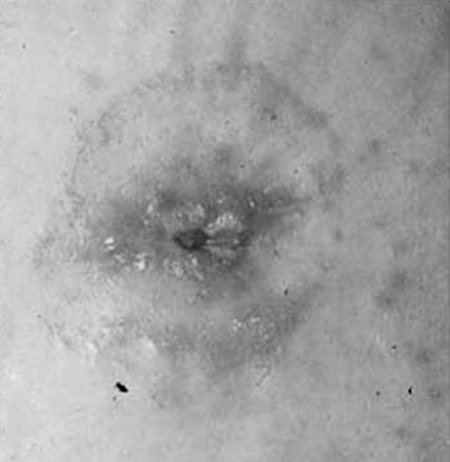
Subcutaneous prolapse is an entity that is somewhere between a parastomal hernia and a stomal prolapse. Externally the patient appears to have a parastomal hernia with a mass abutting a normal or retracted os. The patient may have the same pouching problems as a parastomal hernia. Often, there are few visceral complaints. Nausea, cramps, and abdominal distention are more consistent with incarceration of a separate loop of bowel. Instead, there may be more local discomfort near the stoma, and the patient will have a sense of obstruction at the stoma, much like a stomal prolapse. There may not be a protrusion of the peritoneum through the fascia into the subcutaneous tissue. Instead, the bowel moves directly outward and coils into the extrafascial soft tissues, the equivalent of a “sliding” esophageal hiatal hernia. It is the author's belief this may be associated with irrigation of the stoma, or other manipulation, either therapeutic or self-abusive. The diagnosis will not be made if one does not consider it in advance: “What the mind does not know the eye does not see.”32 A subcutaneous prolapse may be suspected on digitalization of the stoma. The examining finger encounters a convoluted path to the fascial defect or capacious bowel in the subcutaneous space. A computed tomography (CT) scan can confirm the diagnosis (Fig. 1).
Figure 1.
Subcutaneous prolapse of an end sigmoid colostomy.
Making the diagnosis is essential if operative repair is planned. Repair of a subcutaneous prolapse is much like repair of a conventional prolapse. The mucocutaneous junction is separated and the bowel is prolapsed to a comfortable length if it is fixed to the fascia. If the bowel is freely passing through the fascia, the bowel can be returned to the abdomen. Sero-muscular sutures are carefully placed to the fascia, with care not to enter the lumen. The end is then rematured in the usual fashion. If a parastomal hernia exists in conjunction with a subcutaneous prolapse, a mesh repair is performed or the stomal is translocated.
STOMAL PROLAPSE
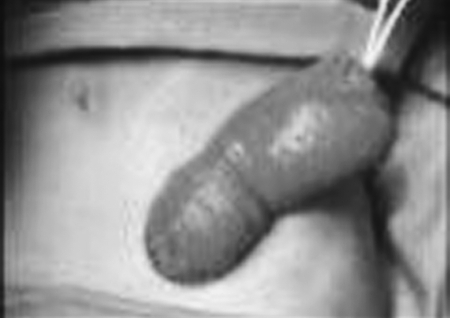
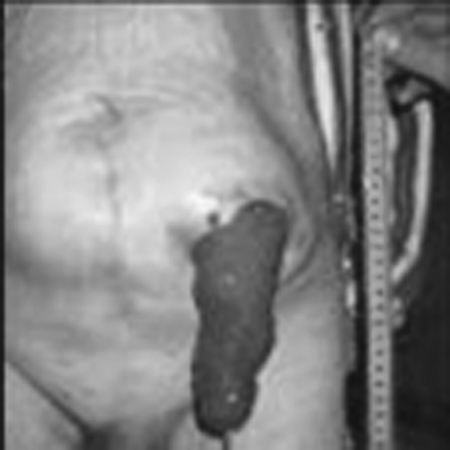
Prolapse is one the more common late complications after stoma creation. The estimated incidence is reported to be between 2 to 26%.3,4,8,33,34 The high variability among rates can be attributed to location in bowel (ileostomy, transverse, or sigmoid colostomy), creation technique (loop vs end stoma), and disease process (benign, malignant, inflammatory), as well as emergent or elective creation. Despite the collected data presented, clear associations are difficult to make. Transverse loop colostomies are believed to carry the highest risk of prolapse, with a rate approaching 30%.10 Nevertheless, prolapse can be seen with any type of stoma (Figs. 2, 3). Usually, the prolapsed segment involves the distal limb of loop colostomy. Some authors believe peritoneal fixation of the stoma mesentery results in decreased incidence of prolapse,35 whereas others have shown that the incidence of prolapse has not been affected by this maneuver.6 Furthermore, adhesions resulting from mesenteric fixation may make the subsequent stoma reversal technically more difficult. It has been suggested that fixation of bowel wall to fascial edges might result in decreasing the risk of a later prolapse.36 Although often functionally benign, stomal prolapse can induce significant emotional distress in many patients. A significantly prolapsed stoma may have no pain, obstruction, or hygiene problem. However, the patient presents for attention due to the obvious abnormal appearance including a visible mass under the clothing. It may however, interfere with appliance fitting and the resultant skin irritation. Prolonged mucosal exposure and trauma may result in ulceration and bleeding. Most often, the prolapsed segment can be reduced manually with little effort—with incarceration being an unusual event. In cases where edema of prolapsed segment makes manual reduction difficult, local osmotic therapy with topical household sugar may aid in reducing edema and facilitate reduction of prolapsed stoma.
Figure 2.
Prolapsed sigmoid loop colostomy.
Figure 3.
