ABSTRACT
Venous thromboembolic disease, which includes deep vein thromboses as well as pulmonary emboli, can be a significant complication in the postoperative patient. In particular, colorectal patients often carry a higher risk for venous thromboembolism when compared with patients undergoing other operative procedures. Features unique to colorectal patients are the high incidence of inflammatory bowel disease or malignancy. Typically, these patients will undergo lengthy pelvic procedures, which also contribute to a cumulative risk of venous thrombosis. It is critical that all patients and the proposed operative procedure are appropriately risk stratified. Risk stratification allows for easier implementation of an appropriate prophylactic strategy. There are a wide range of safe and effective mechanical and pharmacologic measures available. The authors provide very specific recommendations, but note that clinical judgment plays a significant role.
Keywords: Deep vein thrombosis, pulmonary embolism, prophylaxis, venous thromboembolism, colorectal surgery
The burden of deep vein thrombosis (DVT) is a major national health concern. It is estimated that in the United States alone ~250,000 DVTs are diagnosed annually resulting in ~50,000 fatal pulmonary emboli (PE). PEs are the most common preventable cause of in-hospital mortality.1,2,3 Though these numbers are daunting, they are most likely underestimations based on the silent nature of a significant proportion of DVTs as well as available autopsy data.4
Surgical patients are vulnerable to the development of DVTs, and colorectal patients seem to be particularly prone to this complication given their unique risk factor profile. It is well known that DVTs, and certainly PEs, may result in devastating consequences like postthrombotic syndrome, pulmonary hypertension, and even death. Despite these very significant potential complications, multiple trials demonstrate the efficacy of DVT prophylaxis. With this in mind, it seems unreasonable not to proceed with perioperative prophylaxis for our patients. Despite these data, the prospective United States DVT Free Study analyzed 1375 patients who underwent nonorthopedic surgeries and developed postoperative, ultrasound-confirmed DVTs. Of this cohort, less than one half had received some sort of DVT prophylaxis.5 A different study, which looked at compliance rates with the American College of Chest Physicians Consensus Conference on Antithrombotic Therapy found a compliance of only 13%.6 So why is perioperative DVT prophylaxis so underutilized? We propose that venous thromboembolic disease is a common complication in colorectal patients and is associated with significant morbidity and mortality. However, there are effective and safe prophylactic measures that may be easily utilized. In this review we will examine factors specific to colorectal surgery and the prevention of postoperative DVT.
PATHOGENESIS
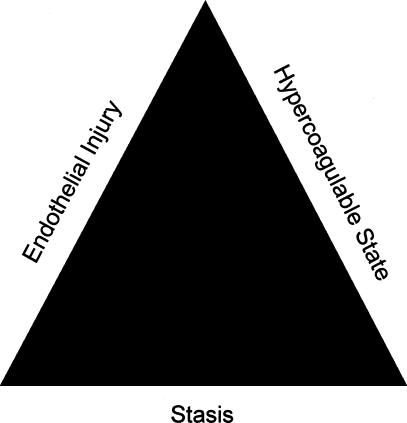
The pathogenesis of venous thromboembolism (VTE) is based on Virchow's triad (Fig. 1). Credited with its discovery, Rudolf Ludwig Karl Virchow never actually proposed or suggested his own named triad.7 In fact, it was not for over 50 years after his death that Virchow's triad of (1) alteration in blood flow (stasis), (2) vascular endothelial injury, and (3) alterations in the constitution of blood (hypercoagulable states) was named for the Father of Modern Pathology.8 Recognized years ago, this triad continues to serve as the foundation to our understanding of this disease process.
Figure 1.
Virchow's triad.
RISK FACTORS
The postoperative patient offers a different set of risk factors when compared with the ambulatory patient, or even the hospitalized medical patient. Postoperatively, patients undergo known physiologic changes that result in a proinflammatory, prothrombotic phase referred to as the perioperative thromboembolic syndrome. This syndrome has been described to last at least one week and is multifactorial in etiology. Intravascular thrombin formation is promoted by the proinflammatory cytokine milieu, the perioperative transient hypotension (not uncommon as a result of general anesthesia as well as perioperative fluid shifts), and activated endothelial cells.9 The perioperative thromboembolic syndrome is clearly based on Virchow's three tenets.
Individual patient factors that contribute to venous thromboembolism are numerous (Table 1). These variables can be used to stratify at-risk patients, and determine the most efficacious method of VTE prophylaxis. Based on the American College of Chest Physician Guidelines, all patients should be classified as low, moderate, high, and highest risk (Table 2). Many surgical patients have multiple risk factors, which act in a cumulative fashion (Table 3). For example, an elderly patient who undergoes a major surgical intervention for a traumatic injury resulting in a period of postoperative immobility will suffer an ~20% risk of proximal DVT and a 0.2 to 5% risk of fatal PE, if no prophylaxis is used.10,11
Table 1.
Risk Factors—Venous Thromboembolism
| Surgery |
| Age > 50 years old |
| Trauma |
| Immobility, paresis |
| Malignancy |
| Cancer therapy |
| Hypercoagulable state |
| Previous deep vein thrombosis |
| Pregnancy |
| Estrogen therapy |
| History of myocardial infarction |
| Atrial fibrillation/cardiac dysfunction |
| Diabetes |
| Acute medical illness |
| Heart or respiratory failure |
| Inflammatory bowel disease |
| Nephrotic syndrome |
| Paroxysmal nocturnal hemoglobinuria |
| Obesity |
| Smoking |
| Varicose veins |
| Central venous catheterization |
Table 2.
