ABSTRACT
Crohn's disease is a chronic inflammatory disease of the intestinal tract that often results in the need for surgical intervention to treat complications of the disease. The transmural nature of the inflammation can lead to intestinal perforation, intraabdominal abscesses, intestinal strictures, and fistula development. Because there is no cure for Crohn's disease, many patients will require multiple operations during their lifetime. Index surgery and reoperative surgery in these patients is often complex and challenging. There are many preoperative planning and technical aspects of Crohn's surgery that can be helpful in achieving a successful clinical outcome. In this paper, we will review some of the important principles in operative and reoperative Crohn's surgery that can assist the surgeon when approaching these challenging cases.
Keywords: Crohn's disease, reoperative surgery, strictureplasty, adhesions
Crohn's disease and ulcerative colitis are the major categories of nonspecific inflammatory bowel diseases (IBD); the etiology of both diseases is unknown. However, unlike ulcerative colitis, which is limited to the rectum and colon, Crohn's disease can involve any point along the entire intestinal tract. Although Crohn's disease predominantly affects the small bowel, it frequently involves the colon, rectum, and perianal region. Sometimes, these areas may be simultaneously involved or may be diseased at separate times during the course of the patient's disease. Unlike ulcerative colitis in which the intestinal manifestations of the disease are cured with removal of the rectum and colon, surgery for Crohn's disease is directed at relieving symptoms related to or treatment for complications of the disease. Surgery should not be considered curative, but rather as an adjunct to maximal medical therapy. The decision to proceed with an operation should only be entertained after careful consideration and consultation with the patient and a gastroenterologist experienced in the medical management of IBD. Furthermore, the surgeon needs to be highly experienced in the many techniques used to treat the multitude of different clinical presentations and complications associated with Crohn's disease to achieve optimal patient outcomes.
Nearly all of the complications associated with Crohn's disease that require surgical intervention are related to the transmural inflammation of the bowel wall that characterizes the disease. When this inflammatory process is allowed to progress it may lead to abscess formation, entero-entero or enterocutaneous fistula, acute bowel perforation, and intestinal strictures. As previously noted, Crohn's disease is not cured by surgery. Therefore, patients may require multiple surgical procedures over their lifetime due to recurrence of the disease. In the preimmunomodulator period, up to 30% of patients with Crohn's disease who underwent surgery would require reoperative surgery to treat their disease within 5 to 10 years.1 The influence of newer therapeutic agents, such as azathioprine and infliximab, on the need for future surgery is currently unknown. Because there is a relatively high reoperation rate in patients with Crohn's disease at present, another primary concern of the surgeon should be to minimize the amount of intestine that is resected, especially small bowel. Thus, the surgeon must carefully plan his or her current operation with the expectation that the patient will require another operation in the future for complications of the disease.
PREOPERATIVE EVALUATION AND PREPARATION
One of the keys to a successful operation or reoperation in Crohn's disease is appropriate preoperative planning and patient preparation. Prior to undertaking surgery, the surgeon should ensure that the patient has received optimal medical therapy for the Crohn's disease. Furthermore, any septic focus should be controlled with antibiotics, percutaneous drainage, or both. Surgery should be delayed as long as possible to allow the inflammation associated with the septic process to resolve. In many situations, percutaneous drainage will obviate the need for surgery.2,3 Hopefully, this method will minimize the amount of associated inflammation and involvement of adjacent bowel and organs allowing for a limited resection of bowel. Equally important is an evaluation of the patient's nutritional state. Patients who are nutritionally compromised should undergo nutritional repletion either by a liquid enteral diet or by parenteral nutrition to reverse their catabolic state prior to surgery. A nutritional repletion delay may not alter the need for surgery; however, it has been shown to reduce postoperative complications in appropriately selected surgical patients.4
Another key for optimizing surgical outcomes is clearly defining the scope and nature of the patient's past and current disease activity. Obtaining a thorough history of prior operations and, if possible, copies of prior operative reports is very helpful in planning surgery. Preoperative knowledge of the intestinal anatomy and that of any fistulas is essential. Contrast studies of the bowel such as fluoroscopic barium studies or computed tomography (CT) enterography will provide important information about the distribution of disease activity or alterations in anatomy due to prior procedures. Fluoroscopic small bowel barium studies can provide information on the number and length of strictures, the presence of any unsuspected enteroenteric fistulas, contained perforations, and an estimate of small bowel length. New technology, such as CT enterography, using a high-resolution scanner and three-dimensional reconstructions, can provide images that substitute for a small bowel series. Furthermore, these studies provide additional information that may not be seen on a small bowel study, such as an occult abscess, evidence of disease distal to a high-grade obstruction, and possible evidence of an unsuspected malignancy. Fistulography for enterocutaneous fistulas can also be a valuable diagnostic tool.