Prolapsed end sigmoid colostomy.
Several treatment options exist for a prolapsed stoma and are dependant upon whether the stoma is temporary or permanent. Prolapse in a temporary stoma can be managed expectantly until the patient is ready for reversal. In cases of prolapse in permanent stomas, surgical correction is recommended to facilitate better stoma device fitting and avoid complications such as ulceration and strangulation.
Stomal prolapse can be treated with local surgical procedures and laparotomy can be avoided in the majority of the cases. A procedure similar to an Altemeier perineal proctectomy is easily performed. A full-thickness circumferential incision is made on the bowel 1 cm from the mucocutaneous junction. The prolapsed bowel is then excised and an anastomosis is performed between the distal end of the intestine and the mucosal edge. The incision at the mucosa near the mucocutaneous junction rather than the skin not only affords a technically easier anastomosis, but also prevents enlargement of the skin opening.37 Abulafi et al38 described an adaptation of Delorme's technique to treat mucosal prolapse. This procedure involves an incision at the mucosa near the mucocutaneous junction followed by excision of the redundant mucosa and plication of the muscular wall. Recently, a few reports have described the use of a linear stapler to amputate the prolapsed segment. This technique can be performed under sedation without need for general anesthesia.36,39 Stomal prolapse may occur in conjunction with a parastomal hernia. The hernia is likely to be the more pressing issue, and often surgical repair of the prolapse requires reduction and repair of the hernia regardless of symptoms. We believe the choice of hernia repair supersedes repair of the prolapse. If technically feasible and tolerable to the patients, we advocate translocation in this situation.
RETRACTION
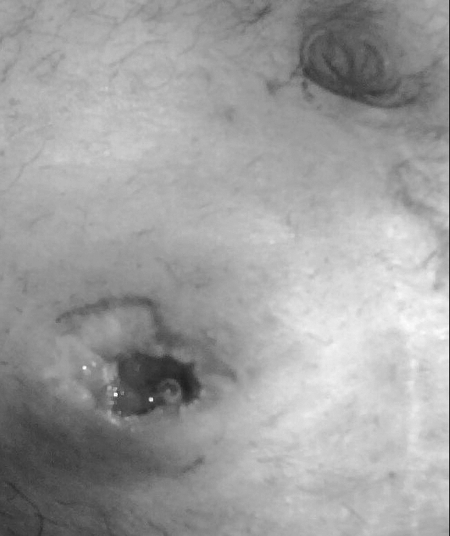
Retraction, although mostly seen acutely after stoma creation, may also occur in chronic stomas. The rate of late retraction is quite variable as expected. Some authors report rates as low as 1%.4 However, series range up to 6% for colostomies and 3 to 17% for ileostomies.10 Simple benign causes are weight gain after stoma formation or a short length of the exteriorized segment (Fig. 4). It is more commonly seen in obese patients where the thickness of the abdominal wall makes exteriorization difficult.3 Depending on the reason for creation of the stoma, a work-up for recurrent Crohn's disease, a malignancy, or ischemia should be undertaken prior to surgical revision. Retraction results in poor appliance fit and soilage. In symptomatic cases, the use of a convex stoma appliance may result in decreased soilage. Definitive treatment usually entails stoma revision with exteriorization of a segment of sufficient length. Local revision with mobilization of the proximal bowel through the stomal incision is possible. However, this requires increasing the size of the skin opening. The terminal ileum can be more easily mobilized for length. Left-sided colostomies often require mobilization of the splenic flexure. If local revision is proposed the patient must be prepared and expected to tolerate full laparotomy. In the majority of cases, laparotomy is the planned approach. If skin integrity is not too severely compromised, the stoma may be recreated using the same location. Stomal revision techniques are the same as for the initial creation, ensuring adequate length and blood supply. In cases where adequate length is hampered by the blood supply, some authors advocate creating an end-loop stoma in which a point 3 to 5 cm proximal to the end of bowel is matured as the os. The distal end is folded in the subcutaneous tissue or left below the fascia.10,40,41
Figure 4.
Retracted ileostomy.
STOMAL STENOSIS AND STRICTURE
Ischemia is the usual underlying factor in stomal stenosis. This may be apparent acutely immediately after the stomal creation, or may not manifest for months if necrosis is not present. Infection and retraction of stoma may also lead to stenosis (Fig. 5).41 The reported incidence is 2 to 14%.4,33,42 As part of the evaluation, recurrent malignancy or Crohn's disease must be ruled out. Stenosis in the subcutaneous aspect is usually treated with dilation initially; however, multiple sessions are usually required and tissue trauma during mechanical dilation invokes fibrosis which, in turn, results in further stenosis. Definitive treatment requires stoma revision in most cases. Damage to the ileum with the everting stitches may create a “Bishop's collar” deformity. Skin-level stricture may be fixed with a local procedure.10 A double “Z-plasty” can be used to enlarge the skin opening. An adequate length of bowel is required to recreate the stoma. This procedure is more complex and may create a convex deformity.
Figure 5.
Stricture of end sigmoid colostomy.
