ABSTRACT
With development over the past 25 years of new surgical techniques and neoadjuvant therapy regimens for rectal cancer, physicians now have a range of treatment options that minimize morbidity and maximize the potential for cure. Accurate pretreatment staging is critical, ensuring adequate therapy and preventing overtreatment. Many options exist for staging primary rectal cancer. However, endorectal ultrasound (ERUS) remains the most attractive modality. It is an extension of the physical examination, and can be performed easily in the office. It is cost effective and is generally well tolerated by the patient, without need for general anesthesia. The authors discuss the data currently available on ERUS, including its accuracy and limitations, as well as the technical aspects of performing ERUS and interpreting the results. They also discuss new ultrasound technologies, which may improve rectal cancer staging in the future.
Keywords: Endorectal ultrasound, rectal cancer, evaluation, staging
Rectal cancer remains a significant health concern worldwide, affecting over 40,000 people annually1 in the United States alone. Recurrence rates approach 50% in the setting of stage II and III disease.2 The narrow confines of the bony pelvis make surgical extirpation challenging. Incomplete resection leads to local recurrence,3 which is associated with high morbidity. Postoperative chemoradiation has been shown to reduce the rate of local relapse from 25% to 16%, prompting the National Institutes of Health (NIH; Bethesda, MD) to issue a consensus statement advocating adjuvant therapy for all stage II and III rectal cancer patients.4,5,6 A recent prospective, randomized trial by the German Rectal Cancer Study Group demonstrated that preoperative chemoradiation results in even lower rates of local recurrence, reduced treatment toxicity, and improved rates of sphincter preservation.7 Indeed, many series reporting on combined modality therapy (CMT)—preoperative chemoradiation followed by total mesorectal excision—demonstrate local recurrence rates of less than 10%8 in the setting of locally advanced lesions. These studies highlight the need for proper staging prior to initiation of treatment.9
Endorectal ultrasound is currently the most widely used and effective diagnostic modality in the assessment of rectal cancer overall. Its accuracy in numerous trials and meta-analyses ranges from 80 to 95% for T-staging and 70 to 75% for N-staging, levels that are slightly higher than the respective 75 to 85% and 60 to 70% observed for magnetic resonance imaging (MRI).10 Additionally, ERUS is capable of evaluating a wide range of pertinent features, providing information in each case that may help direct therapy. In experienced hands, ERUS can accurately measure the size, circumference, and distance of the tumor from various anatomic landmarks (e.g., anal verge, anorectal line). It is capable of examining the anal sphincters for defects as well as tumor infiltration, allowing the surgeon to decide whether a sphincter-sparing resection is safe or feasible.11 ERUS can demonstrate the relationship of tumor to the pelvic peritoneal reflection (PPR), information that will help the clinician determine whether local excision is possible or preoperative chemoradiation necessary.12
STAGING ACCURACY
Many studies have reported on the accuracy of ERUS (Table 1).10,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29 Despite a wide range of results, common patterns have emerged. One is that the accuracy of ERUS T-staging varies in relation to tumor stage; ERUS tends to be least accurate in assessing intramural T2 masses, which are often overstaged.10,13 A meta-analysis of 11 studies found the sensitivity of ERUS in identifying T1, T2, T3, and T4 masses to be 84%, 76%, 96%, and 76%, respectively.18,30 Another meta-analysis of 31 studies reported ERUS sensitivity rates of 76%, 75%, 88%, and 87%, respectively.14 A common pattern in current studies, however, has been declining rates of accuracy.10,15 Harewood's31 recent review examined all estimates of ERUS accuracy published in the English literature between 1985 and 2003. This review, covering 4,118 cases, reported a mean accuracy of 85% for assessing T-stage, and an accuracy of 75% for assessing N-stage. Despite the overall numbers, the author noted that accuracy rates had declined significantly over time, with the lowest rates being reported in the most recent articles. He also noted that accuracy tends to be inversely proportional to sample size. Concern was raised that the accuracy of ERUS may have been inflated in earlier studies due to publication bias.31 Several theories, directed at both the studies themselves and ERUS, have been posited as a means of explanation.
Table 1.
Accuracy of Endorectal Ultrasound
| Study | Year | N | T-Staging (Overall) (%) | T1 | T2 | T3 | T4 | N-Staging (%) |
|---|---|---|---|---|---|---|---|---|
| Badger et al16 | 2007 | 95 | 71.6 | – | – | – | – | 68.8 |
| Landmann et al20 | 2007 | 134 | – | – | – | – | – | 70 |
| Skandarajah et al14 | 2006 | 2718 | 81.8 | 76 | 75 | 88 | 87 | – |
| Ptok et al10,* | 2006 | 3501 | 65.8 | 76.4 (402) | 56.0 (1208) | 71.2 (1780) | 48.6 (111) | – |
| Kulig et al25 | 2006 | 29 | – | 89.2 | 96.2 | – | – | – |
| Giovannini et al22,† | 2006 | 35 | 71.4 | 71.4 | ||||
| Kim et al18 | 2006 | 85 | 69 | 56 | ||||
| Zammit et al24 | 2005 | 117 | 76.4 | – | – | – | – | 73.6 |
| Bali et al26 | 2004 | 29 | 79 | – (0) | 50 (4) | 84 (25) | – (0) | 59 |
| Garcia-Aguilar et al15,* | 2002 | 545 | 69 | 47 (105) | 68 (153) | 70 (131) | 50 (8) | 64 (238) |
| Kim et al23,‡ | 2002 | 33 | – | – | 84.8 | 75.8 | – | 66.7 |
| Hünerbein et al21,§ | 2000 | 30 | 83 | 71.4 (7) | 90.9 (11) | 50 (2) | 100 (1) | – |
| Gualdi et al27 | 2000 | 26 | 76.9 | 76 | ||||
| Akasu et al19 | 2000 | 154 | ||||||
| Blomqvist et al28,‡ | 2000 | 49 | 84.1 | 89.8 | 77.6 | 63.3 | 91.8 | 60 |
| Kruskal et al29 | 2000 | 26 | 76.9 | |||||
| Kim et al13,‡,§ | 1999 | 89 | 81.1 | 100 (4) | 50 (12) | 87 (66) | 71 (7) | 63.5 |
| Massari et al17,§ | 1998 | 75 | 90.7 | 86.7 (15) | 88.9 (18) | 91.4 (35) | 100 (7) | 76 |
Accuracies are really PPVs (positive predictive value) (TP/TP [true positive] + FP [false positive]) for each UTx.