A final important planning step is to discuss the possible need for a temporary or permanent stoma. Collaboration with an experienced enterostomal therapist to insure pre- and postoperative patient education and preoperative stoma site selection is extremely important. Stoma sites should be marked even if there is a small chance that a stoma will be needed because unexpected intraoperative findings may change the scope of the operation and necessitate a stoma. Proper placement of a stoma is important for successful long-term function and quality of life for the patient.
THE OPERATIVE AND REOPERATIVE SETTING
Once the preoperative planning is complete, there are several technical aspects of the conduct of the operation that will assist the surgeon in completing a safe operation. As previously noted, many patients with CD will require multiple operations. Therefore, even in patients who are undergoing their first operation, the surgeon should consider what can be done during the first procedure that will make the subsequent operations easier. We have identified three aspects of the first operation that may be useful: careful measurement of remaining small bowel length, use of antiadhesion barriers (Seprafilm®; Genzyme Corp., Cambridge, MA), and a laparoscopic approach.
A major concern of patients with Crohn's disease is the amount of small bowel at risk for resection and their potential for short bowel syndrome. Although contrast studies may give a qualitative assessment of small bowel length, we believe that at the end of each procedure a careful measurement of the remaining small bowel from the ligament of Trietz to the ileocolonic anastomosis or ileostomy should be performed and documented in the operative note. To accomplish this measurement in an easy and time-efficient manner, we will use a suture of known length, usually 45 cm or 60 cm to manually measure the length of small bowel. This measurement is done with the bowel in a relaxed state; neither stretched nor contracted, and is of course, an approximate value. The normal small bowel length should be at least 350 to 400 cm, but in some patients can be measured at > 600 cm. Once the small bowel length has been reduced to 100 to 125 cm due to repeated resections, the patient may be challenged with profound nutritional limitation that may require chronic intravenous nutritional and fluid support.
Reoperative abdominal surgery is nearly always complicated by adhesion formation between the bowel and the abdominal wall, internal organs, and among loops of bowel (interloop adhesions). Adhesions are estimated to be the leading cause of small bowel obstruction in the Western world. Nearly one third of those patients, who present with a bowel obstruction, will require an operation to relieve the obstruction. Dissection of adhesions can lead to bowel injury, which will require repair or potential resection of bowel and in the worse case situation an unrecognized injury that will result in intraabdominal sepsis, fistula formation, and possible emergency reoperation. To minimize adhesions, several products have been developed for placement within the abdominal cavity at the end of an operation that will inhibit adhesion formation. The most widely used is a sodium hyaluronate and carboxymethylcellulose product, Seprafilm®. Several clinical studies have shown it to be safe and effective in reducing the incidence and severity of adhesions after both abdominal and pelvic surgery.5,6 In a large multiinstitutional randomized controlled trial, the use of Seprafilm® resulted in a significant decrease in the number of postoperative bowel obstructions that required reoperation within 5 years after the index operation.7 However, the overall incidence of small bowel obstruction was the same between the control and Seprafilm® groups.
Lastly, the role of laparoscopy in the surgical management of Crohn's disease has clearly been shown to be safe and feasible. Furthermore, laparoscopy has several short-term benefits, including more rapid resolution of postoperative ileus, shorter hospitalizations, and lower morbidity. Laparoscopy also has better cosmetic outcomes, which is an important consideration for younger patients with Crohn's disease.8 In a few small studies, including the study by Bergamaschi and colleagues, the 5-year incidence of small bowel obstruction rates between open versus laparoscopic ileocolic resections in patients with Crohn's disease was significantly reduced in the laparoscopic group, 35.4% compared with 11.1%.9 Overall, the conduct of the first operation may significantly influence the ease of any subsequent operation in the planning stage and in the operating room.