Colostomy stricture differs in some ways from an ileostomy stricture. The causes are the same; however, local infection and inadequate skin opening may also create the complication. If significant skin complications do not occur, a strictured colostomy can be followed expectantly and treated with dietary modification. Patients can be instructed to irrigate with a cone catheter. In one series, 6 of 10 patients were able to avoid stomal revision.43 In one series, 10 of 203 patients with colostomies developed strictures over 5 years, 50% in the first year. Two of these 10 required local revision. Two others required laparotomy and translocation. The remaining 6 did not require surgical intervention. In another series, 5% required translocation.7
OBSTRUCTION
In addition to adhesive bowel obstructions that all patients with prior surgery can suffer, ostomates may have obstruction at the ostomy itself. The more common causes have already been discussed, parastomal hernia, stricture and stenosis, recurrent Crohn's disease or malignancy, as well as internal hernia or volvulus around the terminal bowel internally. An additional source of obstructive symptoms is food bolus obstruction. In cases where adhesive bowel obstruction is suspected, decompressed bowel may be visualized distally on radiologic studies. In this case, standard management should be undertaken based on severity and length of obstruction.
In the case of food bolus obstruction, intestinal distention will extend near to the stoma. Cases are often preceded by a history of a meal of high residual foods such as poorly chewed nuts, large volumes of popcorn, fibrous vegetables such as broccoli or celery, unpeeled fruit or mushrooms. The first event often occurs 3 to 6 months after the creation of an ileostomy. If the diagnosis is suspected but unclear, an initial work-up with a water-soluble contrast enema via the stoma will be diagnostic and often therapeutic. If a food bolus is not identified, contrast can often be refluxed to a transition point. Subsequent events can be managed with gentle irrigation with water or saline. Patients may require mild anesthesia for comfort and to provide relaxation of the abdominal wall. Surgery is indicated if other significant pathology is identified or the obstruction is not relieved.
STOMAL BLEEDING/PERISTOMAL VARICES
Patient presenting with stomal bleeding may have a source anywhere along the gastrointestinal tract proximal to the stoma and should be dealt with in a similar manner as any other patient with gastrointestinal bleeding. We will confine this discussion to the rare occasion of stomal bleeding arising locally from the stoma site. Local trauma is a frequent cause of visible bleeding from the mucosa of the stoma. Isolated minor trauma-related bleeding will stop with local pressure and time. Significant injury may require evaluation in the operating room. Recurrent local injury is often related to poor pouching techniques, with a stiff appliance encroaching on the mucosa. The result can be exuberant granulation tissue that frequently bleeds and may have the gross appearance of recurrent Crohn's disease. Once recurrent inflammatory bowel disease (IBD) is ruled out, the granulation tissue is treated with local destruction by silver nitrate or judicious electrocautery. The source of the trauma needs to be identified and corrected.
Portal hypertension results in mucosal venous congestion, which can bleed profusely. Furthermore, stomas are a recognized ectopic site for variceal development in patients with portal hypertension. Collateral formation between the mucosal vessels and the peristomal cutaneous vessels results in portosystemic connection at the mucocutaneous junction. Paracolostomy varices were first described in 1968 by Resnick et al.44 In the next year, Eade et al45 described parastomal varices associated with an ileostomy. The median time interval between stoma creation and bleeding was reported to be between 20 and 36 months.46 The overall range of onset has been reported to be from 2 months to 29 years.47 Stomal variceal bleeding is most commonly seen in patients with stomas resulting from IBD with underlying sclerosing cholangitis. However, portal hypertension induced by any etiology can result in peristomal variceal development. Bleeding from these varices is usually painless, profound, and recurrent.48 The majority of these patients also have underlying esophageal varices,46 and it is imperative to rule out esophageal variceal bleeding in a patient with cirrhosis presenting with history of passing blood from the stoma. The majority of cases of peristomal varices can be easily identified on physical exam by a bluish hue in the peristomal skin and presence of caput medusae in the peristomal area.49
The first therapeutic maneuver for an acute episode of bleeding is the application of local pressure. Gauze soaked in dilute solution of epinephrine may also be used as an adjunct to local pressure. Other local procedures include suture ligation of bleeding varix and sclerotherapy.50 Local procedures universally yield short-term success. Rebleeding is the rule rather than the exception. Surgical procedures include mucocutaneous disconnection and stoma relocation. Mucocutaneous disconnection includes the division of portosystemic circulation surrounding the stoma by incising the mucocutaneous junction and continuing dissection along the bowel wall to the level of fascia.51 The cut end of bowel is then resutured to the deep dermal layer. Percutaneous embolization of the variceal vessels is another therapeutic option.52,53 The procedures described so far result in short-term success because mucocutaneous collaterals reform. Definitive therapy involves treatment of the underlying portal hypertension. This can be achieved by either transjugular intrahepatic porto-systemic shunt (TIPS) or liver transplantation53,54,55 and carries the lowest rebleeding rate.51 The need for these procedures, however, is governed not only by the presence of bleeding stomal varices, but also by the presence and severity of esophageal varices as well as the patient's overall condition. Because mortality in these patients is related to the underlying liver disease rather than bleeding from stomal varices, we recommend that the initial approach should be local procedures and TIPS should be reserved for severe, recurrent bleeding, or for patients suffering from concurrent bleeding esophageal varices.
