ABSTRACT
The clinical course of Crohn's disease (CD) is characterized by unpredictable phases of disease activity and quiescence. The majority of CD patients experience mild to moderate disease or are in clinical remission over significant periods during the course of their disease. These patients can be treated conservatively with 5-aminosalicylates or budesonide depending on the disease location. Those patients with more severe forms of the disease who require corticosteroids should be treated more aggressively with early introduction of immunomodulator and/or biologic therapy, which may help to prevent the complications associated with CD. It has been suggested that therapies directed at mucosal healing may favorably modify the natural history of CD. As newer, more effective medications become available and new therapeutic approaches are introduced (top-down therapy), mucosal healing, and not solely clinical remission, may well become the preferred treatment objective.
Keywords: Crohn's disease, medical management
Crohn's disease (CD) is a chronic inflammatory condition of unknown etiology. The disease can affect any part of the gastrointestinal tract from the mouth to the anus, but most commonly involves the ileum and colon. The inflammatory process begins as a mucosal process and may progress to affect the entire wall, typically in a patchy distribution with normal intervening mucosa.
CD affects an estimated 400,000 to 600,000 people in North America; it often presents in the teenage years, although individuals in their 60s and 70s are at increased risk of developing the disease.1,2,3,4 CD carries significant morbidity, with up to 15% of patients being rendered incapable of working after 10 to 20 years of diagnosis.5,6,7 The mortality rate for CD is slightly higher than in the general population.5
The clinical course of CD is characterized by unpredictable phases of disease activity and quiescence. Over the course of the disease ~75% of patients will experience chronic intermittent disease, ~10% will suffer from chronically active disease; the remainder will be asymptomatic over many years.2,8 Over 50% of patients will go on to develop complications in the form of stricturing (stenotic) or perforating (fistulizing, abscess) disease.9,10
The location of inflammation is an important determinant of the type of complications that are likely to develop.9,10,11 Stricturing disease is more likely to occur in patients with small bowel disease involvement; fistulizing disease will occur more frequently in patients with perianal disease involvement.9,10,11 Surgical intervention is generally reserved for patients whose complications are severe or when medical management is unsuccessful. As many as 50% of all CD patients undergo intestinal resection within 5 years from initial diagnosis.2,6,7,8,9,">11,12,13,14 Because this percentage is based on studies at tertiary referral centers, the overall percentage is likely to be lower.
The pathogenesis of CD is not well understood, a fact that has stood in the way of the discovery of an effective cure and the development of more effective treatment strategies. The disease process varies substantially from individual to individual. Unfortunately, at its onset, the frequency and intensity of exacerbations, as well as the precise form that the disease will take and whether complications will develop cannot be known.15 Therefore, medical therapies have been largely directed at controlling symptoms by targeting the hosts immune response in an effort to effectively suppress the inflammatory process. Thus, the goals of medical therapy have been limited to the following:
Induction and maintenance of clinical remission
Reduction of complications (strictures, fistula)
Improving quality of life
Minimizing drug toxicity
Restoring and maintaining nutrition
Limiting the need for surgery and/or hospitalization
MEDICAL THERAPY
Treatment for CD depends upon the location of inflammation, severity of disease, complications, and the response of the patient to medical treatment. Over the years, several classes of medications have been developed for the treatment of CD. These differ largely on the basis of their relative potency in suppressing the host immune response. However, the more potent the medication, the more likely will be the occurrence of adverse affects.
Therefore, the usual recommended approach to medical therapy is to start with the least potent medication for milder disease and to escalate therapy as disease severity increases. Unfortunately, relapses with the conventional forms of therapy are common and research into alternative therapeutic approaches, both to control symptoms and to prevent recurring episodes continues.
The clinical assessment of disease severity and its response and remission for use in clinical studies has been a major challenge. Most trials evaluating medication efficacy use the Clinical Disease Activity Index (CDAI), which is a research tool used to quantify the symptoms of patients with CD.16 The role of CDAI in clinical practice, however, is limited. It is a cumbersome technique and has been criticized for being highly subjective. It has a substantial degree of interobserver variability, it depends upon a continuous variable with arbitrary cut-off values, and it fails to take into account inflammation activity.17,18,19 Studies have shown that better accuracy for assessing disease severity can be achieved when combining clinical data with biochemical markers of inflammation (such as CRP [c-reactive protein]) and endoscopy findings.20 Currently, no gold standard for assessing clinical disease activity in clinical practice has been proposed.
The American College of Physicians has established working definitions for CD activity to guide the physician in a clinical practice setting (Table 1).21 Treatment of stenotic, fistulation, and perianal CD not accounted for by these working definitions will be discussed in later sections by Weiss, Efron, and Vivas.
Table 1.
Working Definitions of Crohn's Disease Activity
| MILD–MODERATE DISEASE |
| Patient is ambulatory and able to tolerate oral alimentation without manifestations of dehydration, toxicity (high fevers, rigors, prostration), abdominal tenderness, painful mass, obstruction, or > 10% weight loss. |
| MODERATE–SEVERE DISEASE |
| Patients have failed to respond to treatment for mild–moderate disease or have more prominent symptoms of fevers, significant weight loss, abdominal pain or tenderness, intermittent nausea or vomiting (without obstructive findings), or significant anemia. |
| SEVERE–FULMINANT DISEASE |
| Patients have persisting symptoms despite the introduction of steroids as outpatients, or present with high fever, persistent vomiting or evidence of intestinal obstruction, rebound tenderness, cachexia or evidence of an abscess. |
| REMISSION |
| Patients are asymptomatic or without inflammatory sequelae. Definition includes patients who have responded to acute medical intervention or have undergone surgical resection without gross evidence of residual disease. Patients requiring steroids to maintain well-being are considered to be steroid-dependent and are usually not considered to be in remission. |
Induction and Maintenance of Remission in Mild to Moderate Crohn's Disease
The treatment guidelines for mild to moderate CD have generated much controversy over the years. Issues of drug safety and tolerance in a setting of lesser disease severity have predominated largely at the expense of therapeutic efficacy. An increased awareness of the detrimental effects of corticosteroids, which were previously used as a first-line therapy, has led to stringent attempts to limit their use for patients with more severe forms of the disease and those who have not responded to other agents. Consequently, the current established first line agents for the treatment of mild to moderate CD include 5-aminosalicylate (5-ASA) compounds, budesonide, and antibiotics.
5-AMINOSALICYLATES (5-ASA)
Sulfasalazine, consisting of 5-ASA linked to sulfapyridine moiety, was the first 5-ASA to be used for the treatment of CD. Several controlled clinical trials using sulfasalazine at dosages between 3 to 6 g per day have demonstrated a benefit over placebo in inducing remission in subgroups of patients with mild to moderate CD.22,23,24 The subgroups that achieved superior rates over placebo at induction of clinical remission included patients with disease involvement of the colon and patients not previously exposed to steroids.22,23 Patients with disease localized to the small bowel and those who had undergone previous surgery were less likely to benefit from sulfasalzine.22,23,25 Unfortunately, adverse side effects are common with sulfasalazine and occur in up to 30% of patients in a dosage-dependent manner, which limits their use over extended periods of time.26,27
Delayed release 5-ASA (mesalamine) exists in the form of enteric-coated preparations that deliver the active 5-ASA component in variable amounts to the small bowel and colon. This class of drug includes those that are released by intraluminal pH > 7 primarily in the terminal ileum and colon (Asacol; Proctor & Gamble Pharmaceuticals, Cincinnati, OH) and those that are released by hydrophilic effects primary in the small bowel (Pentasa-Ferring A/S Corp., Wayne, PA).11,28 The overall benefit of these agents, even at high dosages, in inducing clinical remission in mild to moderate CD was not demonstrated in several randomized controlled trials.29,30,31,32 In these trials, however, a reduction in disease severity as measured by CDAI scores was observed with higher dosages of mesalamine (Pentasa 4 g/day; Asacol 3.2 g/day).29,30,31,32 The clinical significance of this benefit is unclear. Although these agents are safe, a solid evidence base for their use as first-line agents for the induction of clinical remission in patients with mild to moderately active CD is lacking.
BUDESONIDE
Budesonide is a corticosteroid with a strong affinity for corticosteroid receptors and rapid hepatic metabolism.33,34 It acts as a topical steroid and has fewer side effects than conventional corticosteroids.33,34 Budesonide has been formulated into a coated-capsule that facilitates delivery of the medication to the terminal ileum and proximal colon.
In a meta-analysis, budesonide at a dosage of 9 mg/day was effective for induction of remission in patients with mild to moderate CD, particularly in patients with disease involvement of the ileum or ileum and proximal colon.35 The rate of clinical remission was ~50% after 8 weeks of therapy.36,37 In a single randomized controlled trial comparing budesonide with mesalamine favored budesonide for induction of remission in patients with mild to moderately active CD of the ileum and/or proximal colon. A higher rate of severe adverse events was observed in the mesalamine group, a factor that was attributed, in part, to its lower efficacy.38 In a meta-analysis of five randomized controlled trials, budesonide was not as effective as conventional corticosteroids in inducing clinical remission in CD patients, but did produce significantly less adverse events.35
In summary, for CD in the ileum alone and /or proximal colon, budesonide offers an effective therapy for induction of remission in patients with mild to moderate CD. Although budesonide may be less efficacious than conventional corticosteroids for inducing remission, it is associated with far fewer adverse effects.