Accuracies are based on four TN (true negative) groups (T1/T2N0, T3N0, T3N1, T4N1)-T and N accuracy not given independently.
(For N-staging) Accuracy for each Tx = total # of correct calls by ultrasound (TP and TN) for each T per total number of patients.
Accuracies represent sensitivities (TP/TP + FN [false negative]) for each pTx. Overall sensitivity calculated by adding the total TP made per total number of patients.
Some studies may have been subjected to selection bias, as patients with advanced tumors receiving neoadjuvant therapy, as well as those with superficial lesions requiring only local excision, are often excluded from these cohorts, favoring study populations predominantly composed of patients with intramural T2 or T3 tumors.10,15 Given the decreased accuracy of ERUS in assessing T2 tumors, these exclusion criteria could conceivably undermine the actual overall accuracy of ERUS T-staging. Another reason for the lower levels may be due to the sample sizes, and to how each of the studies defines accuracy. In some,10,15 accuracy is equated with positive predictive value, a parameter that can be significantly reduced when applied to a study with a large sample size for a finding with low incidence.
In 2002, Garcia-Aguilar et al reported an ~10 to 15% difference in T-staging accuracy between three board-certified, experienced colorectal surgeons who performed all but 17 of 545 ERUS examinations, a finding of statistical significance.15 The idea that the accuracy of ERUS is operator-dependent has been supported in the literature.14,30 Another report by Orrom et al suggests that standardization of proper technique and criteria are key to maximizing ERUS accuracy. This study involved 59 patients who were divided into three investigative groups. In the first group, for which neither technique nor staging criteria were standardized and multiple examiners were used, overall accuracy was 58%. In the second group, for which a technical protocol was implemented and a single examiner used, accuracy was 77%. In the third group, for which both a technical protocol and a single examiner were used, and an older staging system replaced with a newer one, the accuracy was 95%.32
The lower rates of accuracy reported recently may reflect the impact of less-experienced operators, improper technique, and varied criteria in a time when use of ERUS in the evaluation of rectal cancer has become more widespread. Two of the more recent studies reporting lower accuracies did cull their findings from multicenter populations.10,33 Another group observed that ERUS rates of accuracy in assessing T- and N-stage in 756 patients declined, from 81% and 76% to 71% and 71%, respectively, when the number of ERUS examiners increased from 4 to 6.16 As the medical community continues to embrace ERUS and gains experience using it for work-up of rectal cancer, future studies will hopefully report an improvement in accuracy.
SPECIFICS ON PROCEDURE AND INTERPRETATION
Technique
Prior to ERUS examination, the patient must be prepared with enemas to remove all air, stool, and mucus from the rectum, which, if present, can create artifacts during the study. With the patient in the left lateral decubitus position, a digital rectal examination is performed. Rigid proctoscopy follows, using an instrument measuring 20 mm in diameter to accommodate the ERUS probe. Following proctoscopy, and after estimates of tumor size and distance from the anal verge have been noted, the ERUS probe is prepared. A balloon is placed over the crystal of the ERUS and the probe inserted into the rectum, past the proximal border of the rectal mass, either under direct vision or through the lumen of the proctoscope. Once inserted, the balloon is inflated with water. Care must be taken to remove all air bubbles that might interfere with imaging. The balloon may hold up to 90 mL of fluid, although typically no more than 30 to 60 mL are necessary; the amount of fluid used is established by the diameter of the rectal lumen as determined on proctoscopy, the level of patient discomfort, and the ability to traverse the lesion.34 Imaging of the rectum is initiated as the probe is withdrawn. The tumor is evaluated for size and depth of invasion, and the perirectal fat examined for suspicious lymph nodes. Using the proctoscope to place the probe is preferred, as this ensures that the transducer is advanced past the proximal border, permitting imaging and T-staging of the entire tumor. During the exam, it is essential to keep the probe centered within the rectal lumen to maintain image clarity. Images are usually obtained using an ultrasound frequency of 5 to 15 MHz, depending on which part of the rectum is being examined. Higher frequencies provide better resolution of the sphincter muscles and the rectal wall layers, whereas pararectal tissue and lymph nodes are more accurately assessed using lower frequencies.35,36,37 To evaluate lesions that are low enough to involve the sphincter-tightened anal canal, the balloon may be replaced with a plastic cap and the rectum filled with 150 cc of water. This permits adequate conduction of sound waves through a strictly fluid medium, while minimizing compression and potential distortion of both the lesion and the surrounding anatomy.38
Interpretation of Endorectal Ultrasound Imaging
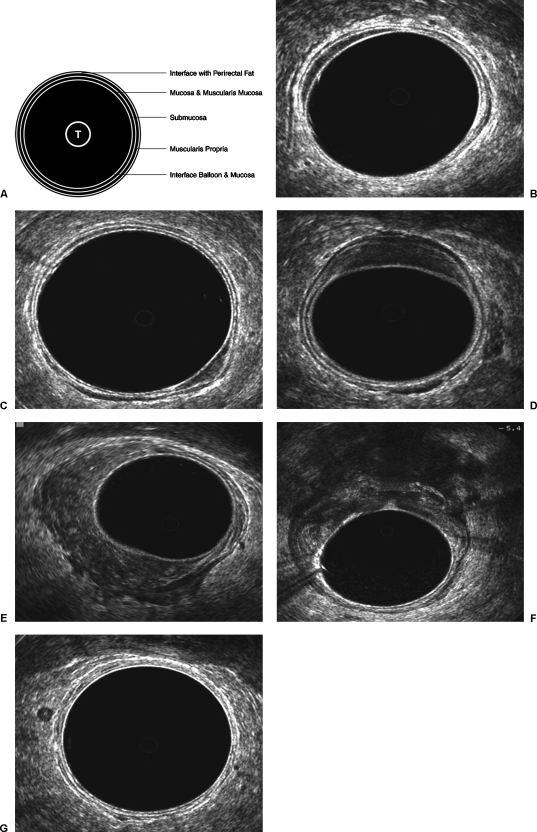
ERUS images of the rectal wall comprise three hyperechoic and two hypoechoic layers, which alternate with each other and correspond to anatomic layers (Fig. 1A–G). Debate continues over how these two sets of layers correspond. The model first described by Hildebrandt and Feifel39 assumes that the mucosa, muscularis mucosa, and submucosa cannot be sonographically differentiated. In this system, the three hyperechoic lines represent interfaces to the anatomic layers that are defined by the two hypoechoic lines. The first hyperechoic line corresponds to the interface between the balloon and the mucosa. The second hypoechoic line corresponds to the mucosa, muscularis mucosa, and submucosa. The third hyperechoic line represents an interface between the submucosa and muscularis propria. The fourth hypoechoic line represents the muscularis propria. The fifth hyperechoic line represents an interface between the muscularis propria and perirectal fat/serosa.40
Figure 1.