REOPERATIONS FOR CROHN'S DISEASE
For any patient with Crohn's disease undergoing reoperation, several minor technical issues can be useful in the operating room to assist during the procedure. We liberally use placement of ureteric stents. These patients often have had inflammatory processes involving the retroperitoneum or prior operative dissection that have altered the normal anatomic course of the ureters. Stents assist in the identification of the ureters and though they may not decrease the incidence of injury, they certainly make it easier to identify an intraoperative injury. They can be placed after the induction of anesthesia and prior to commencement of intraabdominal access. If the operation does or potentially could involve the rectum or left colon, we will position the patient in the Lloyd–Davies, or synchronous position, to have access to the perineum. Lastly, an endoscope should be readily available to allow evaluation of the colon and rectum.
One of the most common causes of reoperation in patients with Crohn's disease is for obstructive symptoms related to stricture formation. Symptomatic strictures often involve the small bowel and less commonly, the colon due to the difference in luminal diameter. The primary surgical treatment of the initial symptomatic presentation of Crohn's disease is resection of the involved bowel with either restoration of intestinal continuity or a temporary stoma. Unfortunately, as previously discussed, a high percentage of patients with Crohn's disease will need to have subsequent surgery to manage recurrence of their disease. In these patients, repeat bowel resection may lead to problems related to a decreased length of the small bowel including vitamin and nutrient malabsorption and volume depletion. Strictureplasty is a useful surgical technique that relieves the obstructive symptoms caused by small bowel strictures while preserving intestinal length, and thus possibly avoiding the complications of repeated small bowel resection.
Lee and Papaioannou10 first reported strictureplasties for the treatment of Crohn's strictures in 1982. The advantage of a strictureplasty is that the procedure relieves the bowel obstruction due to the stricture without loss of bowel, thus minimizing the potential for short bowel syndrome. Any patient with Crohn's disease undergoing surgery for obstructive disease symptoms might be a candidate for a strictureplasty; nevertheless, those patients who will most benefit are those who develop recurrent strictures. The etiology of bowel stricture is most likely related to repeated episodes of inflammation, resolution, and remodeling of the bowel wall that leads to replacement of the normally pliable tissue with a thickened nonpliable bowel wall segment that eventually narrows the lumen. This results in obstructive symptoms, which can only be surgically alleviated.
Strictureplasty may be performed in the ileum, jejunum, duodenum, and even the colon. The nature of the stricture and the amount of the remaining bowel are key factors in determining whether a strictureplasty is appropriate. Strictureplasty is not an option in the setting of bowel perforation or extensive inflammatory phlegmon in the involved segment of bowel.
Initial exploration should include assessment of all evidence; obvious strictures should be marked with sutures (Fig. 1). Each area of involved bowel should be examined for any other pathology that might preclude a strictureplasty such as a fistula or localized abscess. The length of bowel that will remain in situ should to be assessed. If the patient has an adequate bowel length and the strictures are close to each other, it might be safer to perform a single resection and anastomosis rather than multiple strictureplasties. However, if residual bowel length is a concern, then strictureplasties are indicated.
Figure 1.
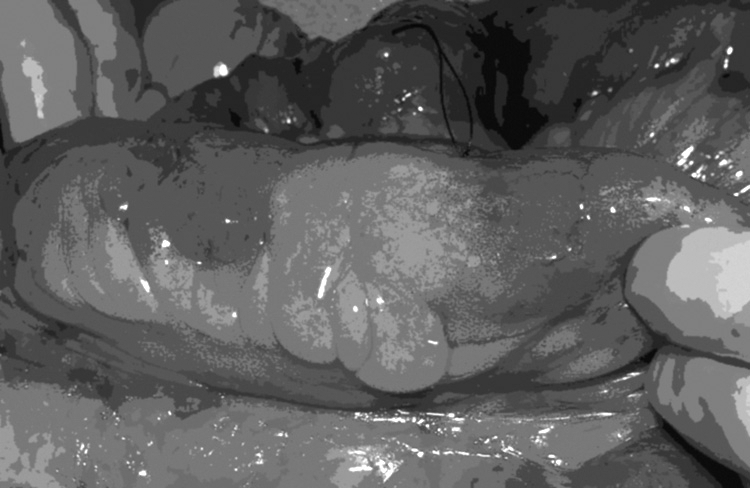
Small bowel Crohn's stricture marked with a suture.
