ABSTRACT
The management of full-thickness rectal prolapse involves surgical intervention in the majority of cases. Many procedures have been described employing both perineal and abdominal approaches. Abdominal procedures result in more durable repair of the prolapse; however, the procedures require general anesthesia and are reserved for younger healthier patients. Laparoscopy has been utilized in the treatment of rectal prolapse since its introduction for colorectal procedures; recent studies have found equivalent long-term results and short-term outcomes.
Keywords: Rectal prolapse, surgery, abdominal approach
Rectal prolapse is defined as a circumferential full-thickness protrusion of the rectum through the anus; descriptions of the condition date back to ancient times. Hippocrates suggested in cases of incarcerated prolapse “having fomented the part with a soft sponge, and anointed it with a snail, bind the man's hands together, and suspend him head down for a short time and the gut will return” and for definitive treatment “a caustic potass is applied to the rectal mucosa and after the reduction of the prolapse the thighs are bound together for three days.” Over the last century, a plethora of techniques have been devised for the treatment of this disease; an indication of our imperfect understanding of the disorder and the absence of an ideal procedure to effectively treat this ailment. Current accepted theories of the etiology of rectal prolapse include the one proposed by Moschcowits1 at the turn of the 20th century. He suggested that rectal prolapse was a sliding hernia through a defect in the pelvic fascia. This theory was challenged by Broden and Snellman,2 who with the aid of cinedefecography, were able to demonstrate that the prolapse is, in fact, a circumferential intussusception of the rectum through the anus.
Irrespective of which theory one might subscribe to, common anatomic findings associated with rectal prolapse include an abnormally deep cul-de-sac, lax and atonic anal sphincters, redundant sigmoid colon, and loss of posterior fixation of the rectum. It is unclear whether some of these findings are a cause or an effect of rectal prolapse. Nonetheless, most of the modern procedures described for the treatment of rectal prolapse attempt to correct some or all of these abnormalities.
CLINICAL FEATURES AND EVALUATION
Rectal prolapse affects patients at extremes of age. It is more common in woman than men with a ratio of 6 to 1. In children the gender distribution is equal. Male patients have an equal incidence per decade of life whereas women have an increased incidence as they age.
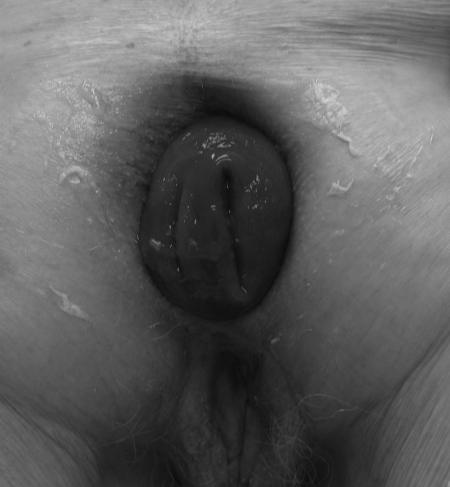
Patients usually complain of a lump protruding through the anus during defecation (Fig. 1). Bleeding and mucus discharge are frequent complaints in chronic prolapse. More than half of rectal prolapse patients complain of fecal incontinence and 15 to 65% of patients have constipation3 other symptoms may be related to the prolapse of other pelvic organs.
Figure 1.
Rectal prolapse.
Physical exam should include a careful assessment of the patient's functional status and their fitness to undergo surgery including a cardiac and pulmonary assessment. The prolapse may be easily visible during the physical exam or alternatively the patient may be asked to sit on a commode and bear down to reproduce the prolapse. While on the commode, the patient is asked to lean forward with the examiner standing behind to confirm the diagnosis. Full-thickness rectal prolapse can be distinguished from partial thickness prolapse by its concentric folds and the presence of a sulcus between the anus and the prolapsed rectum. In case of chronic prolapse, mucosal ulceration and bleeding may be present. The abdomen should be examined and any previous scars, masses, or hernias identified. A digital rectal examination is performed to assess the tone of the anal sphincter muscles. Vaginal examination should be performed to assess for other coexisting abnormalities such as a cystocele or an enterocele, which are frequently present in women with rectal prolapse. Urogynecological consultation can be sought in the presence of these abnormalities and may influence the surgical approach.