Endorectal ultrasound images of rectal cancers. (A) Beynon five-layer model. (B) uT0. (C) uT1. (D) uT2. (E) uT3. (F) uT4. (G) N1.
In a later model described by Beynon et al, the mucosa and submucosa are distinguishable from one another. The second hypoechoic line represents only the mucosa and muscularis mucosa. The third hyperechoic line corresponds to the actual submucosa.40 There are only a few published studies that directly examine the efficacy of one model versus the other. However, in a study by Orrom et al in which 77 patients were divided into three investigative groups, the second and third groups differed only in relation to the staging model used. Accuracy of staging in the group applying the Hildebrandt and Feifel model was 77%; accuracy in the group using the Beynon model was 95%.32
Visualization of Perirectal Anatomy
Perirectal structures may also be visualized on ERUS. The upper anal canal begins with the appearance of the puborectalis, a hyperechoic band that wraps around the posterior half of the anal canal. As the probe approaches the middle portion of the anal canal, the internal anal sphincter appears as a circumferential hypoechoic band external to the perirectal fat. This band vanishes as the probe reaches the lower anal canal; it is replaced by the hyperechoic band of the external anal sphincter, which first appears external to the internal sphincter before disappearing from view.34 Additional structures that may be seen external to the perirectal fat and anteriorly in males include the seminal vesicles, prostate, bladder, and urethra. In females the vagina, uterus, and bladder are less easily visualized.35 Loops of small bowel may occasionally be noted.
Rectal Tumors
On endorectal ultrasound, rectal tumors appear as hypoechoic lesions and are staged according to level of invasion through the rectal wall, corresponding with the stages of the pathologic (TNM = tumor–node–metastasis) model (Table 2). To set these two staging systems apart, ultrasound stages are labeled with the prefix “u”; pathologic stages are labeled with the prefix “p”. Lymph nodes suspected of containing metastases do not always present with distinct sonographic features. In general, however, metastatic lymph nodes tend to be larger (> 3 mm in diameter), hypoechoic, nonhomogeneous, and more circular in shape, with well-defined borders. These features serve to distinguish them from inflammatory lymph nodes, which tend to be more hyperechoic and oval in shape, with indistinct, blurred borders.
Table 2.
American Joint Council on Cancer TNM Rectal Cancer Staging System
| TMN Stage | Histopathology | Ultrasonographic Features |
|---|---|---|
| T, tumor; N, node; M, metastasis. | ||
| Tx | Primary lesion cannot be assessed | Tumor depth cannot be determined |
| T0 | No primary tumor identified | No tumor visualized |
| Tis | Carcinoma in situ (tumor limited to mucosa) | 1st hypoechoic layer is expanded, but 2nd hyperechoic layer is intact |
| T1 | Tumor invades submucosa, but does not involve muscularis propria | Middle hyperechoic layer is stippled or broken in appearance, but generally intact |
| T2 | Tumor invades muscularis propria | Middle hyperechoic layer completely disrupted; tumor may extend into 2nd hypoechoic layer |
| T3 | Tumor invades into perirectal fat/serosa | Outer hyperechoic layer disrupted |
| T4 | Tumor invades into neighboring organs/peritoneal cavity | Tumor extension into neighboring organs |
| Nx | Nodal metastasis cannot be assessed | |
| N0 | No nodal metastasis | |
| N1 | Involvement of 1–3 perirectal/pericolic nodes | |
| N2 | Involvement of ≥ 4 perirectal/pericolic nodes | |
| Mx | Distal metastasis cannot be identified | |
| M0 | No distal metastasis | |
| M1 | Distal metastasis present | |
Endorectal Ultrasound in Follow-Up
ERUS can also be used to monitor for rectal cancer recurrence postoperatively. After surgery, the excision site appears as a pattern of mixed echogenicity, replacing the normal five-layer image. Anastomotic staples typically present as a circumferential line of small, local hyperechoic foci, without shadowing.41,42
PROBLEMS WITH IMAGING AND INTERPRETATION
Staging Criteria
The criteria for staging rectal tumors on ERUS are often equivocal. As such they are open to operator interpretation, particularly in the setting of masses or lymph nodes that, by virtue of their particular sonographic characteristics, do not fall neatly into one stage or another. This subjective aspect of ERUS interpretation means that operator experience, as well as the staging system used, have tremendous impact on the accuracy of ERUS and its role in properly directing therapy.
It can be extremely difficult to make fine distinctions between a deep tumor of one T-stage and an early tumor of the next T-stage. Those who prefer the Hildebrandt and Feifel model believe that both T0 and T1 lesions appear as a thickening of the second hypoechoic layer, and should be grouped together. A deep T1 lesion may present with irregularity and thickening of the hyperechoic submucosa, making it difficult to distinguish from a superficial T2 mass. A deep T2 lesion may look very much like a T3 mass, with “scalloping” of the perirectal fat instead of actual serosal invasion.