The technique used for a strictureplasty is dependent upon the length of the stricture. For strictures less than 4 to 5 cm in length, the procedure is similar to the Heineke–Mikulicz pyloroplasty performed for pyloric stenosis. This strictureplasty is performed by first placing two 3-0 sutures, either nonabsorbable or absorbable, are then placed on the side of the bowel at the midportion of the stricture to act as stay sutures once the bowel is opened along the antimesenteric edge. The bowel is entered by making an antimesenteric longitudinal incision using electrocautery. This incision divides the stricture and should be performed an equal distance both proximally and distally into normal thin-walled nondiseased bowel. Our practice is to send frozen section biopsies from all strictures to exclude the presence of dysplasia or malignancy. Now, the stay sutures are pulled perpendicular to the long axis of the bowel. The enterotomy is then closed in either one or two layers (Fig. 2).
Figure 2.
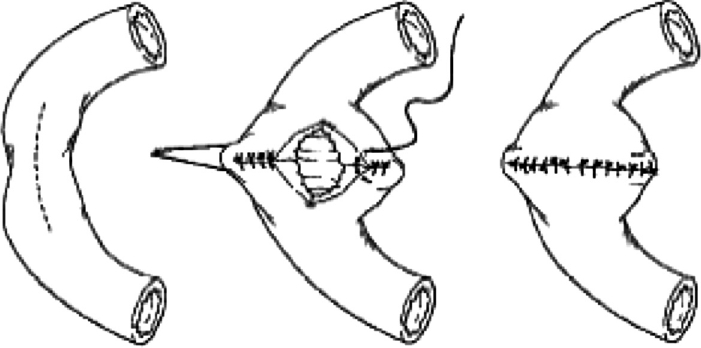
A Heineke–Mikulicz strictureplasty is performed by opening the small bowel along the antimesenteric border of the stricture and then closed transversely. From Tichansky et al,15 with permission.
As previously noted, it is important to identify all the strictures along the bowel. However, visual and tactile external evaluation of the bowel may not identify all small bowel strictures. Several different techniques have been described to intraoperatively evaluate the internal diameter of the small bowel lumen. In essence, these techniques all rely upon the introduction of a device, most commonly a balloon-tipped catheter, into the bowel that can be passed along its length to assess if there are any obstructions to easy passage. The best way of introducing the balloon is through the enterotomy made for the first strictureplasty. We typically use a Baker tube with an inflatable balloon at the distal end (Fig. 3). The tube is manually passed to the end of the small bowel and then the balloon is inflated with 12 to 15 cm of saline, ~1.5 cm in diameter. Any point along the length of the small bowel where easy passage of the balloon is hindered is marked with a suture. Several different devices, including rigid balls, have been described.11 However, none confers any real advantage over the more readily available jejunal tube, which also permits the evacuation of air and fluid from the dilated proximal bowel.
Figure 3.
The Baker tube used to help identify areas of small bowel strictures not easily identified on inspection and palpation of the bowel. The bowel is inflated with 15 cc of normal saline to achieve a diameter of 1 cm.
For strictures greater than 4 to 5 cm, the standard transverse closure of the enterotomy will not be possible. The Finney strictureplasty resembles a side-to-side anastomosis. This strictureplasty may be useful in a patient with a long stricture or for a segment with multiple short strictures closely grouped together with intervening dilated short segments of bowel. The bowel is folded at the stricture with the normal proximal and distal bowel brought along side one another. If a handsewn technique is used there are two options. First, if the strictured area is mildly stenotic and the bowel is of reasonable quality then the entire stricture may be opened along the antimesenteric border and a handsewn essentially side-to-side anastomosis may be performed along the length of the enterotomy. Second, if the stricture is too tight or the bowel is not suitable for suturing, then a true side-to-side anastomosis between the proximal and distal normal bowel can be performed leaving the strictured segment in place as a short bypassed segment. Similarly, if a stapling device is being used, a side-to-side anastomosis can be fashioned between the normal proximal and distal bowel leaving the strictured segment in continuity. The concern with this type of strictureplasty is that it results in a bypassed segment. Though this segment is short, there are concerns about possible complications including bacterial overgrowth and malignant degeneration. This type of strictureplasty also works well when multiple short strictures are formed over a relatively short area with normal intervening segments in a patient in imminent danger of short bowel syndrome.