ANTIBIOTICS
Metronidazole and ciprofloxacin are widely used by clinicians for treatment of mild to moderately active CD. Unfortunately, the available data are limited to small uncontrolled trials that have not consistently demonstrated efficacy with these agents at inducing clinical remission for mild to moderate CD.39,40,41,42,43
A single randomized controlled trial did demonstrate improvement in disease activity with high-dosage metronidazole (20 mg/kg per day) over placebo in the treatment of ileocolitis and colitis.39 However, no difference in clinical remission rates between groups was observed.39 In a small preliminary study, ciprofloxacin at a dosage of 1 g/day proved superior to placebo at inducing clinical remission when combined with other therapies for mild to moderately active CD.43 In a larger study, the combination of ciprofloxacin 1 g/day and metronidazole fared no better than a placebo when added to budesonide for induction of clinical remission in patients with mild to moderately active CD.44 The high frequency of serious adverse effects, however, militates against long-term use of these medications.43,44
Rifaximin, an oral antibacterial agent with negligible systemic absorption, was shown in a recent randomized controlled trial at a dosage of 800 mg twice daily to be superior to placebo at inducing clinical remission in a subgroup of patients with mild to moderately active CD. This subgroup also presented with elevated C-reactive protein serum levels.45 Although these results were encouraging and the medication was well tolerated, further studies are needed to confirm the drug's efficacy.
Sandborn et al46 proposed an evidence-based algorithm for inducing remission in patients with mild to moderate CD that is dependent on disease location. For ileal or ileocecal disease, budesonide was recommended as a first-line therapy at a dosage of 9 mg daily for 8 to 16 weeks. In patients with mild ileocecal disease or disease limited to the colon, sulfasalazine at a dosage of 4 g daily was recommended as an induction therapy for 16 weeks. Mesalamine at a dosage 3.2 to 4.6 g daily was suggested for patients who were intolerant to sulfasalazine, although evidence for such use was lacking. Patients who did respond to first-line therapy were reclassified as having moderate to severe disease and treated accordingly (see next section).
There is no data to support the continued use of 5-ASA, antibiotics, or budesonide in patients with mild to moderate CD who are induced into clinical remission. In several trials, neither sulfasalazine nor mesalamine demonstrated a benefit over placebo for maintenance of remission in mild to moderate CD.22,47,48 Similarly, trials using budesonide at a maintenance dosage of 6 mg/day did not demonstrate significant long-term benefits.49 In a pooled analysis of four randomized, placebo controlled trials, budesonide was found to be superior to placebo at prolonging time to relapse. However, the effect was not sustained at one-year follow-up. No data exists to support a role for antibiotics in maintenance therapy.50
Induction and Maintenance of Remission in Moderate to Severe Crohn's Disease
CORTICOSTEROIDS
Patients who do not respond to therapy for mild to moderate CD disease or whose symptoms are more prominent (Table 1) are commonly treated with oral corticosteroids (CS). Approximately 50% of patients with CD require CS at some point in their disease history.2,51 However, the need for therapy with CS in the first 6 months of disease is considered a bad prognostic factor as these patients are more likely to require surgery.52
In population-based studies and in clinical trials, CS have been shown to be superior to placebo, 5-aminosalicylates, antibiotics, and budesonide at inducing clinical remission in patients with CD.22,23,35,45,53 More than half of patients receiving CS are induced into clinical remission at dosages adjusted to disease severity.18,54,55 Patients with fistulizing and stricturing CD at diagnosis are at an increased risk of CS failure.54
Treatment consists of oral prednisone or prednisolone starting at 40 mg daily titrating up to 1 mg/kg per day for clinical response. Patients who fail to achieve complete clinical response within 8 to 12 weeks are considered treatment failures. After a complete clinical response is reached, dosage is gradually reduced by 5 to 10 mg weekly to a dosage of 20 mg. Dosage then continues to be reduced by 2 to 5 mg weekly until discontinuation.1
After the medication is discontinued a majority of patients relapse and either become steroid dependent and/or require surgery.54,55 With long-term use, CS are associated with a variety of adverse effects including moon face, acne, easily bruised, fluid retention, diabetes mellitus, osteoporosis, osteonecrosis, and adrenal suppression. Therefore, CS should not be considered as maintenance therapy. Their role is strictly to induce remission.
IMMUNOMODULATORS
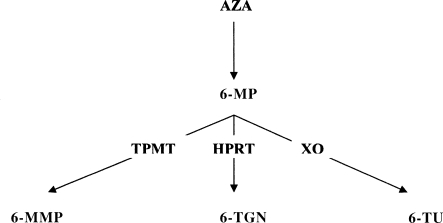
Azathioprine (AZA) and 6-mercaptopurine (6-MP) are the most widely used immunomodulators for patients with CD. Azathioprine is a prodrug of 6-MP, which is metabolized to 6-methylmercaptopurine (6-MMP) and the active 6-thioguanine (6-TG) metabolites (Fig. 1). AZA and 6 MP have shown efficacy over placebo at inducing and maintaining remission and at reducing or eliminating steroid dependence while maintaining a steroid free remission.56,57,58,59 These medications have a delayed onset of action and their maximal effects are often observed after 2 to 3 months.58 As a result, they are used to maintain clinical remission and less frequently for induction of remission in a setting of an acute exacerbation.
Figure 1.
Thiopurine metabolism: azathioprine (AZA) is converted in the body to 6-mercaptopurine (6-MP). 6-MP is metabolized along three competing pathways. (1) Xanthine oxidase (XO) yielding thiouric acid (6-TU), (2) thiopurine methyltransferase (TPMT) yielding 6-methylmercaptopurine (6-MMP), and (3) hypoxanthine-guanine phosphoribosyltransferase (HPRT) yielding 6-thioguanine nucleotides (6-TGN).
The timing for the introduction of 6-MP or AZA in the treatment process has been the subject of much debate. These medications are usually prescribed for patients with steroid refractory or steroid-dependent CD. In two randomized placebo controlled trials in pediatric and adult populations, the addition of 6-MP or AZA to CS significantly lessened the need for CS and improved maintenance of steroid-free remission.60,61 These results suggest that AZA or 6MP should be seriously considered as part of the initial treatment regimen for patients with moderate-to-severe CD.
The benefits of 6-MP and AZA have been demonstrated at dosages of 1.0 to 1.5 mg/kg per day and 2.0 to 2.5 mg/kg per day, respectively.62 However, ~9 to 11% of patients are at risk of developing severe myelosuppression within weeks after introduction of therapy.63,64,65 The patients that are at risk for myelosuppression preferentially metabolize 6-MP to 6-TG instead of 6-MMP due to a complete (0.3% of the population) or partial (9 to 11% of the population) deficiency of the enzyme thiopurine methyl transferase (TPMT).63,64,65 Therefore, many practitioners start 6-MP/AZA at lower dosages and gradually increase dosage at 1- to 2-week intervals while monitoring blood counts. An alternative approach is to determine TPMT activity (through genotype analysis) prior to initiating 6-MP/AZA and adjust the dosage accordingly. This approach allows for therapeutic levels of medication to be reached more quickly and can be cost effective.66,67,68,69 Patients with normal TPMT activity (normal genotype) can begin therapy at the standard dosage. Patients with intermediate enzyme activity (heterozygote genotype) can be started at half the standard dosage; patients with no enzyme activity (homozygote genotype) should be considered for alternative therapies.66
The major side effect of 6-MP/AZA is myelosuppression, which is a more common occurrence in patients with partial or absent TPMT activity. Myelosuppression, however, can still occur in patients with normal TPMT activity at any time over the course of therapy.64 Therefore, the monitoring of blood counts every 2 weeks from initiation of therapy for the first 6 weeks followed by monthly monitoring is advisable. At 3 months, once a stable dosage is achieved monitoring of blood counts should continue at intervals of 8 to 10 weeks. Awareness of drug interactions is essential when administering 6-MP/AZA. Drug interaction is particularly common with concomitant use of allopurinol, which can increase levels of 6-MP by inhibiting xanthine oxidase, thereby potentiating the effect of myelosuppression (Fig. 1). When both drugs are used concomitantly, the dosage of 6-MP/AZA should be decreased by a factor of two thirds or greater from the standard dosage.70 In cases of myelosuppression, medication should be discontinued and alternative therapies considered.
A small subgroup of CD patients do not respond to 6-MP or AZA due to higher than normal TPMT activity that results in the preferential metabolism of 6-MP to 6-MMP instead of the active 6-TG metabolite.71 In these patients, coadministration of allopurinol can safely and effectively optimize 6-TG levels thus increasing the therapeutic efficacy of 6-MP/AZA.72 This approach, however, has not been universally adopted and should be practiced with caution due to the potential for serious adverse effects associated with combination therapy.
There is no clear consensus as to the duration of therapy with 6-MP or AZA in patients who are maintained in clinical remission. Data from a randomized placebo controlled trial demonstrated that patients on AZA in remission for 42 months had fewer relapses when therapy was continued for an additional 18 months.73 The suggestion is that therapy should be continued for at least 3.5 years and beyond.
AZA and 6-MP are relatively safe for long-term maintenance; however, serious though infrequent, adverse effects can occur.66,74 A slight increased risk of lymphoma has been reported in patients treated with 6-MP.75,76 However, this risk must be weighed against the clinical benefits of these medications in CD patients.