A colonoscopy should be performed for complete assessment of the colonic mucosa and to exclude any lead point lesions as a cause for the prolapse. In patients with severe constipation, a colonic transit study should be performed. In the presence of colonic inertia, subtotal colectomy with rectopexy and ileorectal anastomosis may be offered in appropriately selected patients.
Cinedefecography may be performed if the patient presents with symptoms of obstructive defecation; however, this study has a limited impact on clinical decision making in the treatment of full-thickness rectal prolapse. Other studies such as manometry, electromyography, and endoanal ultrasound may be performed to evaluate incontinent patients; however, the sphincters usually begin to regain tone ~1 month after the procedure and many patients regain full continence within 2 to 3 months.2 Full anorectal physiologic assessment and endoanal ultrasound evaluation of the anal sphincters may be undertaken if the patient continues to complain of incontinence after surgical correction of the rectal prolapse.
PATIENT SELECTION
In choosing the best operation for an individual patient, a careful history and preoperative assessment is essential. The overall goal of surgical management is the elimination of the prolapse with relief of associated symptoms. Surgical repair should be associated with minimal morbidity to the patient and result in the lowest possible recurrence rates. Many procedures exist to treat this condition, which implies that none of the currently employed operations are ideal and a patient-tailored approach must be adopted. It has generally been accepted that abdominal operations result in lower recurrence rates; however, they may also be associated with high morbidity.4,5 Therefore, a perineal procedure may be best suited for frail elderly individuals who cannot withstand the stress of an abdominal operation. In this article, we discuss the abdominal approaches for rectal prolapse; perineal approaches are discussed in the following article in this issue.
ABDOMINAL PROCEDURES
Many abdominal operations have been described for the treatment of rectal prolapse. All of these approaches essentially involve varying degrees of mobilization of the rectum, as well as fixation of the rectum to the presacral fascia with or without bowel resection. Fixation can be augmented through anterior or posterior placement of foreign material to incite an inflammatory response and fibrosis. Alternatively, suture rectopexy solely can be utilized to suspend the rectum. Constipated patients should be strongly considered for a resection rectopexy; however, in patients with incontinence a resection should be avoided.6 All of the above procedures can be performed utilizing an open or laparoscopic technique based on the surgeon's experience and comfort.
Mobilization of the Rectum
All of the procedures in use today involve posterior mobilization of the rectum to the level of the levators. Incising the peritoneal lining of the lateral stalks as well as anterior mobilization to the level of the vaginal vault in women and the seminal vesicles in men may be required to achieve the desired rectal mobilization. Division of the lateral stalks may lead to increased incidence of constipation and poor rectal emptying. In a recent Cochrane meta analysis by Brazzilli et al7 of articles reporting on surgical management of rectal prolapse, 10 randomized controlled trials were identified with 324 patients. They concluded that division, rather than preservation, of the lateral ligaments was associated with less recurrent prolapse, but more postoperative constipation, although these findings were made in small numbers.
Dissection is undertaken in the avascular areolar plane surrounding the mesorectum between the parietal and visceral facial planes of the pelvis. The plane can be identified by incising the peritoneum on either side at the base of the rectosigmoid mesentery at the level of the sacral promontory. Dissection is undertaken as far as possible posteriorly. The peritoneum of the lateral stalks is then incised and the incision continued anteriorly into the cul-de-sac to facilitate the posterior dissection. Care must be taken to identify and preserve both the ureters and the autonomic nerves. Anteriorly, the dissection is taken to the level of the vaginal vault or seminal vesicles. The rectum and the anterior peritoneal dissection are handled based on which procedure is chosen.
Rectopexy with or without Mesh
The type of operation chosen depends on which side of the Atlantic the surgeon is trained and practices. In the United Kingdom, the Well's8 posterior Ivalon rectopexy has been the treatment of choice; in the United States the Ripstein9 procedure predominates. The use of mesh (initially nonabsorbable, but later absorbable) has been challenged in recent years. Rectopexy with mesh placement has been associated with erosion of the mesh into the rectum leading to fistula formation, strictures, increased pelvic sepsis,10,11 and wound complications12 prompting many surgeons to increasingly abandon mesh placement.
Ripstein Procedure
This procedure was originally described by Ripstein in 1952;13 it involved an anterior levator plication reinforced with fascia lata. He then modified the procedure in 1963 to what is now known as the classic Ripstein repair.9 This operation is undertaken to restore the posterior curve of the rectum.