When the bowel is markedly dilated proximal to a short stricture the size discrepancy between the proximal and distal normal bowel often precludes a Heineke–Mikulicz strictureplasty. In these instances, a Moskel–Walske–Neumayer strictureplasty is performed. This strictureplasty is essentially a Y-to-V advancement flap closure of the stricture. The stricture is opened along the antimesenteric border as a Y-shaped enterotomy with the Y portion in the dilated bowel just proximal to the stricture. The strictured segment is then pulled apart and the antimesenteric segment of the proximal bowel is advanced into the strictured area and closed in a transverse fashion with one side of the closure being normal bowel along the entire length and the other being the two strictured bowel edges.
Michelassi has developed a unique procedure for dealing with the most difficult types of stricture, the very long stricture (> 20 cm) or the series of strictures in close proximity (Fig. 4).12 In these highly unusual patients, a very long segment of bowel that would result in a prohibitively extensive resection can be retained as a side-to-side isoperistaltic strictureplasty. In this technique, the bowel is completely divided transversely at the middle of the strictured segment. Unlike other strictureplasties, the mesentery is divided perpendicular to the long axis of the bowel to permit the two segments of bowel to be overlapped and positioned side-to-side along the entire length of the divided segments. Both strictured segments are opened along the antimesenteric border and the antimesenteric faces of bowel are sewn one to the other in an isoperistaltic fashion. This technique has the advantage of not having any bypassed bowel. Because a small numbers of patients require this type of strictureplasty, reports of long-term functional results are limited. In a series of 21 patients that had a strictureplasty of > 20 cm performed, the authors found no difference in postoperative complications nor increased recurrence rate when compared with strictureplasties on shorter strictures.13
Figure 4.
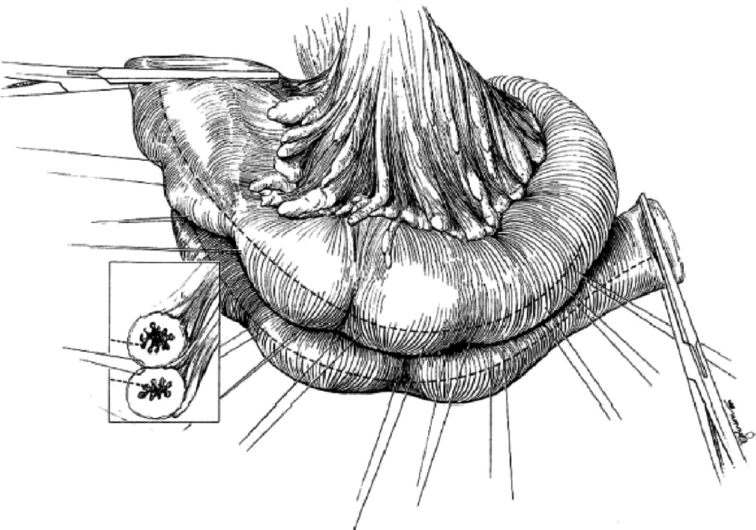
The isoperistaltic side-to-side strictureplasty is used to treat long strictures or multiple strictures that are very close to one another. The diseased segment is divided in the center of the area and overlapped. The antimesenteric border is opened in both segments and then the divided bowel segments are sutured together to construct a common lumen. From Michelassi F, Upadhyay GA. Side-to-side isoperistaltic strictureplasty in the treatment of extensive Crohn's disease. J Surg Res 2004;117:71–78, with permission.