Methotrexatve (MTX) is considered a second-line immunomodulator for patients who do not respond to or cannot tolerate 6MP/AZA. MTX is effective when administered subcutaneously or intramuscularly (oral route has minimal benefit) and usually requires 8 to 16 weeks before the beneficial effects are observed.76,77,78,79,80 In randomized placebo controlled trials of CD patients with chronically active steroid-dependent disease, MTX at a dosage of 25 mg intramuscularly (IM) weekly was more effective than placebo at inducing remission and for maintenance of remission when used at a lower dosage (15 mg IM weekly) over 40 weeks.76,80 MTX treated patients were less likely to require prednisone, which supports the use of MTX as a steroid-sparing agent.76,80
The occurrence of adverse events with MTX is not uncommon.81 In the North American Crohn's Study Group, MTX treatment was withdrawn in 17% of patients (as compared with 2% in the placebo group).80 A major concern with long-term use of MTX is its potential to induce hepatic fibrosis and cirrhosis, as is sometimes seen in patients who are treated with MTX for psoriasis.82 Because some of the toxic effects of MTX are thought to be mediated by the antifolate effect of MTX, folic acid supplementation at a dosage of 1 to 5 mg/day is recommended and is commonly used in conjunction with MTX in clinical practice.83
Therefore, although low-dosage MTX is effective in the treatment in steroid-dependent and AZA-/6MP-intolerant CD patients it remains to be seen whether low-dosage MTX is effective and safe as a long-term maintenance therapy in these patients.
BIOLOGIC THERAPY
Biologic therapy interferes with the body's inflammatory response in CD by targeting specific molecular players in the inflammatory process such as cytokines. Promising targets include tumor necrosis factor (TNF)-α, interleukins, adhesion molecules, and colony-stimulating factors. Biologic therapies offer a distinct advantage in CD treatment. Unlike corticosteroids, which tend to suppress the entire immune system and thereby produce major undesirable side effects, biologic agents act more selectively in the inflammatory pathway.
Infliximab, a chimeric anti-TNF monoclonal antibody, is the most widely used biologic in CD and is effective for rapid induction of remission in patients with moderate to severe CD.84 It is most commonly used in patients who have not responded to 5-ASA, CS, or immunomodulators. Infliximab has also been shown to be effective in maintenance of remission.85,86
A large, prospective, randomized controlled trial (A Crohn's Disease Clinical Trial Evaluating Infliximab in a New Long-term Treatment Regimen [ACCENT-1]) demonstrated superior efficacy of maintenance therapy with infliximab compared with placebo in patients with moderate to severe CD who had responded to initial dosages of infliximab for maintenance of long-term clinical remission.86 Infliximab was also shown to have a significant steroid-sparing effect.86
Infliximab has a rapid onset of action with a median duration of response of 8 weeks. The effective dosing regimen for infliximab is 5 mg/kg intravenous infusion at week 0, 2, and 6 for induction followed by repeat infusions every 8 weeks for maintenance.87 Acute infusion reactions occur in 10 to 13% of patients, usually within 2 hours of treatment. However, less than 2% of patients actually discontinued infliximab because of infusion reactions.88 Severe infusion reactions were seen in only 0.5% of infusions. Infusion reactions can be medically managed with Benadryl (Johnson & Johnson Consumer Companies, Inc., Skillman, NJ) and CS and patients can be maintained on therapy.
A proportion of patients receiving infliximab infusions develop antibodies against infliximab reducing its beneficial effect over the long term.85 Concomitant use of immunomodulators (6-mercaptopurine, azathioprine, or methotrexate) can reduce the risk of infusion reactions as well as the development of antibodies against infliximab.89
Serious adverse effects with infliximab, although uncommon, can occur.86 Prolonged use can lead to complications associated with immunosuppression, especially when used in combination with immunomodulators.90 Any acute infectious complication requires immediate cessation of therapy until complete resolution is achieved. Reactivation of latent tuberculosis, as well as, hepatitis B can occur following therapy with infliximab.91,92,93 Screening patients for tuberculosis prior to treatment is recommended and although no screening guidelines currently exists for hepatitis B, patients should probably be tested for serological markers prior to infliximab therapy and, if positive, considered for prophylaxis of reactivation using antiviral therapy.93
Long-term safety data on the risk of lymphoproliferative malignancy remains to be determined.94,95,96 Rare cases of fatal hepatosplenic T cell lymphoma in young patients have been reported with infliximab and concomitant immunomodulators (AZA/6MP).97
Despite the initial successes reported for infliximab in CD, ~30% of patients fail to respond to treatment. Newer biologic agents such as adalimumab, certolizumab, and natalizumab have demonstrated excellent efficacy and safety in early trials.98,99,100,101,102,103,104,105,106
Adalimumab has been shown in two randomized placebo controlled trials (CLASSIC 1, CLASSIC 2) to be effective for induction and maintenance of clinical remission in patients with moderate to severely active CD.98,99
Induction of remission was achieved after 4 weeks of adalimumab therapy and clinical remission was maintained for up to 56 weeks in patients with moderate to severely active CD naive to other anti-TNF therapy. Adalimumab at a dosage of 40-mg subcutaneously administered every other week was as efficacious as weekly dosing.98,99 In these trials, adalimumab was generally well tolerated.
Recent data from the CHARM trial presented in abstract form demonstrated that adalimumab administered weekly or every other week was effective for maintenance of remission and was steroid sparing in patients with moderate to severely active CD disease. Adalimumab was also found to be effective for closure of fistulae in these patients. An advantage of adalimumab over infliximab is that it can be self-administered through subcutaneous injection with either a prefilled syringe or an auto-injection pen.100 The effective dosage for adalimumab is 160 mg at week 0, 80 mg at week 2, and then 40 mg every other week.
Certolizumab pegol is a humanized anti-TNF Fab' fragment linked to polyethylene glycol that is administered subcutaneously. Preliminary data from two phase 3 randomized control studies (PRECISE 1 and PRECISE 2) showed superior efficacy of certolizumab pegol over placebo at clinical response in patients with moderate to severely active CD.101,102 A clinical response was defined as a decrease in the baseline CDAI score ≥ 100 points. Certolizumab pegol was effective in CD patients regardless of whether they had previously received infliximab therapy.103 The effective dosage of certolizumab pegol was 400 mg administered subcutaneously at weeks 0, 2, and 4 and at every 4 weeks to week 24.
Natalizumab is a humanized monoclonal antibody to α-4 integrin and is administered intravenously. Two phase 2 studies demonstrated that natalizumab may be efficacious in the induction of clinical remission when administered as a single dose of 3 mg/kg at week 0, or as 2 infusions of 3 or 6 mg/kg at weeks 0 and 4 in patients with moderate to severely active CD.104,105
In the ENACT 1 study, natalizumab administered intravenously at a dose of 300 mg at 0, 4, and 8 weeks failed to induce clinical response (defined as a decrease in the baseline CDAI ≥ 70 points) at week 10.106 However, subgroup analysis demonstrated the efficacy of natalizumab versus placebo in patients with an elevated C-reactive protein level greater than the upper limit of normal (2.87 mg/L). The success of α-4-integrin blockade with natalizumab has been tempered by reports of three cases of multifocal leukoencephalopathy caused by the human polyoma JC virus in patients with multiple sclerosis and CD patients treated with this agent.107,108,109,110
New Therapeutic Concepts
Conventional therapeutic algorithms have not effectively changed the natural history of CD nor have they prevented the need for surgery or the development of disease complications. Initiation of CS is usually associated with a poor clinical course with ~30% requiring surgery within one year of commencement of therapy.111 There is evidence to suggest that therapy directed at mucosal healing may alter the natural course of CD.112 Recent studies have shown improved rates of mucosal healing in CD patients treated with infliximab and AZA.113,114,115
It has been suggested that the need for CS is the tipping point at which patients should be treated more aggressively. Early introduction of immunomodulators is an option that has proven effective at maintaining long-term remission in the pediatric population.61 This therapeutic option is termed “top-down therapy” and involves early introduction of biologic therapy in the disease process. A recent landmark study compared the effectiveness of combined immunomodulation and biologic therapy (top-down) with a conventional “step-up” approach (“top-down versus step-up therapy”) in steroid-naïve CD patients with a CDAI ≥ 200.113 The top-down arm of treatment consisted of AZA and three infusions of infliximab, followed by episodic infliximab for disease flare and, if necessary, CS. The step-up algorithm included induction of remission with CS, followed by repeat courses of steroids and AZA in cases where new exacerbations were encountered, and eventually infliximab, if these therapeutic interventions failed.
The top-down approach leads to more frequent and rapid remission rates.113 At 6 and 12 months significantly more patients in the top-down arm were in corticosteroid-free remission compared with patients receiving standard therapy. Beyond this initial 12 months period, however, a significant difference could no longer be detected. This result appeared to be due to a combination of decreasing remission rates in the top-down group and a gradual increasing remission rate in the conventional study group. Conceivably, these differences may have been due to the frequent use of immunomodulators in patients receiving conventional therapy and to the ineffective maintenance-dosing regimen of infliximab as needed in the top-down group.