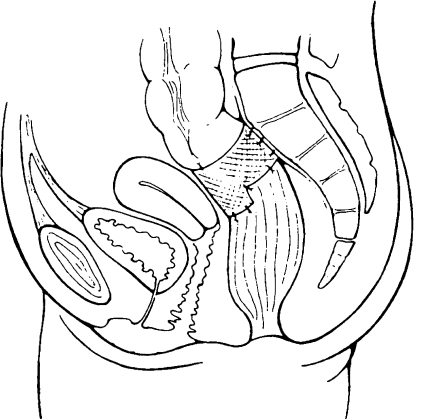
The operation is commenced by mobilizing the rectum to the level of the levator muscle as mentioned above. A piece of prosthetic mesh is placed around the anterior wall of the rectum at the level of the peritoneal reflection. The mesh is then secured to the presacral fascia using nonabsorbable material 1 cm from midline on either side. The anterior wall of the rectum is sutured to the sling to prevent the sling from sliding up using absorbable material ensuring that the sutures do not penetrate the rectal wall. The cul-de-sac is obliterated with nonabsorbable sutures (see Fig. 2).
Figure 2.
Anterior (Ripstein) rectopexy. Reprinted with permission of Elsevier Limited.
In a recent review of the literature, Madiba et al3 reported recurrence rates between 0 to 13%, mortality between 0 to 2.8% with complication rates as high as 52% in some series.14 The operation's influence on preexisting constipation was variable. Results of the Ripstein procedure are summarized in Table 1.14,15,16,17
Table 1.
Results of Anterior Mesh Rectopexy
Wells' Posterior Ivalon Rectopexy
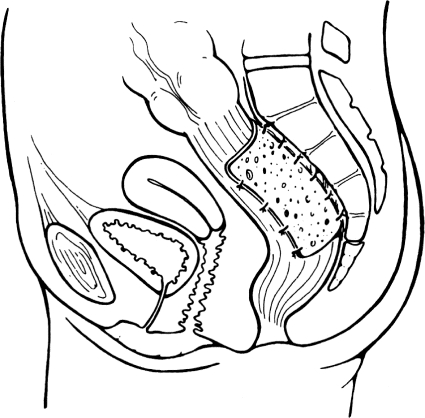
Posterior Ivalon rectopexy as described by Wells8 is the preferred method for correction of rectal prolapse in the United Kingdom. The operation consists of posterior rectal mobilization and fixation with a sheet of Ivalon® (Fabco, New London, CT) mesh to the sacrum. The mesh is secured to the sacral hollow as low as possible with nonabsorbable sutures and then wrapped on either side of the rectum. The anterior portion of the rectum must be kept uncovered to avoid narrowing of the lumen. The peritoneum is closed over the mesh to exclude it from the peritoneal cavity. Meticulous hemostasis is essential, as the formation of pelvic hematoma may contribute to pelvic sepsis and significant morbidity in this procedure (see Fig. 3). Recently, the absorbable mesh has been substituted for Ivalon® mesh with similar results. Recurrence rates, mortality rates, and effects on constipation are comparable to the anterior wrap. However, this procedure is associated with a lower incidence of fecal impaction and strictures. The most feared complication following this procedure is pelvic sepsis secondary to infected mesh, which may require mesh removal. Table 2 summarizes the results of posterior rectopexy.12,18,19,20,21
Figure 3.
Posterior (Wells) rectopexy. Reprinted with permission of Elsevier Limited.
Table 2.
Results of Posterior Mesh Rectopexy
Suture Rectopexy
Cutait22 in 1959 suggested that implanted foreign material was unnecessary and perhaps increased the risk of postoperative pelvic sepsis; he proposed that the mesorectum simply needed to be attached to the presacral fascia with sutures. Following full mobilization of the posterior rectum as described above, the lateral stalks are sutured to the presacral fascia using a nonabsorbable suture material. Two to three sutures are used on either side of the rectum making sure not to narrow the rectum. Recurrence rates are reported to be low (3 to 9%) with significant improvement in continence. The outcome in patients with preexisting constipation is variable with some series reporting worsening constipation following the procedure. Patients with constipation should strongly be considered for a resection rectopexy. Table 3 summarizes the results of sutured rectopexy.12,23,24,25,26
Table 3.