In the majority of incorrect ERUS interpretations, tumors are overstaged. Peritumoral inflammation and desmoplastic changes are commonly to blame, as both are difficult to differentiate from actual tumor borders. pT2 tumors are most susceptible to this sort of misinterpretation. Overstaging may also be the result of preoperative biopsies, which can create hematomas and disrupt sonographic anatomy. Fear of understaging is another cause that has been described.17
Since the addition of ERUS to routine workup for rectal cancer, the number of patients receiving neoadjuvant therapy has risen by roughly one-third. Given the risk of overstaging, it is possible that some patients are being overtreated.1 However, at least one study has demonstrated a positive predictive value for T3 and T4 lesions grouped together, as those primary tumors which typically receive neoadjuvant therapy, approaching 100%. Even with aggressive use of ERUS, ~15% of patients in this group are understaged.1
Nodal Staging
In nodal staging, the criteria for distinguishing malignant lymph nodes from inflammatory nodes are a source of controversy. The criteria for echogenicity and border characteristics are completely subjective, although at least one study has shown that as many as 72% of nodes with hypoechoic patterns are metastatic.16 Nodal size criteria are particularly problematic. Although metastatic lymph nodes do tend to be larger than normal nodes, the 3 to 5 mm diameter used as a cutoff is fairly arbitrary. In rectal cancer, as many as 50% of metastatic lymph nodes identified on histopathology may be smaller than 5 mm; as many as 8% may be smaller than 2 mm. A series by Kim et al reported that roughly 18% of nodes measuring less than 5 mm in diameter harbored metastases.18 Another study by Akasu et al reported even more alarming numbers: they found that the incidence of metastasis in nodes with diameters of ≤ 2 mm, 3 to 5 mm, and ≥ 6 mm was 9.5%, 47%, and 87%, respectively.19 This suggests significant impairment of both sensitivity and accuracy, particularly in light of data suggesting that ERUS can miss up to 20% of these smaller nodes.8,35
A recent study by Landmann et al compared the accuracy of ERUS in assessing nodal metastasis with the pathologic T-stage of the primary lesion. Based on nodal histopathology in 134 patients who had undergone radical resection without neoadjuvant therapy, they found that median lymph node size, median size of metastatic deposit, and accuracy of ERUS N-staging all decreased in the setting of less invasive tumors. In 44 pT3 resections, nodes were roughly 8 mm in size, metastatic deposits were 5.9 mm in size, and the accuracy of ERUS N-staging was 84%. In 21 pT1 resections, these numbers had dwindled to 3.3 mm, 0.3 mm, and 48%, respectively.20 Of 47 specimens with nodal disease, the lymph nodes and metastases were both larger in specimens accurately staged by ERUS.
This recent data brings into serious question the ability of ERUS to accurately predict the presence or absence of nodal disease in T1 lesions, and therefore casts doubt upon the wisdom of using ERUS findings as the basis for performing local excision. One suggestion has been to decrease the size criteria for malignancy. This might improve ERUS sensitivity in detecting nodal disease; however, it would also reduce its specificity and accuracy overall. Akasu et al examined this possibility, measuring ERUS specificity, sensitivity, and accuracy using different size criteria in T1 lesions. At a cutoff of 5 mm, sensitivity, specificity, and accuracy were respectively 38%, 94%, and 89%. At a cutoff of only 3 mm, these values changed to 75%, 49%, and 53%, respectively.19 A solution suggested by the authors was to combine a low cutoff with its high sensitivity, and low specificity with ERUS-guided FNA biopsy (presumably on ERUS-positive nodes) as a means of increasing specificity. One group reported a safe method of performing ERUS-guided fine needle aspiration (FNA) on pararectal nodes, producing a sensitivity of 71% and, more important, a specificity of 89%.19 On the other hand, in a study involving 80 patients, Harewood et al found no evidence to suggest that such biopsies had any benefit.43 They reported an overall accuracy of ~80% for N-staging with computed tomography (CT), ERUS, and ERUS-guided fine needle aspiration (FNA). However, there was no significant difference between any of the three groups. In a more recent article, Siddiqui et al1 concluded that ERUS-guided FNA failed to show any benefit because all perirectal nodes large enough to be visualized had metastatic involvement. Further studies of FNA are warranted before its widespread use.
Technical Sensitivity
Although it is simple in concept, performing an accurate ERUS examination can be difficult. Imaging quality may change with the position of the probe in relation to the surrounding rectal walls, and in relation to the environment within the rectum itself. Such changes can introduce acoustic shadowing and reverberation, hindering interpretation. This is another reason why the accuracy of ERUS is so dependent on operator experience. It may also explain the differences in accuracy that occur when different practitioners interpret tumors located in either the proximal or distal rectum, or tumors that are bulky or stenotic.
The presence of uniform acoustic contact is essential for the production of quality ERUS images, and depends on creating a fluid-filled environment uncontaminated by air, mucus secretions, blood, or feces. Position of the ERUS probe in relation to the tumor is just as critical. The axis of the probe must be perpendicular to the axis of the tumor, with the tip beyond the proximal tumor border. Inadequate bowel preparation results in fecal remnants, creating “reverberation defects” that obscure deep tumor margins and may cause overstaging. Air pockets between the probe and the tumor, whether from air bubbles trapped in the balloon or from unfilled space in the rectal vault due to insufficient inflation, produce strong acoustic shadows and block visualization of the deeper tissues. Tumors situated on the haustral folds are often overstaged because of artifact induced by tangential imaging. Given that depth of tumor invasion can vary between the lower and upper border of the tumor, and given that perirectal nodal metastases can spread superiorly from the primary tumor site, an inability to pass the tip of the probe proximal to the upper tumor border may result in understaging.44
The impact of tumor level on ERUS accuracy is controversial. Studies have suggested impaired visualization of tumors located in both the proximal and distal rectum.36 However, the position of the tumor within the vault may not influence accuracy at all. A study by Sailer et al divided 162 tumors into three groups based on tumor location, and failed to show any differences of statistical significance.36 Reduced accuracy in the staging of low rectal tumors has been attributed to the presence of the ampulla recti, whose surface anatomy makes it difficult to maintain uniform acoustic contact and orient the probe properly. Another explanation is poor definition of the five sonographic layers just above the anorectal line, particularly along the posterior wall.15,30,36 To improve accuracy, some have recommended filling the rectal vault with 150 cc of water to maintain acoustic contact, or using linear ERUS miniprobes (which we will soon describe) to achieve proper orientation. Increasing the probe frequency has also been tried as a means of improving wall definition.9
In the proximal rectum, the valves of Houston may present similar problems with regards to visualization of surface anatomy.44 The most commonly cited explanation is inability of the probe to pass the lesion.10,44 Bulky and stenotic tumors present similar challenges. Staging inaccuracies may occur due to distortion and compression of tumor into the rectal wall by a rigid probe or inflated balloon, a problem that is also seen in the setting of small polypoid lesions. These problems can be managed to some degree with the use of miniprobes or three-dimensional (3D) ERUS (see below), or by filling the rectal vault with water as described earlier.