Rates of mucosal healing were significantly better in the top-down treatment group as compared with the step-up group at 24 months. Overall, the benefits of top-down therapy were reflected in fewer clinical relapses, less steroid use, and better mucosal healing. Whether these beneficial effects can translate into fewer hospitalizations, surgeries, and disease complications remains to be determined.86,90 Furthermore, the risk of serious side effects (i.e., opportunistic infections, lymphoproliferative disease) that are associated with this form of therapy must always be weighed against the potential benefits.
Severe Fulminant Disease
Patients with severe or fulminant CD as described in Table 1 should be hospitalized. Supportive resuscitation with fluids and electrolytes is essential. Intestinal obstruction or abscess should be ruled out before starting parental CS (equivalent to 40 to 60 mg of prednisone) in divided dosages or continuous infusion as there is no difference between both forms of administration.116 Broad spectrum antibiotics should be administered together with CS for patients with an inflammatory mass.117 Patients who do not respond to parenteral steroids may respond to intravenous cyclosporine.118 Patients with worsening symptoms or those who do not respond to therapy should be referred for surgical intervention.
Oral nutrition should be continued if tolerated in patients without evidence of obstruction or peritoneal signs. In patients who are unable to maintain nutritional requirements after 5 to 7 days, elemental feeding or parenteral hyperalimentation should be initiated.
MANAGEMENT OF COMPLICATIONS OF CROHN'S DISEASE
Small bowel obstruction is a serious complication in CD patients. This may be secondary to inflammatory narrowing, fibrostenotic stricture or adhesions from previous surgery. Obstruction in the absence of fever or rebound tenderness does not require emergent surgical intervention. Inflammatory bowel narrowing may respond to nasogastric suction, bowel rest, and CS, whereas in fibrostenotic or adhesive obstruction symptoms will often not respond to medical therapy. Patients in this latter group will experience recurrent obstructive symptoms and surgery will likely be required.
Fistulae develop in approximately one third of patients with CD and usually require lengthy periods of healing. Fistulae are generally divided into two types, external and internal. Internal fistulae are those that terminate in the body and include enteroenteric, gastrocolic, duodenocolic, rectovaginal, and rectovesical. External fistulae terminate on the skin, perianal surface, or stoma and are typically associated with pain and discharge.
The primary management of patients with fistulizing CD depends upon the type of fistulae and combines medical and surgical management with the aim of healing the fistula and keeping it closed. Current medical therapies include antibiotics, AZA and 6-MP, and infliximab. 5-ASA is generally ineffective for the treatment of fistulizing CD.119 CS are not beneficial as a form of therapy and may not only delay wound healing, but may also exacerbate abscess formation.120
Metronidazole and ciprofloxacin are considered first-line therapies in the management of perianal fistulae in CD. Data from case series and uncontrolled trials have demonstrated that metronidazole (500 mg twice daily) and ciprofloxacin (250 mg twice daily) used alone or in combination are effective as a short-term therapy at improving and healing draining perianal fistulae.121,122,123,124 However, relapse is common upon discontinuation and adverse events resulting from prolonged use of metronidazole limit its utility over the long term.125 No data are available upon the effectiveness of antibiotics in the treatment of internal fistulae.
Immunomodulators (AZA 2.5 mg/kg per day or 6-MP1.5 mg/kg per day) are effective for perianal fistula disease, but noticeable improvement is slow and healing is often incomplete.125,126 Recurrence and exacerbation is common after discontinuation of drug.125,126
The data on the effectiveness of immunomodulators in the treatment of internal fistulae and other types of external fistulae is sparse. Limited case studies do suggest that their use may be beneficial for enteroenteric, gastrocolic, enterovesical, and rectovaginal fistulae.127,128,129,130,131 A randomized placebo controlled trial of infliximab 5 to 10 mg/kg administered as an induction regimen at 0, 2, and 6 weeks demonstrated superior efficacy over placebo at reducing fistula discharge by at least 50% and at complete closure of fistula in CD patients with draining enterocutaneous and perianal fistulae.132 Infliximab therapy had a rapid onset of action (usually within 2 weeks) with effects lasting a median of 12 weeks.132 Maintenance therapy with infliximab every 8 weeks may prolong the time to loss of response and is required in many patients for long-term symptom control.125
The data on the efficacy of infliximab therapy in treating internal fistulae is limited. A post hoc analysis of a randomized controlled trial demonstrated that women who achieved closure of their rectovaginal fistulae on infliximab therapy had a prolonged period of fistula closure when infliximab therapy was continued.133 In perianal fistula disease, infliximab therapy is associated with a high rate of abscess formation, probably due to closure of the cutaneous opening of the fistula tract prior to closure of the fistula tract itself.132,133 To avoid this complication, placement of noncutting Setons before initiating infliximab therapy has been recommended.134 Improved rates of fistula closure have been demonstrated with this approach.120,134,135
POSTOPERATIVE MANAGEMENT OF CROHN'S DISEASE
Over 70% of patients who undergo surgical resection for CD will have endoscopic recurrence at 1 year usually in the neo-terminal ileum and/or at the anastomosis.136 Endoscopic recurrence is currently evaluated and graded according to the criteria of Rutgeerts et al (Table 2).136 Endoscopic findings precede clinical symptoms and the severity of the endoscopic recurrence often predicts the clinical course.136
Table 2.
Rutgeerts Scale of Postoperative Endoscopic Recurrence*
| Grade | Endoscopic Findings |
|---|---|
| 0 | No lesions |
| 1 | < 5 aphthous lesions |
| 2 | > 5 aphthous lesions with normal mucosa between the lesions, or skip areas of larger lesions, or lesions confined to the ileocolonic anastomotic lining (< 1 cm) |
| 3 | Diffuse aphthous ileitis with diffusely inflamed mucosa |
| 4 | Diffuse ileal inflammation with larger ulcers, nodules, or narrowing |
Hyperemia and edema alone are not considered as signs of recurrence.
Approximately 70% of CD patients will require a second surgery within 20 years.137,138 Risk factors for postoperative CD recurrence include smoking, ileocolonic disease, and perianal disease.138,139,140,141 Medical therapy in the postoperative course of CD patients has consistently proven limited for the prevention of postoperative recurrence of CD. In a randomized controlled trial, high-dosage mesalamine produced no benefit over placebo.142 A placebo controlled trial study with high-dosage metronidazole demonstrated modest benefits over placebo at reducing clinical and endoscopic postoperative recurrence. However, frequent side effects limit long-term use of this medication.143
Conversely, in a randomized placebo controlled trial 6-MP 50 mg/day was more effective than placebo at preventing postoperative clinical relapse in patients who underwent resection for ileal CD.144 However, the endoscopic recurrence was not statistically different between groups. In this study, the overall efficacy of 6 MP may have been reduced by suboptimal dosing of the drug. In an open-label randomized study comparing AZA to mesalamine, no significant difference was observed in the rates of postoperative clinical relapse of CD between groups.145
The need for an effective therapy in postoperative CD is evident. Randomized controlled trials investigating the role of infliximab or other biologic agents in the prevention of postoperative CD are warranted. Our approach is to initiate immunomodulator therapy or biologic therapy in symptomatic patients, as well as those with grades 2 or greater on the Rutgeerts scale.
CONCLUSION
Whether the goal of CD therapy should be clinical remission alone or clinical remission combined with mucosal healing remains to be determined. Whether new therapeutic concepts directed at mucosal healing will reduce relapses, hospitalizations, surgeries, and CD-associated complications over the long term also remains to be determined. As new effective medications become available, our goal will be mucosal healing not solely clinical remission, as we feel that this approach will favorably modify the natural history of CD.
It is important to realize that the majority of CD patients experience mild to moderate disease or are in clinical remission over significant periods during the course of their disease. These patients can be treated conservatively with 5-ASA or budesonide depending on the disease location. Those patients with more severe forms of the disease who require CS should be treated more aggressively with early introduction of immunomodulator therapy, which may help to prevent the complications associated with CD. In light of the potential for serious adverse effects we feel that the introduction of biologic therapy should be reserved for patients who fail to respond to therapy with immunomodulators, or are unable to tolerate such therapy.
More randomized controlled trials are necessary to assess the value of clinical and biochemical prognostic markers, as well as the efficacy and safety of long-term treatment with the newer biologic agents before improvement in the clinical outcomes of CD can be expected.