Results of Suture Rectopexy Repair
Suture Rectopexy with Resection
The technique was first described by Frykman in 195527 combines direct suture rectopexy with sigmoid resection. The procedure is used today for patients with full-thickness rectal prolapse, long redundant sigmoid colon, and constipation. Advantages of the procedure include suspension of the rectum by straightening the left colon, prevention of sigmoid volvulus, and relief of preoperative constipation.
The procedure is performed in the modified lithotomy position to afford the surgeon access to the anus to perform a stapled anastomosis. The rectum is mobilized as described above. The sigmoid colon is resected and a tension-free anastomosis is created. Preservation of the superior hemorrhoidal vessels should be attempted. Fixation sutures are placed from the lateral stalks to endopelvic fascia in a similar fashion to the suture rectopexy; these sutures can be placed before the bowel resection and tied after the anastomosis. The rectum must be elevated out of the pelvis as high as possible prior to placement of the sutures. The anterior cul-de-sac is closed. The procedure carries the risk of anastomotic leakage; however, it results in low incidence of recurrence and significant improvement in preoperative constipation. Table 4 summarizes the results of resection rectopexy.4,28,29,30,31,32
Table 4.
Results of Suture Rectopexy with Resection
LAPAROSCOPIC APPROACH
With the advent of laparoscopic procedures in colorectal surgery in the 1990s, abdominal rectopexy became one of the early procedures to be attempted laparoscopically with results similar to the open technique.
Ashari et al33 reported their experience in 117 patients over a 10-year period that underwent laparoscopically assisted resection rectopexy for full-thickness rectal prolapse. The overall morbidity rate was 9% and mortality occurred in 1 patient (0.8%). Follow-up was complete in 77 patients; median follow-up time was 5 years. Recurrent full- thickness prolapse was observed in 2 patients (2.5%) with 14 others presenting with mucosal prolapse (18%). Anastomotic dilations were needed in 5 patients (4%). They were also able to show shorter operative times with increasing experience.
Kariv et al34 reported the Cleveland Clinic experience with laparoscopic repair of rectal prolapse. In a case matched series, 111 laparoscopic procedures were compared with 86 open procedures. Of the 111 patients, 42 had posterior mesh fixation and 67 sutured rectopexy including 32 patients who underwent a resection rectopexy for constipation. Conversion was required in 8 patients. Hospital stay was significantly reduced in the laparoscopic group (3.9 versus 6 days). Recurrent full-thickness rectal prolapse requiring reoperation occurred in 9.3% of patients in the laparoscopic group and 4.7% in the open group; the difference was not statistically significant with a mean follow-up of 59 months. No differences were found in functional outcomes between the two groups in the long term.
In a recent meta-analysis comparing outcomes using the laparoscopic technique with an open procedure, no differences in operative morbidity and recurrence rates were found. Six studies consisting of 195 patients (98 open and 97 laparoscopic) were included.35 The length of hospital stay was significantly shorter in the laparoscopic group, whereas the operative times were significantly longer. Two of the studies included addressed the issue of cost; both showed significant cost reductions owing to reduced hospital stays.
CONCLUSION
Rectal prolapse is a relatively uncommon condition with unclear etiology that results in much distress for the patient. Treatment is directed at correcting the prolapsed rectum and relieving the associated functional abnormalities. Many procedures have been devised for the treatment of rectal prolapse; generally, these can be divided into perineal and abdominal approaches. Abdominal procedures are ideal for young healthy patients and are associated with lower recurrence rates when compared with perineal procedures. Patient outcome following the various abdominal procedures seems equivalent; there has been a clear reduction in the utilization of mesh-based procedures in recent years. Resection rectopexy is associated with better functional outcome in constipated patients. Laparoscopic repair of rectal prolapse compares favorably to the open technique in regards to short-term outcomes, and it seems to have similar long-term results. As surgeons become more familiar and comfortable utilizing the laparoscopic technique it may ultimately prove to be the preferred approach in repairing full-thickness rectal prolapse.