The majority of series of strictureplasties are single-institution reports. Dietz et al14 have reported the safety and long-term efficacy of strictureplasty in 314 patients with obstructing small bowel Crohn's disease. They reviewed 1,124 strictureplasties performed in 314 patients. The overall morbidity was 18% and septic complications occurred in 5%. Crohn's disease recurred in 34% of patients with a median follow-up of 7.5 years. The only significant predictor of recurrence was earlier age at the time of the index strictureplasty. An interesting meta-analysis of the available literature was performed by Tichansky et al15 who analyzed 1,825 strictureplasties performed in 506 patients as reported in 15 different articles. They found that 90% of all strictures treated with strictureplasty were less than 10 cm in length. The Heineke–Mikulicz technique was used in 85% and 13% were treated with a Finney strictureplasty. Forty-four percent of all procedures included a concurrent bowel resection.
The primary role of strictureplasty is to preserve small bowel length in patients who have severe diffuse medically refractory disease or in patients who have recurrent disease. When strictureplasty is used appropriately it is an extremely important and useful operation for patients with complex Crohn's disease.
Very rarely, a patient may present with severe diffuse inflammatory or stricture disease of a long portion of the central small intestine. In these situations, an in-continuity bypass is an option to preserve small bowel. Although there are no data available to support this assumption, it is hoped that an in-continuity bypass would reduce the risk of bacterial overgrowth and malignancy, yet retain function of the absorptive surface in the partially bypassed segment. Furthermore, this type of procedure can be reversed at a subsequent operation if the disease burden of the bypassed segment decreases sufficiently.
CROHN'S FISTULAS
Due to the transmural inflammatory nature of Crohn's disease, involved segments of bowel may become densely adherent to other bowel loops or the abdominal wall. If there is continued inflammation, this might lead to localized perforation that result in fistula formation between the two loops of bowel, enteroenteric fistula, or the abdominal wall, enterocutaneous fistulas. Historically, Crohn's fistulas had been managed by prolonged period of bowel rest, proximal intestinal diversion, or resection. Recently, the anti-TNF-α antibody infliximab has shown efficacy in closure of both abdominal wall and perianal enterocutaneous fistulas.16 In this study, nearly 55% of all patients had closure of all fistulas and nearly 70% had a significant reduction on the number of draining fistulas. Therefore, prior to surgery for fistulas all patients should be given an adequate trial (> 3 months) of infliximab.
Enteroenteric fistulas may not be clinically apparent if the two segments of bowel are in relative close proximity to one another or the fistula itself is not that large. However, if the two involved segments result in a bypass of a significant amount of normal bowel, the patient may experience persistent diarrhea and malnutrition. In this setting, surgical correction is warranted. As discussed previously, defining the anatomy of the intestinal tract and the fistulas is essential in planning any operation. For enteroenteric fistulas, often only one of the loops of bowel is involved with Crohn's disease. Therefore, at the time of surgery the fistula is divided and the fistulizing segment of bowel is resected while the “innocent” bystander segment of bowel need only have the site of the fistula removed and then the bowel can be closed. Removing the actively diseased bowel segments, while preserving the normal segment, will lead to a high rate of success with minimal morbidity and mortality.17 This same technique can be employed for ileocolic fistulas, often involving the sigmoid colon.