PARASTOMAL SKIN CONDITIONS
Pyoderma Gangrenosum
Peristomal ulceration can occur late after the creation of an ostomy, and may be attributable to many causes. Pyoderma gangrenosum (PG) is a specific condition frequently associated with IBD. PG was first described associated with Crohn's disease in 197056 and as a peristomal complication associated with Crohn's disease in 1984.57 PG is a painful ulcerating condition seen in ~2% of IBD patients.58 It may occur with other conditions and as a peristomal condition may have no other predisposing diagnosis.59 Beginning as small erythematous pustules or papules, rapid coalescence results in superficial ulceration, surrounding induration and undermining at the edges. The sharply demarcated violaceous edges with bright erythematous margins give the classic “cookie cutter” appearance. This allows the diagnosis to be based on physical appearance alone in more than 80% of cases.60
The best treatment of peristomal PG has not been determined due to conflicting results from the few series that have been published. Available options include local débridement and unroofing of undermining ulcers combined with intralesional injection of steroids (Kenalog 40 mg/mL; Bristol Myers Squibb, Princeton, NJ). It is widely held that the onset of PG is related to disease activity in the bowel distant from the stoma. Treatment of PG would then be related to systemic treatment of the underlying disease. However, in one of the larger series of 16 patients with peristomal PG, there was no discernable correlation between incidents with or without IBD and active or quiescent disease.59 In the same series, 2 of 16 patients had complete response to local treatment alone. The remaining 14 of the 16 patients received systemic medical therapy in conjunction with local treatment. Response varied from none, partial, and complete rsponse to treatment. Five of the 14 patients were treated with infliximab. Of these, 4 patients had some response each with a varied combination of therapies. Seven of the 16 patients underwent stomal relocation due to partial or no response. Five of the 7 patients had Crohn's disease, resolution was seen in 3 and recurrence in 2 paients. Overall final complete response with all therapies in these 16 patients was 87%. Because no one therapy is clearly curative, all are considered viable alternatives.
Peristomal Dermatitis
Peristomal dermatitis is the result of an irritation, inflammation, and breakdown of the skin, usually seen associated with ileostomies. It is a reaction of the skin to irritating bowel contents. Because colostomy output is more formed and contains less bile acid, it is less irritating and is in less contact with the skin. Therefore, dermatitis is much less commonly associated with colostomies. Significant episodes of peri-ileostomy dermatitis occur in 5 to 25% of patients.10 Cumulative long-term risk is 34%.5 Initial evaluation and management should address the quality of the patients pouching technique and choice of appliance. Often simple reteaching or an alternative appliance will resolve the issue (see the Alteration in Quality of Life section below). If pouching problems are related to a retracted or poorly formed stoma and a solution cannot be achieved with specially shaped appliances, adhesive seals, or the creative application of stoma paste, surgical revision is indicated.
Candidal Infection
Candida albicans is part of normal intestinal flora. However, overgrowth in the peristomal skin is the most common infection. This is thought to be due to the breakdown in the normal skin barrier due to use of antibiotics, extensive cleansing with solvents to remove appliance components, contact dermatitis as described above, and the warm moist environment found under the appliance. The appearance can be similar to contact dermatitis, but often is more raised and the edges will demonstrate well-circumscribed papules and pustules, or satellite lesions. Treatment is achieved with the application of antifungal powder prior to placement of the appliance. Antifungal creams may also be effective, but they often interfere with adherence of the appliance and must dry fully prior to placement.
Allergic Dermatitis
Many surgical patients manifest a host of allergic reactions to the solvents and adhesives that are dressing tape components, to skin barriers, and to any medical material that comes into contact with the skin. Contact allergies can range from mild erythema and itching to profound skin breakdown, blistering, burning, and pain. The hallmark of allergic dermatitis is that the skin changes are limited to, but fully extend to the extent of contact with the allergen (i.e., creating a perfect reddened area to match the size and shape of the stoma appliance). Because there are many components to a complete stomal appliance system, identification of the offending substance may require systematic removal or replacement of each component in turn. Alternatively, a patch test can be performed by applying the most suspected component to the skin distant to the stoma and maintaining covered contact for at least 2 days. Treatment is simple removal of the allergen. Symptomatic relief can be achieved with topical steroid creams and oral antihistamines.
DIVERSION COLITIS
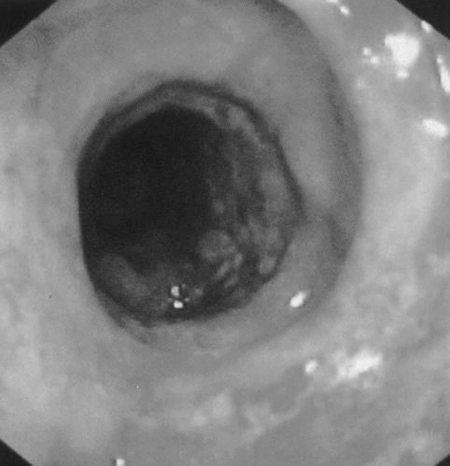
Diversion colitis, or starvation colitis occurs in the retained colon downstream from a completely diverting ileostomy or colostomy. An inflammatory process occurs in the previously normal diverted segment in patients without an idiopathic or infectious colitis. In 1981, in the seemingly first report of 10 cases of this condition, 9 of the patients were asymptomatic. One complained of “mucoid rectal discharge.” Two subsequently developed symptoms.61 However, the pathologic findings had been described in 1972.62 It is now recognized that some aspect of diversion colitis is very common, occurring to some degree in over 90% of patients with a diverted segment. Severity of diversion colitis varies from mild in 52% of patients to moderate in 44% to severe in 4% of patients.63 Many patients remain asymptomatic and symptoms may not correlate with endoscopic appearance. The most common complaint is bleeding from the rectum, with or without mucus discharge. Additional symptoms may include tenesmus and abdominal pain.64 Endoscopically, the mucosa appears very similar to ulcerative colitis, with an erythematous granular appearance (Fig. 6). Endoscopy with biopsy is important to rule out infectious and antibiotic-associated colitis, IBD, and malignancy. The pathophysiology is believed to result from a deficiency in short-chain fatty acids, in particular n-butyrate, propionate, and acetate. Normally, these nutrients are provided to the colon by bacterial breakdown of dietary carbohydrates. As such, treatment includes administration of short-chain fatty acids in enema form twice daily for 2 to 4 weeks with varied results.16,65,66 Additional treatments include 5-ASA enemas or suppositories. If possible, the definitive treatment is reestablishment of intestinal continuity. Asymptomatic patients do not require any intervention.