REFERENCES
- 1.Moschcowits A V. The pathogenesis, anatomy and cure of prolapse of the rectum. Surg Gynecol Obstet. 1912;15:7–21. [Google Scholar]
- 2.Broden B, Snellman B. Procidentia of the rectum studied with cinedefecography: a contribution to the discussion of causative mechanism. Dis Colon Rectum. 1968;11:330–347. doi: 10.1007/BF02616986. [DOI] [PubMed] [Google Scholar]
- 3.Madiba T E, Baig M K, Wexner S D. Surgical management of rectal prolapse. Arch Surg. 2005;140:63–73. doi: 10.1001/archsurg.140.1.63. [DOI] [PubMed] [Google Scholar]
- 4.Kim D S, Tsang C B, Wong W D, Lowry A C, Goldberg S M, Madoff R D. Complete rectal prolapse: evolution of management and results. Dis Colon Rectum. 1999;42:460–469. doi: 10.1007/BF02234167. [DOI] [PubMed] [Google Scholar]
- 5.Azimuddin K, Khubchandani I T, Rosen L, Stasik J J, Riether R D, Reed J F., III Rectal prolapse: a search for the “best” operation. Am Surg. 2001;67:622–627. [PubMed] [Google Scholar]
- 6.Brown A J, Anderson J H, McKee R F, Finlay I G. Strategy for selection of type of operation for rectal prolapse based on clinical criteria. Dis Colon Rectum. 2004;47:103–107. doi: 10.1007/s10350-003-0013-x. [DOI] [PubMed] [Google Scholar]
- 7.Brazzilli M, Bachoo P, Grant A. Surgery for complete rectal prolapse in adults. Cochrane Database Sys Rev. 2000;2:CD001758. doi: 10.1002/14651858.CD001758. [DOI] [PubMed] [Google Scholar]
- 8.Wells C. New operation for rectal prolapse. Proc R Soc Med. 1959;52:602–603. [PMC free article] [PubMed] [Google Scholar]
- 9.Ripstein C B. Treatment of massive rectal prolapse. Am J Surg. 1952;83:68–71. doi: 10.1016/0002-9610(52)90161-x. [DOI] [PubMed] [Google Scholar]
- 10.Kuijpers H C. Treatment of complete rectal prolapse: to narrow, to wrap, to suspend, to fix, to encircle, to plicate or to resect? World J Surg. 1992;16:826–830. doi: 10.1007/BF02066977. [DOI] [PubMed] [Google Scholar]
- 11.Gordon P H, Hoexter B. Complications of the Ripstein procedure. Dis Colon Rectum. 1978;21:277–280. doi: 10.1007/BF02586703. [DOI] [PubMed] [Google Scholar]
- 12.Novell J R, Osborne M J, Winslet M C, Lewis A AM. Prospective randomized trail of Ivalon sponge versus sutured rectopexy for full thickness rectal prolapse. Br J Surg. 1994;81:904–906. doi: 10.1002/bjs.1800810638. [DOI] [PubMed] [Google Scholar]
- 13.Ripstein C B, Lanter B. Etiology and surgical therapy for massive prolapse of the rectum. Ann Surg. 1963;157:259–264. doi: 10.1097/00000658-196302000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts P L, Schoetz D J, Coller J A, Veindenheimer M C. Ripstein procedure. Lahey Clinic experience: 1963–1985. Arch Surg. 1988;123:554–557. doi: 10.1001/archsurg.1988.01400290036005. [DOI] [PubMed] [Google Scholar]
- 15.Ripstein C B. Procidentia: definitive corrective surgery. Dis Colon Rectum. 1972;15:334–346. doi: 10.1007/BF02587406. [DOI] [PubMed] [Google Scholar]
- 16.Tjandra J J, Fazio V W, Church J M, Milson J W, Oakley J R, Lavery I C. Ripstein procedure is an effective treatment for rectal prolapse without constipation. Dis Colon Rectum. 1993;36:501–507. doi: 10.1007/BF02050018. [DOI] [PubMed] [Google Scholar]
- 17.Schultz I, Mellgren A, Dolk A, Johansson C, Holmstrom B. Long- term results and functional outcomes after Ripstein rectopexy. Dis Colon Rectum. 2000;43:35–43. doi: 10.1007/BF02237241. [DOI] [PubMed] [Google Scholar]
- 18.Morgan C N, Porter N H, Klugman D J. Ivalon (polyvinyl alcohol) sponge in the repair of complete rectal prolapse. Br J Surg. 1972;59:841–846. doi: 10.1002/bjs.1800591102. [DOI] [PubMed] [Google Scholar]