REFERENCES
- 1.Hanauer S B. Inflammatory bowel disease. N Engl J Med. 1996;334:841–848. doi: 10.1056/NEJM199603283341307. [DOI] [PubMed] [Google Scholar]
- 2.Loftus E V, Schoenfeld P, Sandborn W J. The epidemiology and natural history of Crohn's disease in population-based patient cohorts from North America: a systematic review. Aliment Pharmacol Ther. 2002;16:51–60. doi: 10.1046/j.1365-2036.2002.01140.x. [DOI] [PubMed] [Google Scholar]
- 3.Loftus C G, Loftus E V, Harmsen W S, et al. Update on the incidence and prevalence of Crohn's disease and ulcerative colitis in Olmsted County, Minnesota, 1940–2000. Inflamm Bowel Dis. 2007;13:254–261. doi: 10.1002/ibd.20029. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein C N. The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am J Gastroenterol. 2006;101:1559–1568. doi: 10.1111/j.1572-0241.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- 5.Persson P G, Bernell O, Leijonmarck C E, Farahmand B Y, Hellers G, Ahlbom A. Survival and cause-specific mortality in inflammatory bowel disease: a population-based cohort study. J Gastroenterol. 1996;110:1339–1345. doi: 10.1053/gast.1996.v110.pm8613037. [DOI] [PubMed] [Google Scholar]
- 6.Binder V, Hendriksen C, Kreiner S. Prognosis in Crohn's disease–based on results from a regional patient group from the county of Copenhagen. Gut. 1985;26:146–150. doi: 10.1136/gut.26.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munkholm P, Langholz E, Davidsen M, Binder V. Disease activity courses in a regional cohort of Crohn's disease patients. Scand J Gastroenterol. 1995;30:699–706. doi: 10.3109/00365529509096316. [DOI] [PubMed] [Google Scholar]
- 8.Silverstein M D, Loftus E V, Sandborn W J, et al. Clinical course and costs of care for Crohn's disease: Markov model analysis of a population-based cohort. J Gastroenterol. 1999;117:49–57. doi: 10.1016/s0016-5085(99)70549-4. [DOI] [PubMed] [Google Scholar]
- 9.Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis. 2002;8:244–250. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Moum B, Ekbom A, Vatn M H, et al. Clinical course during the 1st year after diagnosis in ulcerative colitis and Crohn's disease. Results of a large, prospective population-based study in southeastern Norway, 1990–93. Scand J Gastroenterol. 1997;32:1005–1012. doi: 10.3109/00365529709011217. [DOI] [PubMed] [Google Scholar]
- 11.Henriksen M, Jahnsen J, Lygren I, et al. The Ibsen Study Group. Clinical course in Crohn's disease: results of a five-year population-based follow-up study (the IBSEN study) Scand J Gastroenterol. 2007;42:602–610. doi: 10.1080/00365520601076124. [DOI] [PubMed] [Google Scholar]
- 12.Sands B E, Arsenault J E, Rosen M J, et al. Risk of early surgery for Crohn's disease: implications for early treatment strategies. Am J Gastroenterol. 2003;98:2712–2718. doi: 10.1111/j.1572-0241.2003.08674.x. [DOI] [PubMed] [Google Scholar]
- 13.Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn's disease. Ann Surg. 2000;231:38–45. doi: 10.1097/00000658-200001000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agrez M V, Valente R M, Pierce W, Melton L J, III, Heerden J A van, Beart R W., Jr Surgical history of Crohn's disease in a well-defined population. Mayo Clin Proc. 1982;57:747–752. [PubMed] [Google Scholar]
- 15.Carbonnel F, Macaigne G, Beaugerie L, Gendre J P, Cosnes J. Crohn's disease severity in familial and sporadic cases. Gut. 1999;44:91–95. doi: 10.1136/gut.44.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Best W R, Becktel J M, Singleton J W, Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. J Gastroenterol. 1976;70:439–444. [PubMed] [Google Scholar]
- 17.Hodgson H J, Mazlam M Z. Review article: assessment of drug therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 1991;5:555–584. doi: 10.1111/j.1365-2036.1991.tb00525.x. [DOI] [PubMed] [Google Scholar]
- 18.Modigliani R, Mary J Y, Simon J F, et al. Clinical, biological, and endoscopic picture of attacks of Crohn's disease. Evolution on prednisolone. Groupe d'Etude Therapeutique des Affections Inflammatoires Digestives. J Gastroenterol. 1990;98:811–818. doi: 10.1016/0016-5085(90)90002-i. [DOI] [PubMed] [Google Scholar]
- 19.Jorgensen L G, Fredholm L, Petersen P H, et al. How accurate are clinical activity indices for scoring of disease activity in inflammatory bowel disease (IBD)? Clin Chem Lab Med. 2005;43:403–411. doi: 10.1515/CCLM.2005.073. [DOI] [PubMed] [Google Scholar]
- 20.Cellier C, Sahmoud T, Froguel E, et al. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn's disease. A prospective multicentre study of 121 cases. The Groupe d'Etudes Therapeutiques des Affections Inflammatoires Digestives. Gut. 1994;35:231–235. doi: 10.1136/gut.35.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanauer S B, Sandborn W. Practice Parameters Committee of the American College of Gastroenterology. Management of CD in adults. Am J Gastroenterol. 2001;96:635–643. doi: 10.1111/j.1572-0241.2001.3671_c.x. [DOI] [PubMed] [Google Scholar]
- 22.Summers R W, Switz D M, Sessions J T, Jr, et al. National Cooperative Crohn's Disease Study: results of drug treatment. J Gastroenterol. 1979;77:847–869. [PubMed] [Google Scholar]
- 23.Malchow H, Ewe K, Brandes J W, et al. European Cooperative Crohn's Disease Study (ECCDS): results of drug treatment. J Gastroenterol. 1984;86:249–266. [PubMed] [Google Scholar]
- 24.Hees P A Van, Lier H J Van, Elteren P H Van, et al. Effect of sulphasalazine in patients with active Crohn's disease: a controlled double-blind study. Gut. 1981;22:404–409. doi: 10.1136/gut.22.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anthonisen P, Barany F, Folkenborg O, et al. The clinical effect of salazosulphapyridine (Salazopyrin R) in Crohn's disease. A controlled double-blind study. Scand J Gastroenterol. 1974;9:549–554. [PubMed] [Google Scholar]
- 26.Taffet S L, Das K M. Sulfasalazine. Adverse effects and desensitization. Dig Dis Sci. 1983;28:833–842. doi: 10.1007/BF01296907. [DOI] [PubMed] [Google Scholar]
- 27.Das K M, Eastwood M A, McManus J P, Sircus W. The metabolism of salicylazosulphapyridine in ulcerative colitis. I. The relationship between metabolites and the response to treatment in inpatients. Gut. 1973;14:631–641. doi: 10.1136/gut.14.8.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gearry R B, Ajlouni Y, Nandurkar S, Iser J H, Gibson P R. 5-Aminosalicylic acid (mesalazine) use in Crohn's disease: a survey of the opinions and practice of Australian gastroenterologists. Inflamm Bowel Dis. 2007;13:1009–1015. doi: 10.1002/ibd.20135. [DOI] [PubMed] [Google Scholar]
- 29.Tremaine W J, Schroeder K W, Harrison J M, Zinsmeister A R. A randomized, double-blind, placebo-controlled trial of the oral mesalamine (5-ASA) preparation, Asacol, in the treatment of symptomatic Crohn's colitis and ileocolitis. J Clin Gastroenterol. 1994;19:278–282. doi: 10.1097/00004836-199412000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Singleton J W, Hanauer S B, Gitnick G L, et al. Mesalamine capsules for the treatment of active Crohn's disease: results of a 16-week trial. Pentasa Crohn's Disease Study Group. J Gastroenterol. 1993;104:1293–1301. doi: 10.1016/0016-5085(93)90337-c. [DOI] [PubMed] [Google Scholar]
- 31.Singleton J. Second trial of mesalamine therapy in the treatment of active Crohn's disease. J Gastroenterol. 1994;107:632–633. doi: 10.1016/0016-5085(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 32.Hanauer S B, Strömberg U. Oral Pentasa in the treatment of active Crohn's disease: a meta-analysis of double-blind, placebo-controlled trials. Clin Gastroenterol Hepatol. 2004;2:379–388. doi: 10.1016/s1542-3565(04)00122-3. [DOI] [PubMed] [Google Scholar]
- 33.Dahlberg E, Thalen A, Brattsand R, et al. Correlation between chemical structure, receptor binding, and biological activity in some novel, highly active, 16, 17-acetyl-substituted glucocorticoids. Mol Pharmacol. 1984;25:70–78. [PubMed] [Google Scholar]
- 34.Brattsand R. Overview of newer glucocorticosteroid preparations for inflammatory bowel disease. Can J Gastroenterol. 1990;4:407–414. [Google Scholar]
- 35.Kane S V, Schoenfeld P, Sandborn W J, Tremaine W, Hofer T, Feagan B G. The effectiveness of budesonide therapy for Crohn's disease. Aliment Pharmacol Ther. 2002;16:1509–1517. doi: 10.1046/j.1365-2036.2002.01289.x. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg G R, Feagan B G, Martin F, et al. Oral budesonide for active Crohn's disease. Canadian Inflammatory Bowel Disease Study Group. N Engl J Med. 1994;331:836–841. doi: 10.1056/NEJM199409293311303. [DOI] [PubMed] [Google Scholar]