Figure 6.
Severe diversion colitis.
ALTERATION IN QUALITY OF LIFE
Typically, surgeons view complications as unexpected outcomes related to failures of surgical technique, impaired patient healing, or infection. However, despite an excellent technical outcome, there is often significant negative impact on the patient's quality of life (QOL), which can be viewed as a late stomal complication.
The impact to the patient is viewed relative to another state (e.g., life prior to surgery, life with incontinence, life with a restorative anastomosis.) In a specific population of 45 patients with colonic dysmotility secondary to spinal cord injury, creation of a stoma improved quality of life in the significant majority. Ileostomies, right- and left-sided colostomies were created and in all groups there was a statistically significant improvement in QOL scores; the majority would have preferred to have had their diversion sooner.67 Viewing a stoma as an alternative to fecal incontinence, the Cleveland Clinic Florida (Weston, FL), performed a cross-sectional survey of 39 colostomates and 71 fecal incontinence patients using the fecal incontinence quality of life (FIQOL) tool. Results showed statistically significantly better results only for coping and embarrassment. There were no major differences in perceived state of health, physical, emotional or mental well being. The authors concluded that a well-created stoma is a viable alternative to incontinence.68 An alternative view is that even a well-made stoma is only moderately better than incontinence.
Other surveys evaluating QOL have found significant negative impact with less dramatic results. In a mail questionnaire in the UK, 391 of 542 eligible individuals with stomas responded. Greater than 50% reported that permanent ileostomy or colostomy had little or no effect on their work or ability to get work. Eight percent of those with colostomies and 11% of those with ileostomies reported a complete change. Travel was affected in ~22%. Overall impact on lifestyle varied widely on a visual analog scale. A colostomy has an average 40% impact on lifestyle (a range of 10 to 70% lifestyle impact). Similarly, ileostomy had a 50% impact on lifestyle (20 to 80%). In both groups, 17% reported a greater than 90% impact on lifestyle.69
Given the impact that having a stoma has on QOL, postsurgical care and education by a dedicated enterostomal therapist can offer significant improvement. It has been shown that preoperative marking and education by an enterostomal therapist is of benefit for postoperative outcome and QOL.70 Many patients are given immediate instruction in stoma care prior to discharge, with one or two postoperative visits as needed to initially become competent. However, in a prospective evaluation of 43 patients with well-constructed, well-functioning, uncomplicated end ileostomies and colostomies, there was a significant positive impact of late postsurgical evaluation and teaching. None of these patients had previously seen an enterostomal therapist and all were at least 3 months postoperative. Patients were offered two QOL questionnaires before and 3 months after a course of enterostomal care, which included visits at time 0, 1 week, and invitation to return at 1, 3, and 6 months. The authors found significant improvement in every area studied, including travel, sports, dressing issues, skin irritation, odor, and sexual issues.71
Sexual dysfunction remains a separate and daunting issue following stomal creation, even if patients are able to overcome the other lifestyle changes associated with their stoma. Sexual function and intimacy are difficult to assess in the postoperative patient as much of the dysfunction may be due to neurologic changes associated with pelvic surgery. However, sexual limitations are higher than expected for pelvic dissection without stoma creation. Out of a general QOL survey 44% of patients neglected to answer sexually related questions; of those who answered, 43% had postoperative sexual problems. Impotence ranged from 17% for colostomies to 53% (14 of 25) for ileostomies.69 Other sources of sexual dysfunction include body image, pain, and fear of leakage. If the surgeon or the enterostomal therapist is sensitive to these issues and has the courage to address these embarrassing questions, many of these patients can be directed to appropriate counseling and see improvement in all aspects of their quality of life.
REFERENCES