- 19.Penfold J CB, Hawley P R. Experience of Ivalon-sponge implant for complete rectal prolapse at St. Mark's Hospital, 1960–1970. Br J Surg. 1972;59:846–848. doi: 10.1002/bjs.1800591103. [DOI] [PubMed] [Google Scholar]
- 20.Yoshioka K, Heyen F, Keighley M RB. Functional results after abdominal rectopexy for rectal prolapse. Dis Colon Rectum. 1989;32:835–838. doi: 10.1007/BF02554550. [DOI] [PubMed] [Google Scholar]
- 21.Aitola P T, Hiltunen K M, Matikainen M J. Functional results of operative treatment of rectal prolapse over an 11-year period: emphasis on trans-abdominal approach. Dis Colon Rectum. 1999;42:655–660. doi: 10.1007/BF02234145. [DOI] [PubMed] [Google Scholar]
- 22.Cutait D. Sacro-promontory fixation of the rectum for complete rectal prolapse. Proc R Soc Med. 1959;52(Suppl):105. [Google Scholar]
- 23.Blatchford G J, Perry R E, Thorson A G, Christensen M A. Rectopexy without resection for rectal prolapse. Am J Surg. 1989;158:574–576. doi: 10.1016/0002-9610(89)90196-7. [DOI] [PubMed] [Google Scholar]
- 24.Graf W, Karlbom U, Pahlman L, Nilsson S, Ejerblad S. Functional results after abdominal suture rectopexy for rectal prolapse or intussusception. Eur J Surg. 1996;162:905–911. [PubMed] [Google Scholar]
- 25.Khanna A K, Misra M K, Kumar K. Simplified sutured sacral rectopexy for complete rectal prolapse in adults. Eur J Surg. 1996;162:143–146. [PubMed] [Google Scholar]
- 26.Briel J W, Schouten W R, Boerma M O. Long-term results of suture rectopexy in patients with fecal incontinence associated with incomplete rectal prolapse. Dis Colon Rectum. 1997;40:1228–1232. doi: 10.1007/BF02055169. [DOI] [PubMed] [Google Scholar]
- 27.Frykman H M. Abdominal proctopexy and primary sigmoid resection for rectal procidentia. Am J Surg. 1955;90:780–789. doi: 10.1016/0002-9610(55)90700-5. [DOI] [PubMed] [Google Scholar]
- 28.Husa A, Salinio P, Von Smitten K. Abdominal rectopexy and sigmoid resection (Frykman-Goldberg operation) for rectal prolapse. Acta Chir Scand. 1988;154:221–224. [PubMed] [Google Scholar]
- 29.Luukkonen P, Mikkonen U, Jarvinen H. Abdominal rectopexy with sigmoidectomy vs rectopexy alone for rectal prolapse: a progressive randomized study. Int J Colorectal Dis. 1992;7:219–222. doi: 10.1007/BF00341225. [DOI] [PubMed] [Google Scholar]
- 30.McKee R F, Lauder J C, Poon F W, Aichison M A, Finlay I G. A prospective randomized study of abdominal rectopexy with and without sigmoidectomy in rectal prolapse. Surg Gynecol Obstet. 1992;174:145–148. [PubMed] [Google Scholar]
- 31.Huber F T, Stein H, Siewert J R. Functional results after treatment of rectal prolapse with rectopexy and sigmoid resection. World J Surg. 1995;19:138–143. doi: 10.1007/BF00316999. [DOI] [PubMed] [Google Scholar]
- 32.Yakut M, Kaymakcioglu N, Simsek A, Tan A, Sen D. Surgical treatment of rectal prolapse. A retrospective analysis of 94 cases. Int Surg. 1998;83:53–55. [PubMed] [Google Scholar]
- 33.Ashari L H, Lumley J W, Stevenson A RL, Stitz R W. Laparoscopic assisted resection rectopexy for rectal prolapse: ten year experience. Dis Colon Rectum. 2005;48:982–987. doi: 10.1007/s10350-004-0886-3. [DOI] [PubMed] [Google Scholar]
- 34.Kariv Y, Delaney C P, Casillas S, et al. Long term outcomes after laparoscopic and open surgery for rectal prolapse. Surg Endosc. 2006;20:35–42. doi: 10.1007/s00464-005-3012-2. [DOI] [PubMed] [Google Scholar]
- 35.Purkayastha S, Tekkis P, Athanasiou T, et al. A comparison of open vs lap abdominal rectopexy for full-thickness rectal prolapse: A meta-analysis. Dis Colon Rectum. 2005;48:1930–1940. doi: 10.1007/s10350-005-0077-x. [DOI] [PubMed] [Google Scholar]