Crohn's-associated abdominal wall enterocutaneous fistulas, either spontaneous or postsurgical, can be devastating problems for the patient. In a recent review of a single-institution's experience with abdominal wall fistulas of all types, Draus and colleagues18 noted that even with maximal medical, wound, and surgical care in 106 patients, 7(7%) patients died of fistula complications. In Crohn's, as well as in all types of abdominal wall enterocutaneous fistulas, surgery should be delayed until all intraabdominal sepsis has been resolved, the patient's nutritional state is improved, aggressive local wound care has minimized the amount of skin involvement, and a course of maximal medical therapy has been completed. Early reports regarding the treatment of enterocutaneous fistulas had high recurrences rates; however, recent reports using a multimodality combined nutritional, medical, and surgical approach have demonstrated closure rates as high as 80%.19
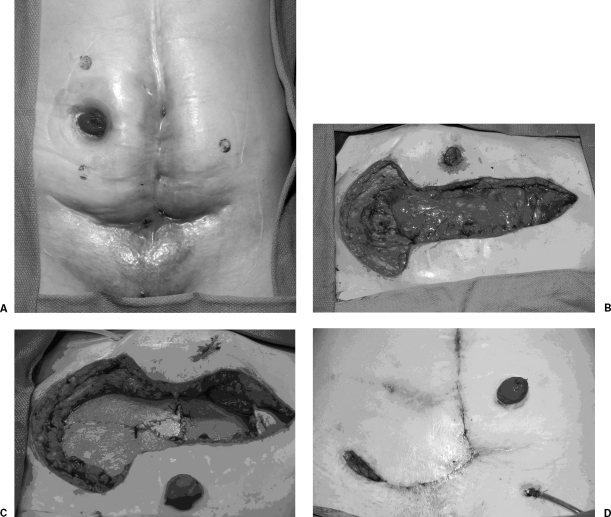
The goal of surgery in the treatment of Crohn's abdominal wall fistulas is to resect the diseased bowel and repair the abdominal wall defect. Unfortunately, resection of a portion of the abdominal wall could leave a defect that may preclude successful closure of the abdomen. The use of prosthetic material such as Prolene (Ethicon, Cincinnati, OH) or Gore-Tex mesh (W.L. Gore & Associates, Inc., Elkton, MD) is contraindicated due to the frequent presence of local sepsis or because of the relatively high frequency of future operations or fistulas. To address this problem, we have begun to use a human-derived dermal graft, Alloderm® (Life Cell Corp., Branchburg, NJ), to reconstruct the abdominal wall (Figs. 5A,B,C,D) This material has numerous advantages in this unique patient population. It is a biologic material that regenerates new native tissue as it is slowly replaced. It also resists intestinal adhesion formation and will tolerate local infection without becoming chronically infected. Once the abdominal wall fistula complex has been removed, the Alloderm® is sutured under tension as a partial underlay to the surrounding healthy abdominal wall tissue with permanent suture. Large skin flaps are then advanced over the closure and numerous drains. In situations where there is not enough skin to close over the Alloderm®, the vacuum-assisted closure device (VAC™; KCI International Inc., San Antonio, TX) may be applied directly to the material. This can then be allowed to heal secondarily or allow the patient to proceed quickly to a skin graft.
Figure 5.
(A) Complex abdominal wall Crohn's disease fistula, (B) that has undergone radical excision of the abdominal wall/fistula complex, and (C) has been repaired with implantation of Alloderm®. (D) The wound is shown 3 weeks after operation.
CONCLUSIONS
Operations for Crohn's disease, in particular reoperative surgery, can be challenging situations for the surgeon. Several elements contribute to a successful outcome. Before the operation, the surgeon must make sure that the patient's physiologic condition has been maximized both with regard to medical management of his or her Crohn's disease and the patient's nutritional state. Adequate time should elapse to optimize resolution of active sepsis and inflammation. A careful history of any prior surgery and detailed radiographic information prior to surgery are very helpful in operative planning. Because many patients with Crohn's disease will undergo future operations, the conduct of the first surgery can greatly assist the subsequent ones. We strongly recommend the use of antiadhesion barriers and/or laparoscopy at the index surgery and during each subsequent intervention. Finally, the most common indications for reoperations in Crohn's disease are strictures and enterocutaneous fistulas. The surgeon involved in the care of these complex patients should be aware of several techniques to treat these conditions, which will minimize the loss of bowel and minimize septic complications.