OTHER ROLES FOR ENDORECTAL ULTRASOUND
Evaluation of Premalignant Lesions
Several studies have examined the utility of ERUS in evaluating biopsy-negative villous adenomas. Approximately 30 to 40% of rectal villous adenomas contain malignancy, and roughly 10% of biopsy-negative adenomas are misdiagnosed, even when polyps with malignant features (such as induration and ulceration) are excluded.45 The goal of using ERUS for these biopsy-negative lesions prior to excision would be to better identify foci of invasive tumor in the primary lesion or in the surrounding lymph nodes, thus minimizing the risk of inadequate resection. Although there are skeptics, it is believed that, with the use of higher-resolution transducers, ERUS is capable of distinguishing reliably between T0 and T1 masses. Current studies report favorable outcomes. In a meta-analysis of 258 biopsy-negative rectal adenomas, 24% of which were ultimately found to harbor undiagnosed invasive tumor, ERUS correctly identified tumor deposits in 81% of the lesions.45 In another series of 60 patients with pT0/pT1 lesions, ERUS detected invasive elements with 89% sensitivity and 88% specificity.37 Overstaging of benign lesions was most likely (1) after snare excision, when fibrosis from the scars mimic tumor penetration into deeper tissue layers; (2) due to location of lesions near the anal sphincters, obscuring visualization of the sonographic layers. These problems can be avoided by performing ERUS prior to excision, and by using higher-frequency transducers.
Evaluating Response to Neoadjuvant Chemoradiotherapy
The ability of ERUS to accurately evaluate tumor response to neoadjuvant chemoradiation prior to surgical resection is hampered primarily by the effects of the chemoradiation itself: tumor necrosis, fibrosis, and peritumoral inflammation caused by therapy can significantly compromise staging accuracy. These reactions may all appear sonographically indistinguishable from residual tumor, obscuring differentiation of the five layers of the rectal wall and resulting in overstaging. Various studies have cited accuracy rates ranging from 48 to 62%, and overstaging rates ranging from 37 to 83%, with lower rates in the setting of tumors responsive to therapy (29 to 41%) versus tumors that are nonresponsive (67 to 82%).30,38,46,47 Despite its lower accuracy in this setting, many practitioners believe that ERUS is useful as a bridge between the two treatment modalities. Residual tumor, when present, is thought to be consistently limited to the region of fibrosis, permitting investigators to determine the maximum possible depth of invasion, the closest possible distance from the anorectal ring, and the possibility of sphincter involvement. This idea has been supported in at least two studies.48,49 In one group, certain sonographic changes in the posttherapy ERUS images were noted to correlate with response of the tumor to chemoradiotherapy. If these sonographic changes are regularly confirmed in future studies as indicating response to therapy, use of ERUS in this setting may offer some patients the possibility of avoiding resection entirely.48
NEW DEVELOPMENTS IN ENDORECTAL ULTRASOUND
Endorectal Ultrasound-Guided Needle Biopsies
The potential role of ERUS-guided needle biopsy in assessing suspicious lymph nodes has already been discussed. Although it is attractive in theory, there is considerable doubt as to whether the technique will ultimately improve the accuracy of ERUS N-staging. However, this technique has been used in the evaluation of primary lesions, specifically peri-anastomotic foci suspicious for local recurrence. ERUS-guided needle biopsy is technically difficult to perform with the radial probes typically used for staging because the path of the needle cannot be visualized on transverse imaging. Linear probes, however, as shown by Akasu,19 have been utilized with some success. Some investigators employ multiplane transducers, using the transverse plane images to view the anatomy and the longitudinal plane to guide the needle.50 Studies have shown that this technique increases the specificity and accuracy of ERUS in detecting local recurrence.50 One recent report also demonstrated some success in assessing metastasis to the iliac lymph nodes, raising the possibility of being able to identify those patients who might be best treated with expanded radiation fields, or with palliative therapy.47
Three-Dimensional Endorectal Ultrasound
Proponents of three-dimensional ERUS (3D-ERUS) maintain that it provides superior visual images of tumor volume and the spatial relationships of tumor to surrounding anatomical structures. Two types of 3D-image construction have been reported. In some studies, transverse scanning is performed with a rigid ERUS probe to create a consecutive array of parallel sections stacked along an axis perpendicular to the images themselves. In other studies, rotational probing is performed, combining 360-degree transverse scanning with 100-degree longitudinal views.18 When reconstructed, the images have resolutions superior to those of MRI.51
3D-ERUS reportedly has several advantages over standard ERUS. Its ability to generate images in multiple planes may increase accuracy by improving diagnostic confidence, as has already been shown with MRI.21 Although the sample sizes have all been relatively small, several studies report that the accuracy of 3D-ERUS is superior to that of standard ERUS.18,22,52 The longitudinal scan planes, unique to 3D-ERUS, can precisely assess tumor size and position, facilitating accurate staging in the setting of bulky and stenotic tumors.21 Perhaps more important, the stereoscopic images generated by 3D-ERUS allow measurement and visualization of certain anatomic features, reducing interpreter error and offering potential predictive value. 3D-ERUS imaging facilitates the observer's ability to distinguish blood vessels from lymph nodes, and may enhance the precision of ERUS-guided needle biopsies.18,23 In one study, investigators were able to accurately measure the extent of circumferential infiltration, a feature shown to correlate with T-staging, lymphovascular invasion, histologic tumor differentiation, and nodal metastasis. The same group identified conical protrusions along the deep tumor margins whose numbers correlated with infiltration grade as well as T- and N-status.18,23 Like MRI, such images provide better definition of the mesorectal fascia, permitting evaluation of circumferential resection margins.6,22
These early studies suggest that 3D-ERUS may be capable of combining the high-resolution images of the rectal wall and cost-effectiveness of standard ERUS with the multiplanar and stereoscopic imaging capabilities of MRI. In time, this may make 3D-ERUS the premier imaging modality used in rectal cancer management.