- 1.Turnbull G B. Ostomy statistics: the $64,000 question. Ostomy Wound Manage. 2003;49(6):22–23. [PubMed] [Google Scholar]
- 2.Bass E M, Del Pino A, Tan A, Pearl R K, Orsay C P, Abcarian H. Does preoperative stoma marking and education by the enterostomal therapist affect outcome? Dis Colon Rectum. 1997;40(4):440–442. doi: 10.1007/BF02258389. [DOI] [PubMed] [Google Scholar]
- 3.Arumugam P J, Bevan L, MacDonald L, et al. A prospective audit of stomas-analysis of risk factors and complication and their management. Colorectal Dis. 2003;5:49–52. doi: 10.1046/j.1463-1318.2003.00403.x. [DOI] [PubMed] [Google Scholar]
- 4.Park J J, Del Peno A, Orsay C P, et al. Stomal Complications: the Cook County experience. Dis Colon Rectum. 1999;42:1575–1580. doi: 10.1007/BF02236210. [DOI] [PubMed] [Google Scholar]
- 5.Leong A PK, Londono-Schimmer E E, Phillips R KS. Lifetable analysis of stomal complications following ileostomy. Br J Surg. 1994;81:727–729. doi: 10.1002/bjs.1800810536. [DOI] [PubMed] [Google Scholar]
- 6.Porter J A, Salvati E P, Rubin R J, Eisenstat T E. Complications of Colostomies. Dis Colon Rectum. 1989;32:299–303. doi: 10.1007/BF02553484. [DOI] [PubMed] [Google Scholar]
- 7.Londono-Schimmer E E, Leong A PK, Phillips R KS. Lifetable analysis of stomal complications following colostomy. Dis Colon Rectum. 1994;37:916–920. doi: 10.1007/BF02052598. [DOI] [PubMed] [Google Scholar]
- 8.Duchesne J C, Wang Y Z, Weintraub S L, Boyle M, Hunt J P. Stoma complications: a multivariate analysis. Am Surg. 2002;68(11):961–966. [PubMed] [Google Scholar]
- 9.Carne P W, Robertson G M, Frizelle F A. Parastomal hernia. Br J Surgery. 2003;90(7):784–793. doi: 10.1002/bjs.4220. [DOI] [PubMed] [Google Scholar]
- 10.Shellito P C. Complications of abdominal stomal surgery. Dis Colon Rectum. 1998;41:1562–1572. doi: 10.1007/BF02237308. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig K A, Milsom J W, Garcia–Ruiz A, Fazio V W. Laparoscopic techniques for fecal diversion. Dis Colon Rectum. 1996;39:285–288. doi: 10.1007/BF02049469. [DOI] [PubMed] [Google Scholar]
- 12.Carne P W, Frye J N, Robertson G M, Frizelle F A. Parastomal hernia following minimally invasive stoma formation. ANZ J Surg. 2003;73(10):843–845. doi: 10.1046/j.1445-2197.2003.02779.x. [DOI] [PubMed] [Google Scholar]
- 13.Geisler D J, Reilly J C, Vaughn S G, Glennon E J, Kondylis P D. Safety and outcome of use of nonabsorbable mesh for the repair of fascial defects in the presence of open bowel. Dis Colon Rectum. 2003;46:1118–1123. doi: 10.1007/s10350-004-7290-x. [DOI] [PubMed] [Google Scholar]
- 14.Gogenur I, Mortensen J, Harvald T, Rosenberg J, Fischer A. Prevention of parastomal hernia by placement of a polypropylene mesh at the primary operation. Dis Colon Rectum. 2006;49:1131–1135. doi: 10.1007/s10350-006-0615-1. [DOI] [PubMed] [Google Scholar]
- 15.Marimuthu K, Vijayasekar C, Ghosh D, Mathew G. Prevention of parastomal hernia using preperitoneal mesh: a prospective observational study. Colorectal Dis. 2006;8:672–675. doi: 10.1111/j.1463-1318.2006.00996.x. [DOI] [PubMed] [Google Scholar]
- 16.Janes A, Cengiz Y, Israelsson L A. Preventing parastomal hernia with a prosthetic mesh; a randomised study. Arch Surg. 2004;139:1356–1358. doi: 10.1001/archsurg.139.12.1356. [DOI] [PubMed] [Google Scholar]
- 17.Janes A, Cengiz Y, Israelsson L A. Ramdomised clinical trial of the use of a prosthetic mesh to prevent parastomal hernia. Br J Surgery. 2004;91:280–282. doi: 10.1002/bjs.4417. [DOI] [PubMed] [Google Scholar]
- 18.Rosin J D, Bonardi R A. Paracolostomy hernia repair with Marlex mesh: a new technique. Dis Colon Rectum. 1977;20:299–302. doi: 10.1007/BF02586428. [DOI] [PubMed] [Google Scholar]
- 19.Leslie D. The parastomal hernia. Aust N Z J Surg. 1981;51:485–486. doi: 10.1111/j.1445-2197.1981.tb05991.x. [DOI] [PubMed] [Google Scholar]
- 20.Moisidis E, Curiskis J I, Brooke-Cowden G L. Improving the reinforcement of parastomal tissues with Marlex mesh. Dis Colon Rectum. 2000;43(1):55–60. doi: 10.1007/BF02237244. [DOI] [PubMed] [Google Scholar]
- 21.Rubin M S. In: Cataldo PA, MacKeigan JM, editor. Intestinal Stomas. 2nd ed. New York: Marcel Dekker; 2004. Parastomal hernias. pp. 277–305.
- 22.Rubin M S, Schoetz D J, Matthews J B. Parastomal hernia: is stomal translocation superior to fascial repair? Arch Surg. 1994;129:413–419. doi: 10.1001/archsurg.1994.01420280091011. [DOI] [PubMed] [Google Scholar]
- 23.Taylor R L, Rombeau J L, Turnbull R B. Transperitoneal relocation of the ileal stoma without formal laparotomy. Surg Gynecol Obstet. 1978;146(6):953–958. [PubMed] [Google Scholar]