REFERENCES
- 1.Michelassi F, Balestracci T, Chappell R, Block G E. Primary and recurrent Crohn's disease. Experience with 1379 patients. Ann Surg. 1991;214:230–238. doi: 10.1097/00000658-199109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahai A, Belair M, Gianfelice D, Cote S, Gratton J, Lahaie R. Percutaneous drainage of intra-abdominal abscesses in Crohn's disease: short and long-term outcomes. Am J Gastroenterol. 1997;92:275–278. [PubMed] [Google Scholar]
- 3.Gutierrez A, Lee H, Sands B. Outcome of surgical versus percutaneous drainage of abdominal and pelvic abscesses in Crohn's disease. Am J Gastroenterol. 2006;101:2283–2289. doi: 10.1111/j.1572-0241.2006.00757.x. [DOI] [PubMed] [Google Scholar]
- 4.Heyland D K, Montalvo M, MacDonald S, Keefe L, Su X Y, Drover J W. Total parenteral nutrition in the surgical patient: a meta-analysis. Can J Surg. 2001;44:102–111. [PubMed] [Google Scholar]
- 5.Becker J M, Dayton M T, Fazio V W, et al. Prevention of postoperative abdominal adhesions by sodium hyaluronate-based adhesion barrier: a prospective, randomized, double-blind multicenter study. J Am Coll Surg. 1996;183:297–306. [PubMed] [Google Scholar]
- 6.Diamond M P. Reduction of adhesions after uterine myomectomy by Seprafilm® membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66:904–910. [PubMed] [Google Scholar]
- 7.Fazio V W, Cohen Z, Fleshman J W, et al. Reduction in adhesive small-bowel obstruction by Seprafilm® adhesion barrier after intestinal resection. Dis Colon Rectum. 2006;49:1–11. doi: 10.1007/s10350-005-0268-5. [DOI] [PubMed] [Google Scholar]
- 8.Tan J J, Tjandra J J. Laparoscopic surgery for Crohn's disease: a meta-analysis. Dis Colon Rectum. 2007;50:576–585. doi: 10.1007/s10350-006-0855-0. [DOI] [PubMed] [Google Scholar]
- 9.Bergamaschi R, Pessaux P, Arnaud J P. Comparision of conventional and laparoscopic ileocolic resection for Crohn's disease. Dis Colon Rectum. 2003;46:1129–1133. doi: 10.1007/s10350-004-7292-8. [DOI] [PubMed] [Google Scholar]
- 10.Lee E C, Papaionnou N. Minimal surgery for chronic obstruction inpatients with extensive or universal Crohn's disease. Ann R Coll Surg Engl. 1982;64(4):229–233. [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Granero E, Esclapez P, Garcia-Armengol J, et al. Simple technique for the intraoperative detection of Crohn's stricture with a calibration sphere. Dis Colon Rectum. 2000;43:1168–1170. doi: 10.1007/BF02236569. [DOI] [PubMed] [Google Scholar]
- 12.Michelassi F. Side-to-side isoperistaltic strictureplasty for multiple Crohn's strictures. Dis Colon Rectum. 1996;39:344–349. doi: 10.1007/BF02049480. [DOI] [PubMed] [Google Scholar]
- 13.Shatari T, Clark M A, Yamamoto T, et al. Long strictureplasty is as safe and effective as short strictureplasty in small-bowel Crohn's disease. Colorectal Dis. 2004;6:438–441. doi: 10.1111/j.1463-1318.2004.00664.x. [DOI] [PubMed] [Google Scholar]
- 14.Dietz D W, Laureti S, Strong S A, et al. Safety and longterm efficacy of strictureplasty in 314 patients with obstructing small bowel Crohn's disease. J Am Coll Surg. 2001;192:330–338. doi: 10.1016/s1072-7515(01)00775-x. [DOI] [PubMed] [Google Scholar]
- 15.Tichansky D, Cagir B, Yoo E, Marcus S M, Fry R D. Strictureplasty for Crohn's disease: meta-analysis. Dis Colon Rectum. 2000;43:911–919. doi: 10.1007/BF02237350. [DOI] [PubMed] [Google Scholar]
- 16.Present D H, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's Disease. N Engl J Med. 1999;340:1398–1405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- 17.Michelassi F, Stella M, Balestracci T, Giuliante F, Marongna P, Block G E. Incidence, diagnosis, and treatment of enteric and colorectal fistulae in patients with Crohn's disease. Ann Surg. 1993;218:660–666. doi: 10.1097/00000658-199321850-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Draus J M, Huss S A, Harty N J, Cheadle W G, Larson G M. Enterocutaneous fistula: are treatments improving? Surgery. 2006;140:570–578. doi: 10.1016/j.surg.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Lynch A C, Delaney C P, Senagore A J, Connor J T, Remzi F H, Fazio V W. Clinical outcomes and factors predictive of recurrence after enterocutaneous fistula surgery. Ann Surg. 2004;240:825–831. doi: 10.1097/01.sla.0000143895.17811.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]