Alternative Approaches in Sonography of Rectal Tumors
The difficulty in evaluating stenotic, bulky and proximal rectal tumors using the traditional rigid ERUS probe has prompted clinicians to experiment with alternative approaches to ultrasound. Transvaginal sonography employing longitudinal probes has been used in women, with some success, to evaluate stenotic tumors or rule out local recurrence following abdominoperineal resection.53 Miniaturized probes, or “miniprobes,” and probes on flexible scopes have also been developed and tested because of their ability to traverse these lesions. Flexible scopes are hampered by poor accuracy (49 to 59% T-staging accuracy; 60 to 78% N-staging accuracy).24,30 Miniprobes may be inserted through colonoscopes to assess tumors in the colon as well, and to evaluate iliac lymph nodes for M-staging.47 Although some studies have reported T- and N-staging accuracies of 82 to 90% and 67 to 87%, respectively, the probes emit only high frequencies (e.g., 12.5 to 30 MHz), and therefore have poor depth of penetration and poor ability to differentiate between T3 and T4 lesions.24,54 Given the high resolution for the superficial wall, however, many surgeons believe that these miniprobes will ultimately play a role in determining the presence of malignancy in broad-based rectal polyps. Miniprobes may also serve similar functions in the evaluation of colonic polyps, potentially offering some patients the option of endoscopic local excision over colectomy.54
Predictive Sonographic Features
A small number of studies have attempted to identify sonographic features that might serve as predictors of tumor invasiveness, response to neoadjuvant therapy, and long-term outcomes. One group noted small numbers of 1–3 mm hypoechoic spots in perirectal fat at the tumor margins. These spots were found to correlate positively with lymphovascular invasion, the presence of nodal or distal metastasis, and frequency of local recurrence.55 In another study, investigators used Doppler ultrasound to grade the vascularity of 29 uT3-staged rectal tumors. Examining tumor response to chemoradiotherapy, they noted significantly higher rates of response in tumors that were more extensively vascularized and had less vascular resistance.56
CONCLUSION
Endorectal ultrasound remains the most effective diagnostic tool for evaluating rectal cancer. It is easy to perform, well-tolerated, inexpensive, and readily usable in the clinic environment. Although it is operator-dependent, with a steep learning curve, the dedicated practitioner can master ERUS readily. Recent studies have reported lower levels of accuracy associated with ERUS than were reported previously; however, this is probably due to its more widespread use by less-experienced physicians, and we expect that the reported accuracy will improve over time. In addition to its value in tumor staging, studies have shown ERUS to be useful in evaluating adenomas for foci of malignancy, assessing tumor response to neoadjuvant therapy, and in posttreatment surveillance. The ongoing development of ERUS-guided biopsies, miniprobes, and 3D-ERUS offers the potential for further improvement in staging of lymph nodes and poorly accessible tumors, as well as prediction of response to therapy. It is clear that ERUS will remain a key element in the treatment of rectal cancer for some time.
REFERENCES
- 1.Siddiqui A A, Fayiga Y, Huerta S. The role of endoscopic ultrasound in the evaluation of rectal cancer. Int Semin Surg Oncol. 2006;3:36–42. doi: 10.1186/1477-7800-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gavioli M, Losi L, Luppi G, et al. Preoperative therapy for lower rectal cancer and modifications in distance from anal sphincter. Int J Radiat Oncol Biol Phys. 2007;69:370–375. doi: 10.1016/j.ijrobp.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 3.Quirke P, Durdy P, Dixon M F, Williams N S. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2:996–999. doi: 10.1016/s0140-6736(86)92612-7. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst. 1988;80:21–29. doi: 10.1093/jnci/80.1.21. [DOI] [PubMed] [Google Scholar]
- 5.Krook J E, Moertel C G, Gunderson L L, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–715. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- 6.NIH Consensus Conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264:1444–1450. [PubMed] [Google Scholar]
- 7.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 8.Engelen S M, Beets G L, Beets-Tan R GH. Role of preoperative local and distant staging in rectal cancer. Onkologie. 2007;30:141–145. doi: 10.1159/000099026. [DOI] [PubMed] [Google Scholar]
- 9.Bartram C, Brown G. Endorectal ultrasound and magnetic resonance imaging in rectal cancer staging. Gastroenterol Clin North Am. 2002;31:827–839. doi: 10.1016/s0889-8553(02)00027-4. [DOI] [PubMed] [Google Scholar]
- 10.Ptok H, Marusch F, Meyer F, et al. Feasibility and accuracy of TRUS in the pre-treatment staging for rectal carcinoma in general practice. Eur J Surg Oncol. 2006;32:420–425. doi: 10.1016/j.ejso.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Rieger N, Tjandra J, Solomon M. Endoanal and endorectal ultrasound: applications in colorectal surgery. ANZ J Surg. 2004;74:671–675. doi: 10.1111/j.1445-1433.2004.02884.x. [DOI] [PubMed] [Google Scholar]