- 24.Kaufman J J. Repair of parastomal hernia by translocation of the stoma without laparotomy. J Urol. 1983;129(2):278–279. doi: 10.1016/s0022-5347(17)52049-2. [DOI] [PubMed] [Google Scholar]
- 25.Botet X, Boldo E, Llaurado J M. Colonic parastomal hernia repair by translocation without formal laparotomy. Br J Surg. 1996;83(7):981. doi: 10.1002/bjs.1800830730. [DOI] [PubMed] [Google Scholar]
- 26.Porcheron J, Payan B, Balique J G. Mesh repair of paracolostomal hernia by laparoscopy. Surg Endosc. 1998;12(10):1281. doi: 10.1007/s004649900838. [DOI] [PubMed] [Google Scholar]
- 27.Gould J C, Ellison E C. Laparoscopic parastomal hernia repair. Surg Laparosc Endosc Percutan Tech. 2003;13(1):51–54. doi: 10.1097/00129689-200302000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Safadi B. Laparoscopic repair of parastomal hernias: early results. Surg Endosc. 2004;18(4):676–680. doi: 10.1007/s00464-003-8518-x. [DOI] [PubMed] [Google Scholar]
- 29.LeBlanc K A, Bellanger D E, Whitaker J M, Hausmann M G. Laparoscopic parastomal hernia repair. Hernia. 2005;9(2):140–144. doi: 10.1007/s10029-004-0295-5. [DOI] [PubMed] [Google Scholar]
- 30.Voitk A. Simple technique for laparoscopic hernia repair. Dis Colon Rectum. 2000;43:1451–1453. doi: 10.1007/BF02236646. [DOI] [PubMed] [Google Scholar]
- 31.Mancini G J, McClusky D A, 3rd, Khaitan L, et al. Laparoscopic parastomal hernia repair using a nonslit mesh technique. Surg Endosc. 2007;21:1487–1491. doi: 10.1007/s00464-007-9419-1. [DOI] [PubMed] [Google Scholar]
- 32.Old Hindu proverb
- 33.Cheung M T. Complications of an abdominal stoma: an analysis of 322 stomas. Aust N Z J Surg. 1995;65(11):808–811. doi: 10.1111/j.1445-2197.1995.tb00566.x. [DOI] [PubMed] [Google Scholar]
- 34.Robertson I, Leung E, Hughes D, et al. Prospective analysis of stoma-related complications. Colorectal Dis. 2005;7(3):279–285. doi: 10.1111/j.1463-1318.2005.00785.x. [DOI] [PubMed] [Google Scholar]
- 35.Makela J T, Turku P H, Laitinen S T. Analysis of late stomal complications following ostomy surgery. Ann Chir Gynaecol. 1997;86(4):305–310. [PubMed] [Google Scholar]
- 36.Maeda K, Maruta M, Utsumi T, Sato H, Masumori K, Aoyama H. Pathophysiology and prevention of loop stomal prolapse in the transverse colon. Tech Coloproctol. 2003;7(2):108–111. doi: 10.1007/s10151-003-0020-x. [DOI] [PubMed] [Google Scholar]
- 37.Corman M L. Colon and Rectal Surgery. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- 38.Abulafi A M, Sherman I W, Fiddian R V. Delorme operation for prolapsed colostomy. Br J Surg. 1989;76:1321–1322. doi: 10.1002/bjs.1800761234. [DOI] [PubMed] [Google Scholar]
- 39.Hata F, Kitagawa S, Nishimori H, et al. A novel, easy, and safe technique to repair a stoma prolapse using a surgical stapling device. Dig Surg. 2005;22(5):306–309. discussion 310. doi: 10.1159/000088626. [DOI] [PubMed] [Google Scholar]
- 40.Hebert J C. A simple method for preventing retraction of an end colostomy. Dis Colon Rectum. 1988;31:328–329. doi: 10.1007/BF02554373. [DOI] [PubMed] [Google Scholar]
- 41.Efron J E. Ostomies and stomal therapy. 2004 ASCRS core subjects. Available at: www.fascrs.org. Accessed August 10, 2007. Available at: www.fascrs.org
- 42.Caricato M, Ausania F, Ripetti V, et al. Retrospective analysis of long-term defunctioning stoma complications after colorectal surgery. Colorectal Dis. 2007;9(6):559–561. doi: 10.1111/j.1463-1318.2006.01187.x. [DOI] [PubMed] [Google Scholar]
- 43.Allen-Mersh T G, Thompson J P. Surgical treatment of colostomy complications. Br J Surg. 1988;75(5):416–418. doi: 10.1002/bjs.1800750507. [DOI] [PubMed] [Google Scholar]
- 44.Resnick R H, Ishihara A, Chalmers T C, Schimmel E M. Boston inter-hospital liver group. A controlled trial of colon bypass in chronic hepatic encephalopathy. Gastroenterology. 1968;54:1057–1069. [PubMed] [Google Scholar]
- 45.Eade M N, Alexander-Williams J, Cooke W T. Bleeding from an ileostomy caput medusae. Lancet. 1969;2:1166–1168. doi: 10.1016/s0140-6736(69)92489-1. [DOI] [PubMed] [Google Scholar]
- 46.Conte J V, Arcomano T A, Naficy M A, Holt R W. Treatment of bleeding stomal varices. Report of a case and review of the literature. Dis Colon Rectum. 1990;33(4):308–314. doi: 10.1007/BF02055474. [DOI] [PubMed] [Google Scholar]
- 47.Loehner D L, Schoetz D J. In: Mackegan JM, Cataldo PA, editor. Intestinal Stomas. St. Louis, MO: St Louis Quality Medical Pub; 1993. Unusual problems in stoma management. pp. 339–349.