- 37.Tremaine W J, Hanauer S B, Katz S, et al. Budesonide CIR capsules (once or twice daily divided-dosage) in active Crohn's disease: a randomized placebo-controlled study in the United States. Am J Gastroenterol. 2002;97:1748–1754. doi: 10.1111/j.1572-0241.2002.05835.x. [DOI] [PubMed] [Google Scholar]
- 38.Thomsen O O, Cortot A, Jewell D, et al. A comparison of budesonide and mesalamine for active Crohn's disease. N Engl J Med. 1998;339:370–374. doi: 10.1056/NEJM199808063390603. [DOI] [PubMed] [Google Scholar]
- 39.Sutherland L, Singleton J, Sessions J, et al. Double blind, placebo controlled trial of metronidazole in Crohn's disease. Gut. 1991;32:1071–1075. doi: 10.1136/gut.32.9.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allan R, Cooke W T. Evaluation of metronidazole in the management of Crohn's disease. Gut. 1977;18:A422. [Google Scholar]
- 41.Ambrose N S, Allan R N, Keighley M R, et al. Antibiotic therapy for treatment in relapse of intestinal Crohn's disease. A prospective randomized study. Dis Colon Rectum. 1985;28:81–85. doi: 10.1007/BF02552649. [DOI] [PubMed] [Google Scholar]
- 42.Blichfeldt P, Blomhoff J P, Myhre E, et al. Metronidazole in Crohn's disease. A double blind cross-over clinical trial. Scand J Gastroenterol. 1978;13:123–127. doi: 10.3109/00365527809179816. [DOI] [PubMed] [Google Scholar]
- 43.Arnold G L, Beaves M R, Pryjdun V O, et al. Preliminary study of ciprofloxacin in active Crohn's disease. Inflamm Bowel Dis. 2002;8:10–15. doi: 10.1097/00054725-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Steinhart A H, Feagan B G, Wong C J, et al. Combined budesonide and antibiotic therapy for active Crohn's disease: a randomized controlled trial. J Gastroenterol. 2002;123:33–40. doi: 10.1053/gast.2002.34225. [DOI] [PubMed] [Google Scholar]
- 45.Prantera C, Lochs H, Campieri M, et al. Antibiotic treatment of Crohn's disease: results of a multicentre, double blind, randomized, placebo-controlled trial with rifaximin. Aliment Pharmacol Ther. 2006;15(23):1117–1125. doi: 10.1111/j.1365-2036.2006.02879.x. [DOI] [PubMed] [Google Scholar]
- 46.Sandborn W J, Feagan B G. Review article: mild to moderate Crohns disease defining the basis for a new treatment algorithm. Aliment Pharmacol Ther. 2003;18:263–277. doi: 10.1046/j.1365-2036.2003.01661.x. [DOI] [PubMed] [Google Scholar]
- 47.Camma C, Giunta M, Rosselli M, Cottone M. Mesalamine in the maintenance treatment of Crohn's disease: a meta-analysis adjusted for confounding variables. J Gastroenterol. 1997;113:1465–1473. doi: 10.1053/gast.1997.v113.pm9352848. [DOI] [PubMed] [Google Scholar]
- 48.Rijk M C, Hogezand R A van, Lier H J van, Tongeren J H van. Sulphasalazine and prednisone compared with sulphasalazine for treating active Crohn disease. A double-blind, randomized, multicenter trial. Ann Intern Med. 1991;114:445–450. doi: 10.7326/0003-4819-114-6-445. [DOI] [PubMed] [Google Scholar]
- 49.Greenberg G R, Feagan B G, Martin F, et al. Oral budesonide as maintenance treatment for Crohn's disease: a placebo-controlled, dosage-ranging study. Canadian Inflammatory Bowel Disease Study Group. J Gastroenterol. 1996;110:45–51. doi: 10.1053/gast.1996.v110.pm8536887. [DOI] [PubMed] [Google Scholar]
- 50.Sandborn W J, Löfberg R, Feagan B G, Hanauer S B, Campieri M, Greenberg G R. Budesonide for maintenance of remission in patients with Crohn's disease in medically induced remission: a predetermined pooled analysis of four randomized, double-blind, placebo-controlled trials. Am J Gastroenterol. 2005;100:1780–1787. doi: 10.1111/j.1572-0241.2005.41992.x. [DOI] [PubMed] [Google Scholar]
- 51.Munkholm P, Langholz E, Davidsen M, Binder V. Frequency of glucocorticoid resistance and dependency in Crohn's disease. Gut. 1994;35:360–362. doi: 10.1136/gut.35.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sands B E, Arsenault J E, Rosen M J, et al. Risk of early surgery for Crohn's disease: implications for early treatment strategies. Am J Gastroenterol. 2003;98:2712–2718. doi: 10.1111/j.1572-0241.2003.08674.x. [DOI] [PubMed] [Google Scholar]
- 53.Rutgeerts P, Löfberg R, Malchow H, et al. A comparison of budesonide with prednisolone for active Crohn's disease. N Engl J Med. 1994;331:842–845. doi: 10.1056/NEJM199409293311304. [DOI] [PubMed] [Google Scholar]
- 54.Ho G T, Chiam P, Drummond H, Loane J, Arnott I D, Satsangi J. The efficacy of corticosteroid therapy in inflammatory bowel disease: analysis of a 5-year UK inception cohort. Aliment Pharmacol Ther. 2006;24:319–330. doi: 10.1111/j.1365-2036.2006.02974.x. [DOI] [PubMed] [Google Scholar]
- 55.Faubion W A, Loftus E V, Harmsen W S, Zinsmeister A R, Sandborn W J. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. J Gastroenterol. 2001;121:255–260. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- 56.Sandborn W, Sutherland L, Pearson D, May G, Modigliani R, Prantera C. Azathioprine or 6-mercaptopurine for inducing remission of Crohn's disease. Cochrane Database Syst Rev. 2000;2:CD000545. doi: 10.1002/14651858.CD000545. [DOI] [PubMed] [Google Scholar]
- 57.Present D H, Korelitz B I, Wisch N, Glass J L, Sachar D B, Pasternack B S. Treatment of Crohn‘s disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med. 1980;302:981–987. doi: 10.1056/NEJM198005013021801. [DOI] [PubMed] [Google Scholar]
- 58.Pearson D C, May G R, Fick G H, Sutherland L R. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med. 1995;123:132–142. doi: 10.7326/0003-4819-123-2-199507150-00009. [DOI] [PubMed] [Google Scholar]
- 59.Candy S, Wright J, Gerber M, Adams G, Gerig M, Goodman R. A controlled double-blind study of azathioprine in the management of Crohn's disease. Gut. 1995;37:674–678. doi: 10.1136/gut.37.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ewe K, Press A G, Singe C C, et al. Azathioprine combined with prednisolone or monotherapy with prednisolone in active Crohn's disease. J Gastroenterol. 1993;105:367–372. doi: 10.1016/0016-5085(93)90709-l. [DOI] [PubMed] [Google Scholar]
- 61.Markowitz J, Grancher K, Kohn N, Lesser M, Daum F. A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn's disease. J Gastroenterol. 2000;119:895–902. doi: 10.1053/gast.2000.18144. [DOI] [PubMed] [Google Scholar]
- 62.Sandborn W J. A review of immune modifier therapy for inflammatory bowel disease: azathioprine, 6-mercaptopurine, cyclosporine, and methotrexate. Am J Gastroenterol. 1996;91:423–433. [PubMed] [Google Scholar]
- 63.Black A J, McLeod H L, Capell H A, et al. Thiopurine methyltransferase genotype predicts therapy-limiting severe toxicity from AZA. Ann Intern Med. 1998;129:716–718. doi: 10.7326/0003-4819-129-9-199811010-00007. [DOI] [PubMed] [Google Scholar]
- 64.Colombel J F, Ferrari N, Debuysere H, et al. Genotypic analysis of thiopurine S-methyltransferase in patients with Crohn's disease and severe myelosuppression during azathioprine therapy. J Gastroenterol. 2000;118:1025–1030. doi: 10.1016/s0016-5085(00)70354-4. [DOI] [PubMed] [Google Scholar]
- 65.Campbell S, Kingstone K, Ghosh S. Relevance of thiopurine methyltransferase activity in inflammatory bowel disease patients maintained on low-dosage azathioprine. Aliment Pharmacol Ther. 2002;16:389–398. doi: 10.1046/j.1365-2036.2002.01177.x. [DOI] [PubMed] [Google Scholar]
- 66.Dubinsky M C, Lamothe S, Yang H Y, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. J Gastroenterol. 2000;118:705–713. doi: 10.1016/s0016-5085(00)70140-5. [DOI] [PubMed] [Google Scholar]
- 67.Regueiro M, Mardini H. Determination of thiopurine methyltransferase genotype or phenotype optimizes initial dosing of azathioprine for the treatment of Crohn's disease. J Clin Gastroenterol. 2002;35:240–244. doi: 10.1097/00004836-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 68.Winter J, Walker A, Shapiro D, Gaffney D, Spooner R J, Mills P R. Cost-effectiveness of thiopurine methyltransferase genotype screening in patients about to commence azathioprine therapy for treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20:593–599. doi: 10.1111/j.1365-2036.2004.02124.x. [DOI] [PubMed] [Google Scholar]
- 69.Dubinsky M C, Reyes E, Ofman J, Chiou C F, Wade S, Sandborn W J. A cost-effectiveness analysis of alternative disease management strategies in patients with Crohn's disease treated with azathioprine or 6-mercaptopurine. Am J Gastroenterol. 2005;100:2239–2247. doi: 10.1111/j.1572-0241.2005.41900.x. [DOI] [PubMed] [Google Scholar]