- 12.Gerdes B, Langer P, Kopp I, Bartsch D, Stinner B. Localization of the peritoneal reflection in the pelvis by endorectal ultrasound. Surg Endosc. 1998;12:1401–1404. doi: 10.1007/s004649900868. [DOI] [PubMed] [Google Scholar]
- 13.Kim N K, Kim M J, Yun S H, Sohn S K, Min J S. Comparative study of transrectal ultrasonography, pelvic computerized tomography, and magnetic resonance imaging in preoperative staging of rectal cancer. Dis Colon Rectum. 1999;42:770–775. doi: 10.1007/BF02236933. [DOI] [PubMed] [Google Scholar]
- 14.Skandarajah A R, Tjandra J J. Preoperative loco-regional imaging in rectal cancer. ANZ J Surg. 2006;76:497–504. doi: 10.1111/j.1445-2197.2006.03744.x. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Aguilar J, Pollack J, Lee S H, et al. Accuracy of endorectal ultrasonography in preoperative staging of rectal tumors. Dis Colon Rectum. 2002;45:10–15. doi: 10.1007/s10350-004-6106-3. [DOI] [PubMed] [Google Scholar]
- 16.Badger S A, Devlin P B, Neilly P J, Gilliland R. Preoperative staging of rectal carcinoma by endorectal ultrasound: is there a learning curve? Int J Colorectal Dis. 2007;22:1261–1268. doi: 10.1007/s00384-007-0273-3. [DOI] [PubMed] [Google Scholar]
- 17.Massari M, De Simone M, Cioffi U, Rosso L, Chiarelli M, Gabrielli F. Value and limits of endorectal ultrasonography for preoperative staging of rectal carcinoma. Surg Laparosc Endosc. 1998;8:438–444. [PubMed] [Google Scholar]
- 18.Kim J C, Kim H C, Yu S C, et al. Efficacy of 3-dimensional endorectal ultrasonography compared with conventional ultrasonography and computed tomography in preoperative rectal cancer staging. Am J Surg. 2006;192:89–97. doi: 10.1016/j.amjsurg.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 19.Akasu T, Kondo H, Moriya Y, et al. Endorectal ultrasonography and treatment of early stage rectal cancer. World J Surg. 2000;24:1061–1068. doi: 10.1007/s002680010151. [DOI] [PubMed] [Google Scholar]
- 20.Landmann R G, Wong W D, Hoepfl J, et al. Limitations of early rectal cancer nodal staging may explain failure after local excision. Dis Colon Rectum. 2007;50:1520–1525. doi: 10.1007/s10350-007-9019-0. [DOI] [PubMed] [Google Scholar]
- 21.Hünerbein M, Pegios W, Rau B, Vogl T J, Felix R, Schlag P M. Prospective comparison of endorectal ultrasound, three-dimensional endorectal ultrasound, and endorectal MRI in the preoperative evaluation of rectal tumors. Surg Endosc. 2000;14:1005–1009. doi: 10.1007/s004640000345. [DOI] [PubMed] [Google Scholar]
- 22.Giovannini M, Bories E, Pesenti C, Moutardier V, Lelong B, Delpéro J R. Three-dimensional endorectal ultrasound using a new freehand software program: results in 35 patients with rectal cancer. Endoscopy. 2006;38:339–343. doi: 10.1055/s-2005-870412. [DOI] [PubMed] [Google Scholar]
- 23.Kim J C, Cho Y K, Kim S Y, Park S K, Lee M G. Comparative study of three-dimensional and conventional endorectal ultrasonography used in rectal cancer staging. Surg Endosc. 2002;16:1280–1285. doi: 10.1007/s00464-001-8277-5. [DOI] [PubMed] [Google Scholar]
- 24.Zammit M, Jenkins T, Urie A, O'Dwyer P J, Molloy R G. A technically difficult endorectal ultrasound is more likely to be inaccurate. Colorectal Dis. 2005;7:486–491. doi: 10.1111/j.1463-1318.2005.00869.x. [DOI] [PubMed] [Google Scholar]
- 25.Kulig J, Richter P, Gurda-Duda A, Gach T, Klek S. The role and value of endorectal ultrasonography in diagnosing T1 rectal tumors. Ultrasound Med Biol. 2006;32:469–472. doi: 10.1016/j.ultrasmedbio.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Bali C, Nousias V, Fatouros M, Stefanou D, Kappas A M. Assessment of local stage in rectal cancer using endorectal ultrasonography (EUS) Tech Coloproctol. 2004;8:S170–S173. doi: 10.1007/s10151-004-0147-4. [DOI] [PubMed] [Google Scholar]
- 27.Gualdi G F, Casciani E, Guadalaxara A, d'Orta C, Polettini E, Pappalardo G. Local staging of rectal cancer with transrectal ultrasound and endorectal magnetic resonance imaging: comparison with histologic findings. Dis Colon Rectum. 2000;43:338–345. doi: 10.1007/BF02258299. [DOI] [PubMed] [Google Scholar]
- 28.Blomqvist L, Machado M, Rubio C, et al. Rectal tumour staging: MR imaging using pelvic phased-array and endorectal coils vs. endoscopic ultrasonography. Eur Radiol. 2000;10:653–660. doi: 10.1007/s003300050979. [DOI] [PubMed] [Google Scholar]
- 29.Kruskal J B, Sentovich S M, Kane R A. Staging of rectal cancer after polypectomy: usefulness of endorectal US. Radiology. 1999;211:31–35. doi: 10.1148/radiology.211.1.r99ap1231. [DOI] [PubMed] [Google Scholar]
- 30.Steele S R, Martin M J, Place R J. Flexible endorectal ultrasound for predicting pathologic stage of rectal cancers. Am J Surg. 2002;184:126–130. doi: 10.1016/s0002-9610(02)00903-0. [DOI] [PubMed] [Google Scholar]
- 31.Harewood G C. Assessment of publication bias in the reporting of EUS performance in staging rectal cancer. Am J Gastroenterol. 2005;100:808–816. doi: 10.1111/j.1572-0241.2005.41035.x. [DOI] [PubMed] [Google Scholar]
- 32.Orrom W J, Wong W D, Rothenberger D A, Jensen L L, Goldberg S M. Endorectal ultrasound in the preoperative staging of rectal tumors. A learning experience. Dis Colon Rectum. 1990;33:654–659. doi: 10.1007/BF02150740. [DOI] [PubMed] [Google Scholar]
- 33.Marusch F, Koch A, Schmidt U, et al. Routine use of transrectal ultrasound in rectal carcinoma: results of a prospective multicenter study. Endoscopy. 2002;34:385–390. doi: 10.1055/s-2002-25292. [DOI] [PubMed] [Google Scholar]