- 48.Ackerman N B, Graeber G M, Fey J. Enterostomal varices secondary to portal hypertension: progression of disease in conservatively managed cases. Arch Surg. 1980;115(12):1454–1455. doi: 10.1001/archsurg.1980.01380120028007. [DOI] [PubMed] [Google Scholar]
- 49.Roberts P L, Martin F M, Schoetz D J, Jr, Murray J J, Coller J A, Veidenheimer M C. Bleeding stomal varices. The role of local treatment. Dis Colon Rectum. 1990;33(7):547–549. doi: 10.1007/BF02052204. [DOI] [PubMed] [Google Scholar]
- 50.Morgan T R, Feldshon S D, Tripp M R. Recurrent stomal variceal bleeding. Successful treatment using injection sclerotherapy. Dis Colon Rectum. 1986;29(4):269–270. doi: 10.1007/BF02553036. [DOI] [PubMed] [Google Scholar]
- 51.Beck D E, Fazio V W, Grundfest-Broniatowski S. Surgical management of bleeding stomal varices. Dis Colon Rectum. 1988;31(5):343–346. doi: 10.1007/BF02564880. [DOI] [PubMed] [Google Scholar]
- 52.Samaraweera R N, Feldman L, Widrich W C, et al. Stomal varices: percutaneous transhepatic embolization. Radiology. 1989;170(3 Pt 1):779–782. doi: 10.1148/radiology.170.3.2783784. [DOI] [PubMed] [Google Scholar]
- 53.Alkari B, Shaath N M, El-Dhuwaib Y, et al. Transjugular intrahepatic porto-systemic shunt and variceal embolisation in the management of bleeding stomal varices. Int J Colorectal Dis. 2005;20(5):457–462. doi: 10.1007/s00384-004-0669-2. [DOI] [PubMed] [Google Scholar]
- 54.Ryu R K, Nemcek A A, Jr, Chrisman H B, et al. Treatment of stomal variceal hemorrhage with Trends Pharmacol Sci: case report and review of the literature. Cardiovasc Intervent Radiol. 2000;23(4):301–303. doi: 10.1007/s002700010073. [DOI] [PubMed] [Google Scholar]
- 55.Labori K J, Carlsen E. Treatment of bleeding peristomal varices. Eur J Surg. 2002;168(11):654–656. doi: 10.1080/11024150201680017. [DOI] [PubMed] [Google Scholar]
- 56.Mountain J C. Cutaneous ulceration in Crohn's disease. Gut. 1970;11:18–26. doi: 10.1136/gut.11.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGarity W C, Robertson D B, McKeown P P, Amerson J R, Darden W A. Pyoderma gangrenosum of the parastomal site in patients with Crohn's disease. Arch Surg. 1984;119:1186–1188. doi: 10.1001/archsurg.1984.01390220064014. [DOI] [PubMed] [Google Scholar]
- 58.Callen J P. Pyoderma gangrenosum. Lancet. 1998;351:581–585. doi: 10.1016/S0140-6736(97)10187-8. [DOI] [PubMed] [Google Scholar]
- 59.Kiran R P, O'Brien-Ermlich B, Achkar J P, Fazio V W, Delaney C P. Management of peristomal pyoderma gangrenosum. Dis Colon Rectum. 2005;48:1397–1403. doi: 10.1007/s10350-004-0944-x. [DOI] [PubMed] [Google Scholar]
- 60.Cairns B A, Herbst C A, Sartor B R, Briggaman R A, Koruda M J. Peristomal pyoderma gangrenosum and inflammatory bowel disease. Arch Surg. 1994;129:769–772. doi: 10.1001/archsurg.1994.01420310101019. [DOI] [PubMed] [Google Scholar]
- 61.Glotzer D J, Glick M E, Goldman H. Proctitis and colitis following diversion of the fecal stream. Gastroenterology. 1981;80(3):438–441. [PubMed] [Google Scholar]
- 62.Morson B C, Dawson I M. Gastrointestinal Pathology. 1st ed. London: Blackwell Scientific Pub; 1972. p. 485.
- 63.Whelan R L, Abramson D, Kim D S, Hashimi H F. Diversion colitis. Surg Endosc. 1994;8:19–24. doi: 10.1007/BF02909487. [DOI] [PubMed] [Google Scholar]
- 64.Longo W E, Oliver G C. In Wolfe BG, Fleshman JW, Beck DE, Pemberton JH, Wexner SD, editor. The ASCRS Textbook of Colon and Rectal Surgery. New York: Springer; 2007. Less common benign disorders of the colon and rectum. pp. 601–621.
- 65.Guillemot F, Colomberl J F, Lecemte M, et al. Treatment of diversion colitis by short-chain fatty acids. Dis Colon Rectum. 1991;34:861–864. doi: 10.1007/BF02049697. [DOI] [PubMed] [Google Scholar]
- 66.Harig J M, Soergel K H, Komorowoski R A, Wood C M. Treatment of diversion colitis with short-chain fatty acid irrigation. N Engl J Med. 1989;320:23–28. doi: 10.1056/NEJM198901053200105. [DOI] [PubMed] [Google Scholar]
- 67.Safadi B Y, Rosito O, Nino-Murcia M, Wolfe V A, Perkash I. Which stoma works better for colonic dysmotility in the spinal cord injured patients? Am J Surg. 2003;186:437–442. doi: 10.1016/j.amjsurg.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 68.Colquhoun P, Kaiser R, Efron J, et al. Is the quality of life better in patients with colostomy than patients with fecal incontinence? World J Surgery. 2006;30(10):1925–1928. doi: 10.1007/s00268-006-0531-5. [DOI] [PubMed] [Google Scholar]
- 69.Nugent K P, Daniels P, Stewart B, Patankar R, Johnson C D. Quality of life in stoma patients. Dis Colon Rectum. 1999;42:1569–1574. doi: 10.1007/BF02236209. [DOI] [PubMed] [Google Scholar]
- 70.Bass E M, Del Pin A, Tan A, Pearl R K, Orsay C P, Abcarian H. Does preoperative stoma markin and education by the enterostomal therapist affect outcome? Dis Colon Rectum. 1997;40(4):440–442. doi: 10.1007/BF02258389. [DOI] [PubMed] [Google Scholar]
- 71.Karadağ A, Bülent Menteş B, Üner A, İrkörücü O, Ayaz S, Özkan S. Impact of stomatherapy on quality of life in patients with permanent colostomies or ileostomies. Int J Colorectal Dis. 2003;18:234–238. doi: 10.1007/s00384-002-0462-z. [DOI] [PubMed] [Google Scholar]