- 70.Cummins D, Sekar M, Halil O, Banner N. Myelosuppression associated with azathioprine-allopurinol interaction after heart and lung transplantation. Transplantation. 1996;61:1661–1662. doi: 10.1097/00007890-199606150-00023. [DOI] [PubMed] [Google Scholar]
- 71.Dubinsky M C, Yang H, Hassard P V, et al. 6-MP metabolite profiles provide a biochemical explanation for 6-MP resistance in patients with inflammatory bowel disease. J Gastroenterol. 2002;122:904–915. doi: 10.1053/gast.2002.32420. [DOI] [PubMed] [Google Scholar]
- 72.Sparrow M P, Hande S A, Friedman S, et al. Allopurinol safely and effectively optimizes thioguanine metabolites in inflammatory bowel disease patients not responding to azathioprine and mercaptopurine. Aliment Pharmacol Ther. 2005;22:441–446. doi: 10.1111/j.1365-2036.2005.02583.x. [DOI] [PubMed] [Google Scholar]
- 73.Lémann M, Mary J Y, Colombel J F, et al. Groupe D'Etude Thérapeutique des Affections Inflammatoires du Tube Digestif. A randomized, double-blind, controlled withdrawal trial in Crohn's disease patients in long-term remission on azathioprine. J Gastroenterol. 2005;128:1812–1818. doi: 10.1053/j.gastro.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 74.Chebli J M, Gaburri P D, De Souza A F, et al. Long-term results with azathioprine therapy in patients with corticosteroid-dependent Crohn's disease: open-label prospective study. J Gastroenterol Hepatol. 2007;22:268–274. doi: 10.1111/j.1440-1746.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- 75.Korelitz B I, Mirsky F J, Fleisher M R, Warman J I, Wisch N, Gleim G W. Malignant neoplasms ubsequent to treatment of inflammatory bowel disease with 6-mercaptopurine. Am J Gastroenterol. 1999;94:3248–3253. doi: 10.1111/j.1572-0241.1999.01530.x. [DOI] [PubMed] [Google Scholar]
- 76.Farrell R J, Ang Y, Kileen P, et al. Increased incidence of non-Hodgkin's lymphoma in inflammatory bowel disease patients on immunosuppressive therapy but overall risk is low. Gut. 2000;47:514–519. doi: 10.1136/gut.47.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feagan B G, Rochon J, Fedorak R N, et al. MTX for the treatment of Crohn's disease. The North American Crohn's Study Group Investigators. N Engl J Med. 1995;332:292–297. doi: 10.1056/NEJM199502023320503. [DOI] [PubMed] [Google Scholar]
- 78.Oren R, Arber N, Odes S, et al. Methotrexate in chronic active ulcerative colitis: a double-blind, randomized, Israeli multicenter trial. J Gastroenterol. 1996;110:1416–1421. doi: 10.1053/gast.1996.v110.pm8613046. [DOI] [PubMed] [Google Scholar]
- 79.Arora S, Katkov W, Cooley J, et al. Methotrexate in Crohn's disease: results of a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. 1999;46:1724–1729. [PubMed] [Google Scholar]
- 80.Feagan B G, Fedorak R N, Irvine E J, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn's disease. North American Crohn's Study Group Investigators. N Engl J Med. 2000;342:1627–1632. doi: 10.1056/NEJM200006013422202. [DOI] [PubMed] [Google Scholar]
- 81.Schröder O, Stein J. Low dosage methotrexate in inflammatory bowel disease: current status and future directions. Am J Gastroenterol. 2003;98:530–537. doi: 10.1111/j.1572-0241.2003.07305.x. [DOI] [PubMed] [Google Scholar]
- 82.Roenigk H H, Jr, Auerbach R, Maibach H, et al. Methotrexate in psoriasis: Revised guidelines. J Am Acad Dermatol. 1988;19:145–156. doi: 10.1016/s0190-9622(88)80237-8. [DOI] [PubMed] [Google Scholar]
- 83.Ede A E Van, Laan R FJM, Blom H J, et al. Methotrexate in rheumatoid arthritis: an update with focus on mechanisms involved in toxicity. Semin Arthritis Rheum. 1998;27:277–292. doi: 10.1016/s0049-0172(98)80049-8. [DOI] [PubMed] [Google Scholar]
- 84.Targan S R, Hanauer S B, Deventer S J van, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 85.Rutgeerts P, D'Haens E R, Targan S R, et al. Efficacy and safety of retreatment with anti-tumour necrosis factor antibody to maintain remission in Crohn's disease. J Gastroenterol. 1999;117:761–769. doi: 10.1016/s0016-5085(99)70332-x. [DOI] [PubMed] [Google Scholar]
- 86.Hanauer S B, Feagan B G, Lichtenstein G R, et al. Maintenance infliximab for Crohn's disease: the ACCENT 1 randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 87.Sandborn W J, Hanauer S B. Infliximab in the treatment of Crohn's disease: a user's guide for clinicians. Am J Gastroenterol. 2002;97:2962–2972. doi: 10.1111/j.1572-0241.2002.07093.x. [DOI] [PubMed] [Google Scholar]
- 88.Cohen R D. Efficacy and safety of repeated infliximab infusions for Crohn's disease: 1-year clinical experience. Inflamm Bowel Dis. 2001;7(Suppl 1):S17–S22. doi: 10.1002/ibd.3780070505. [DOI] [PubMed] [Google Scholar]
- 89.Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003;348:601–608. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 90.Sandborn W J, Loftus E V. Balancing the risks and benefits of infliximab in the treatment of inflammatory bowel disease. Gut. 2004;53:780–782. doi: 10.1136/gut.2003.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Keane J, Gershan S, Wise R P. Tuberculosis associated with infliximab, a tumour necrosis factor-α neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 92.Madonia S, Orlando A, Scimeca D, Olivo M, Rossi F, Cottone M. Occult hepatitis B and infliximab-induced HBV reactivation. Inflamm Bowel Dis. 2007;13:508–509. doi: 10.1002/ibd.20035. [DOI] [PubMed] [Google Scholar]
- 93.Esteve M, Saro C, González-Huix F, Suarez F, Forné M, Viver J M. Chronic hepatitis B reactivation following infliximab therapy in Crohn's disease patients: need for primary prophylaxis. Gut. 2004;53:1363–1365. doi: 10.1136/gut.2004.040675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bebb J R, Logan R P. Review article: does the use of immunosuppressive therapy in inflammatory bowel disease increase the risk of developing lymphoma? Aliment Pharmacol Ther. 2001;15:1843–1849. doi: 10.1046/j.1365-2036.2001.01125.x. [DOI] [PubMed] [Google Scholar]
- 95.Colombel J F, Loftus E V, Tremaine W J, et al. The safety profile of infliximab in patients with Crohn's disease: the Mayo clinic experience in 500 patients. J Gastroenterol. 2004;126:19–31. doi: 10.1053/j.gastro.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 96.Lichtenstein G R, Feagan B G, Cohen R D, et al. Serious infections and mortality in association with therapies for Crohn's disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4:621–630. doi: 10.1016/j.cgh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 97.Belhadj K, Reyes F, Farcet J P, et al. Hepatosplenic gamma delta T-cell lymphoma is a rare clinicopathologic entity with poor outcome: report on a series of 21 patients. Blood. 2003;102:4261–4269. doi: 10.1182/blood-2003-05-1675. [DOI] [PubMed] [Google Scholar]
- 98.Hanauer S B, Sandborn W J, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC I trial. Gastroenterology. 2006;130:323–333. doi: 10.1053/j.gastro.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 99.Sandborn W J, Hanauer S B, Lukas M, et al. Maintenance of remission over 1 year in patients with active Crohn's disease treated with adalimumab: results of a blinded, placebo-controlled trial. Am J Gastroenterol. 2005;100:843. [Google Scholar]
- 100.Colombel J, Sandborn W J, Rutgeerts P, et al. Adalimumab induces and maintains clinical response and remission in patients with active Crohn's disease: results of the CHARM trial. J Gastroenterol. 2006;130:Abstract 686d. [Google Scholar]
- 101.Sandborn W J, Feagan B G, Stoinov S, et al. Certolizumab pegol administered subcutaneously is effective and well tolerated in patients with active Crohn's disease: results from a 26-week, placebo-controlled Phase III study (PRECISE 1) J Gastroenterol. 2006;130:A-107. [Google Scholar]
- 102.Schreiber S, Khaliq-Kareemi M, Lawrance I C, et al. Maintenance therapy with certolizumab pegol for Crohn's disease. N Engl J Med. 2007;357:239–250. doi: 10.1056/NEJMoa062897. [DOI] [PubMed] [Google Scholar]
- 103.Hanauer S B, Colombel J F, Sandborn W J, Panes J, McColm J A, Schreiber S. Subcutaneous certolizumab pegol is effective in anti-TNF naive patients and patients with prior infliximab use. Am J Gastroenterol. 2006;101:Abstract 36B. [Google Scholar]
- 104.Gordon F H, Lai C W, Hamilton M I, et al. A randomized placebo-controlled trial of a humanized monoclonal antibody to alpha4 integrin in active Crohn's disease. J Gastroenterol. 2001;121:268–274. doi: 10.1053/gast.2001.26260. [DOI] [PubMed] [Google Scholar]
- 105.Ghosh S, Goldin E, Gordon F H, et al. Natalizumab for active Crohn's disease. N Engl J Med. 2003;348:24–32. doi: 10.1056/NEJMoa020732. [DOI] [PubMed] [Google Scholar]