- 34.Schaffzin D M, Wong W D. Surgeon-performed ultrasound: endorectal ultrasound. Surg Clin North Am. 2004;84:1127–1149. doi: 10.1016/j.suc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Giovannini M, Ardizzone S. Anorectal ultrasound for neoplastic and inflammatory lesions. Best Pract Res Clin Gastroenterol. 2006;20:113–135. doi: 10.1016/j.bpg.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Sailer M, Leppert R, Bussen D, Fuchs K H, Thiede A. Influence of tumor position on accuracy of endorectal ultrasound staging. Dis Colon Rectum. 1997;40:1180–1186. doi: 10.1007/BF02055164. [DOI] [PubMed] [Google Scholar]
- 37.Starck M, Bohe M, Simanaitis M, Valentin L. Rectal endosonography can distinguish benign rectal lesions from invasive early rectal cancers. Colorectal Dis. 2003;5:246–250. doi: 10.1046/j.1463-1318.2003.00416.x. [DOI] [PubMed] [Google Scholar]
- 38.Schaffzin D M, Wong W D. Endorectal ultrasound in the preoperative evaluation of rectal cancer. Clin Colorectal Cancer. 2004;4:124–132. doi: 10.3816/ccc.2004.n.015. [DOI] [PubMed] [Google Scholar]
- 39.Hildebrandt U, Feifel G. Preoperative staging of rectal cancer by intrarectal ultrasound. Dis Colon Rectum. 1985;28:42–46. doi: 10.1007/BF02553906. [DOI] [PubMed] [Google Scholar]
- 40.Kim H J, Wong W D. Role of endorectal ultrasound in the conservative management of rectal cancers. Semin Surg Oncol. 2000;19:358–366. doi: 10.1002/ssu.6. [DOI] [PubMed] [Google Scholar]
- 41.de Anda E H, Lee S H, Finne C O, Rothenberger D A, Madoff R D, Garcia-Aguilar J. Endorectal ultrasound in the follow-up of rectal cancer patients treated by local excision or radical surgery. Dis Colon Rectum. 2004;47:818–824. doi: 10.1007/s10350-004-0514-2. [DOI] [PubMed] [Google Scholar]
- 42.Löhnert M S, Doniec J M, Henne-Bruns D. Effectiveness of endoluminal sonography in the identification of occult local rectal cancer recurrences. Dis Colon Rectum. 2000;43:483–491. doi: 10.1007/BF02237191. [DOI] [PubMed] [Google Scholar]
- 43.Harewood G C, Wiersma M J. Cost-effectiveness of endoscopic ultrasonography in the evaluation of proximal rectal cancer. Am J Gastroenterol. 2002;97:874–882. doi: 10.1111/j.1572-0241.2002.05603.x. [DOI] [PubMed] [Google Scholar]
- 44.Santoro G A, D'Elia A, Battistella G, Di Falco G. The use of a dedicated rectosigmoidoscope for ultrasound staging of tumours of the upper and middle third of the rectum. Colorectal Dis. 2006;9:61–66. doi: 10.1111/j.1463-1318.2006.01012.x. [DOI] [PubMed] [Google Scholar]
- 45.Worrell S, Horvath K, Blakemore T, Flum D. Endorectal ultrasound detection of focal carcinoma within rectal adenomas. Am J Surg. 2004;187:625–629. doi: 10.1016/j.amjsurg.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Rau B, Hünerbein M, Barth C, et al. Accuracy of endorectal ultrasound after preoperative radiochemotherapy in locally advanced rectal cancer. Surg Endosc. 1999;13:980–984. doi: 10.1007/s004649901151. [DOI] [PubMed] [Google Scholar]
- 47.Bhutani M S. Recent developments in the role of endoscopic ultrasonography in diseases of the colon and rectum. Curr Opin Gastroenterol. 2007;23:67–73. doi: 10.1097/MOG.0b013e328011630b. [DOI] [PubMed] [Google Scholar]
- 48.Gavioli M, Bagni A, Piccagli I, Fundaro S, Natalini G. Usefulness of endorectal ultrasound after preoperative radiotherapy in rectal cancer; comparison between sonographic and histopathologic changes. Dis Colon Rectum. 2000;43:1075–1083. doi: 10.1007/BF02236553. [DOI] [PubMed] [Google Scholar]
- 49.Assenat E, Thézenas S, Samalin E, et al. The value of endoscopic rectal ultrasound in predicting the lateral clearance and outcome in patients with lower-third rectal adenocarcinoma. Endoscopy. 2007;39:309–313. doi: 10.1055/s-2007-966211. [DOI] [PubMed] [Google Scholar]
- 50.Hünerbein M, Totkas S, Moesta K T, Ulmer C, Handke T, Schlag P M. The role of transrectal ultrasound-guided biopsy in the postoperative follow-up of patients with rectal cancer. Surgery. 2001;129:164–169. doi: 10.1067/msy.2001.110428. [DOI] [PubMed] [Google Scholar]
- 51.Christensen A F, Nielsen M B, Svendsen L B, Engelholm S A. Three-dimensional anal endosonography may improve detection of recurrent anal cancer. Dis Colon Rectum. 2006;49:1527–1532. doi: 10.1007/s10350-006-0661-8. [DOI] [PubMed] [Google Scholar]
- 52.Manger T, Stroh C. Accuracy of endorectal ultrasonography in the preoperative staging of rectal cancer. Tech Coloproctol. 2004;8:S14–S15. doi: 10.1007/s10151-004-0099-8. [DOI] [PubMed] [Google Scholar]
- 53.Dhamanaskar K P, Thurston W, Wilson S R. Transvaginal sonography as an adjunct to endorectal sonography in the staging of rectal cancer in women. AJR Am J Roentgenol. 2006;187:90–98. doi: 10.2214/AJR.04.1363. [DOI] [PubMed] [Google Scholar]
- 54.Hünerbein M. Endorectal ultrasound in rectal cancer. Colorectal Dis. 2003;5:402–405. doi: 10.1046/j.1463-1318.2003.00516.x. [DOI] [PubMed] [Google Scholar]
- 55.Sunouchi K, Sakaguchi M, Higuchi Y, Namiki K, Muto T. Small spot sign of rectal carcinoma by endorectal ultrasonography: histologic relation and clinical impact on postoperative recurrence. Dis Colon Rectum. 1998;41:649–653. doi: 10.1007/BF02235276. [DOI] [PubMed] [Google Scholar]
- 56.Barbaro B, Valentini V, Coco C, et al. Tumor vascularity evaluated by transrectal color doppler US in predicting therapy outcome for low-lying rectal cancer. Int J Radiat Oncol Biol Phys. 2005;63:1304–1308. doi: 10.1016/j.ijrobp.2005.04.050. [DOI] [PubMed] [Google Scholar]