- 106.Sandborn W J, Colombel J F, Enns R, et al. Natalizumab induction and maintenance therapy for Crohn's disease. N Engl J Med. 2005;353:1912–1925. doi: 10.1056/NEJMoa043335. [DOI] [PubMed] [Google Scholar]
- 107.Langer-Gould A, Atlas S W, Green A J, Bollen A W, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 108.Kleinschmidt-DeMasters B K, Tyler K L. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- 109.Assche G Van, Ranst M Van, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N Engl J Med. 2005;353:362–368. doi: 10.1056/NEJMoa051586. [DOI] [PubMed] [Google Scholar]
- 110.Yousry T A, Habil M, Major E O, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med. 2006;354:924–933. doi: 10.1056/NEJMoa054693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Faubion W A, Loftus E V, Harmsen W S, Zinsmeister A R, Sandborn W J. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. J Gastroenterol. 2001;121:255–260. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- 112.Rutgeerts P, Diamond R H, Bala M, et al. Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn's disease. Gastrointest Endosc. 2006;63:433–442. doi: 10.1016/j.gie.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 113.Hommes D W, Baert F, Assche G van, et al. Management of recent onset Crohn's disease: a controlled randomized trial comparing step-up and top-down therapy. J Gastroenterol. 2005;128(Suppl 2):A577. [Google Scholar]
- 114.D'Haens G, Geboes K, Rutgeerts P. Endoscopic and histologic healing of Crohn's (ileo-) colitis with azathioprine. Gastrointest Endosc. 1999;50:667–671. doi: 10.1016/s0016-5107(99)80017-0. [DOI] [PubMed] [Google Scholar]
- 115.D'Haens G, Geboes K, Ponette E, et al. Healing of severe recurrent ileitis with azathioprine therapy in patients with Crohn's disease. J Gastroenterol. 1997;112:475–481. doi: 10.1016/s0016-5085(97)70027-1. [DOI] [PubMed] [Google Scholar]
- 116.Chun A, Chadi R M, Korelitz B I, et al. Intravenous corticotrophin vs hydrocortisone in the treatment of hospitalized patients with Crohn's disease: a randomized double-blind study and follow up. Inflamm Bowel Dis. 1998;4:177–181. doi: 10.1097/00054725-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 117.Felder J B, Adler D J, Korelitz B I. The safety of corticosteroid therapy in crohn's disease with an abdominal mass. Am J Gastroenterol. 1991;86:1450–1455. [PubMed] [Google Scholar]
- 118.Egan L J, Sandborn W J, Tremaine W J. Clinical outcome following treatment of refractory inflammatory and fistulizing Crohn's disease with intravenous cyclosporine. Am J Gastroenterol. 1998;93:442–448. doi: 10.1111/j.1572-0241.1998.00442.x. [DOI] [PubMed] [Google Scholar]
- 119.Feagan B G. 5-ASA therapy for active Crohn's disease: old friends, old data, and a new conclusion. Clin Gastroenterol Hepatol. 2004;2:376–378. doi: 10.1016/s1542-3565(04)00121-1. [DOI] [PubMed] [Google Scholar]
- 120.Regueiro M, Mardini H. Treatment of perianal fistulizing Crohn's disease with infliximab alone or as an adjunct to exam under anesthesia with Seton placement. Inflamm Bowel Dis. 2003;9:98–103. doi: 10.1097/00054725-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 121.Bernstein L H, Frank M S, Brandt L J, Boley S J. Healing of perineal Crohn's disease with metronidazole. J Gastroenterol. 1980;79:357–365. [PubMed] [Google Scholar]
- 122.Jakobovits J, Schuster M M. Metronidazole therapy for Crohn's disease and associated fistulae. Am J Gastroenterol. 1984;79:533–540. [PubMed] [Google Scholar]
- 123.Brandt L J, Bernstein L H, Boley S J, Frank M S. Metronidazole therapy for perineal Crohn's disease: a follow-up study. J Gastroenterol. 1982;83:383–387. [PubMed] [Google Scholar]
- 124.Solomon M J, McLeod R S, O'Connor B I, Steinhart A H, Greenberg G R, Cohen Z. Combination ciprofloxacin and metronidazole in severe perianal Crohn's disease. Can J Gastroenterol. 1993;7:571–573. [Google Scholar]
- 125.Sands B E, Anderson F H, Bernstein C N, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 126.Pearson D C, May G R, Fick G H, Sutherland L R. Azathioprine and 6-mercaptopurine in Crohn's disease. A meta-analysis. Ann Intern Med. 1995;123:132–142. doi: 10.7326/0003-4819-123-2-199507150-00009. [DOI] [PubMed] [Google Scholar]
- 127.Korelitz B I, Present D H. Favorable effect of 6-mercaptopurine on fistulae of Crohn's disease. Dig Dis Sci. 1985;30:58–64. doi: 10.1007/BF01318372. [DOI] [PubMed] [Google Scholar]
- 128.O'Brien J, Bayless T, Bayless J. Use of azathioprine in the treatment of Crohn's disease. J Gastroenterol. 1991;101:39–46. doi: 10.1016/0016-5085(91)90457-v. [DOI] [PubMed] [Google Scholar]
- 129.Greenstein A J, Present D, Sachar D, et al. Gastric fistulas in Crohn's disease. Report of cases. Dis Colon Rectum. 1989;32:888–892. doi: 10.1007/BF02554563. [DOI] [PubMed] [Google Scholar]
- 130.Margolin M L, Korelitz B. Management of bladder fistula in Crohn's disease. J Clin Gastroenterol. 1989;11:399–402. doi: 10.1097/00004836-198908000-00010. [DOI] [PubMed] [Google Scholar]
- 131.Glass R E, Ritchie J K, Lennard-Jones J E, Hawley P R, Todd I P. Internal fistulas in Crohn's disease. Dis Colon Rectum. 1985;28:557–561. doi: 10.1007/BF02554140. [DOI] [PubMed] [Google Scholar]
- 132.Present D H, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398–1405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- 133.Sands B E, Blank M A, Patel K, Deventer S JH van. Long-term treatment of rectovaginal fistulas in Crohn's disease: response to infliximab in the ACCENT II study. Clin Gastroenterol Hepatol. 2004;2:912–920. doi: 10.1016/s1542-3565(04)00414-8. [DOI] [PubMed] [Google Scholar]
- 134.Wise P E, Schwartz D A. Management of perianal Crohn's disease. Clin Gastroenterol Hepatol. 2006;4:426–430. doi: 10.1016/j.cgh.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 135.Topstad D R, Panaccione R, Heine J A, Johnson D R, MacLean A R, Buie W D. Combined Seton placement, infliximab infusion, and maintenance immunosuppressives improve healing rate in fistulizing anorectal Crohn's disease: a single center experience. Dis Colon Rectum. 2003;46:577–583. doi: 10.1007/s10350-004-6611-4. [DOI] [PubMed] [Google Scholar]
- 136.Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of postoperative course of Crohn's disease. J Gastroenterol. 1990;99:956–963. doi: 10.1016/0016-5085(90)90613-6. [DOI] [PubMed] [Google Scholar]
- 137.Whelan G, Farmer R G, Fazio V W, Goormastic M. Recurrence after surgery in Crohn's disease. Relationship to location of disease (clinical pattern) and surgical indication. J Gastroenterol. 1985;88:1826–1833. doi: 10.1016/0016-5085(85)90007-1. [DOI] [PubMed] [Google Scholar]
- 138.Mekhjian H S, Switz D M, Watts H D, Deren J J, Katon R M, Beman F M. National Cooperative Crohn's Disease Study: factors determining recurrence of Crohn's disease after surgery. J Gastroenterol. 1979;77:907–913. [PubMed] [Google Scholar]
- 139.Yamamoto T. Factors affecting recurrence after surgery for Crohn's disease. World J Gastroenterol. 2005;11:3971–3979. doi: 10.3748/wjg.v11.i26.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ryan W R, Allan R N, Yamamoto T, Keighley M R. Crohn's disease patients who quit smoking have a reduced risk of reoperation for recurrence. Am J Surg. 2004;187:219–225. doi: 10.1016/j.amjsurg.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 141.Polle S W, Slors J F, Weverling G J, Gouma D J, Hommes D W, Bemelman W A. Recurrence after segmental resection for colonic Crohn's disease. Br J Surg. 2005;92:1143–1149. doi: 10.1002/bjs.5050. [DOI] [PubMed] [Google Scholar]
- 142.Lochs H, Mayer M, Fleig W E, et al. Prophylaxis of postoperative relapse in Crohn's disease with mesalamine: European Cooperative Crohn's Disease Study VI. J Gastroenterol. 2000;118:264–273. doi: 10.1016/s0016-5085(00)70208-3. [DOI] [PubMed] [Google Scholar]
- 143.Rutgeerts P, Hiele M, Geboes K, et al. Controlled trial of metronidazole treatment for prevention of Crohn recurrence after ileal resection. J Gastroenterol. 1995;108:1617–1621. doi: 10.1016/0016-5085(95)90121-3. [DOI] [PubMed] [Google Scholar]
- 144.Hanauer S B, Korelitz B I, Rutgeerts P, et al. Postoperative maintenance of Crohn's disease remission with 6-mercaptopurine, mesalamine, or placebo: a 2-year trial. J Gastroenterol. 2004;127:723–729. doi: 10.1053/j.gastro.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 145.Ardizzone S, Maconi G, Sampietro G M, et al. Azathioprine and mesalamine for prevention of relapse after conservative surgery for Crohn's disease. J Gastroenterol. 2004;127:730–740. doi: 10.1053/j.gastro.2004.06.051. [DOI] [PubMed] [Google Scholar]