Levels of Thromboembolism Risk*
| Low Risk | Moderate Risk | High Risk | Highest Risk |
|---|---|---|---|
| Modified from Geerts et al.10,23 | |||
| Minor surgery | Minor surgery | Surgery age > | Surgery in |
| Age < 40 | with risk factors | 60 or age 40– | patient with |
| No additional risk factors | Surgery age 40–60, no risk factors | 60 with risk factors | multiple risk factors |
| Hip/knee arthroplasty | |||
| Major trauma | |||
See Table 1 for additional risk factors.
Table 3.
Deep Vein Thrombosis (DVT)/Pulmonary Emboli (PE) Risk Without Prophylaxis
Certain colorectal patients are particularly prone to the development of VTE. This phenomenon, as expected, is multifactorial. Frequently colorectal patients carry a diagnosis of malignancy. Patients admitted for cancer surgery have twice the risk of DVT and three times the risk of fatal PE than patients undergoing similar procedures for benign disease. Iversen and Thorlacius-Ussing12 found a 20% prevalence of DVT in colorectal cancer patients after resection of their primary tumor. Stender et al13 completed preoperative screening compression ultrasounds in patients with newly diagnosed colorectal cancer and found an alarming 7.8% prevalence of preoperative DVTs. This suggests that malignancy itself is a risk for DVT, and perioperative prophylaxis of VTEs may have little effect on cancer patients in whom DVTs are already present.
Inflammatory bowel disease (IBD) is another common diagnosis among colorectal patients, which caries an increased risk of VTE. Although the mechanism of VTE formation in IBD is unclear, it is widely accepted that this risk is real. In a cohort study from Manitoba, Berstein et al demonstrated a ~0.5% annual incidence of VTE in both Crohn's and ulcerative colitis (UC) patients. This translated into a significant increased risk of both DVT and PE in the IBD cohort.14 Solem et al from the Mayo Clinic showed that the extent of disease, particularly in UC, portends a higher risk of VTE.15
Many colorectal patients seem to bring a disease-induced hypercoagulable state to the operating table. Intraoperatively and postoperatively, additional risks accumulate. These patients may undergo lengthy pelvic procedures in the modified lithotomy position, which can contribute to the cumulative risk of VTE.
LAPAROSCOPIC SURGERY
The risk of VTE associated with laparoscopic surgery is unkown.16 Some hold the opinion that the risk is lower given the ability to recover and return to normal activities quicker. However, others contend that the risk is greater. This increased risk is largely based on the physiologic effects of creating a pneumoperitoneum. Increased intraabdominal pressure from both pneumoperitoneum (potentially compressing the vena cava) and the reverse Trendelenburg position (often for extended periods of time) have been shown to result in a reduced peak femoral vein systolic velocity promoting lower extremity venous stasis.17 Despite this, there are no prospective studies comparing minimally invasive procedures to conventional open procedures. Several cohort studies looking at the incidence of VTE in laparoscopic cholecystectomy patients have failed to demonstrate a significantly increased risk. In a literature review of over 150,000 laparoscopic cholecystectomy patients, Lindberg et al demonstrated a DVT risk of 0.03% and a PE risk of 0.06%.18 This would suggest the risk of VTE associated with laparoscopic cholecystectomy is very low. However, many colorectal patients are very different from standard general surgery patients, as discussed above.
PROPHYLAXIS
To effectively protect a patient from the formation of VTE, one must first risk stratify their patient. This is important because of the many effective prophylactic measures available to our patients (Table 4). Different strategies range from early postoperative mobilization and compression stocking to intravenous anticoagulation therapy, or even the placement of removable vena cava filters. One must carefully weigh the risks of VTE formation given their patient and the planned procedure with the invasiveness of the prophylactic measure and its potential complications.
Table 4.
Methods of Venous Thromboembolism Prophylaxis
| EARLY MOBILIZATION |
| MECHANICAL |
| Graded compression stocking |
| Sequential compression devices |
| Venous foot pump |
| Vena cava filter |
| PHARMACOLOGIC |
| Dextran |
| Aspirin |
| Warfarin |
| Heparin window |
| Low-dose heparin 5,000 U 1–2 h preop then 5,000 U BID/TID |
| Low-molecular-weight heparins |
| Enoxaparin 30 mg SC BID or 40 mg SC QD |
| Ardeparin 50U per kg SC BID |
| Dalteparin 2500 IU SC 1–2 h preop then QD |
| Danaparoid 750 U SC 1–4 h preop then BID |
| Fondaparinux 2.5 mg SC QD postop |
| Ximelagatran 24 mg PO BID |
BID, twice a day; IU, international unit; preop, preoperative; postop, postoperative; QD, once a day; SC, subcutaneously; TID, three times a day; U, unit.
Preoperative prophylaxis for the development of VTE may be divided into two strategies, mechanical and pharmacologic. Mechanical measures include graded compression stocking (GCS), intermittent pneumatic compression devices (IPCD), venous foot pumps (VFP), and simple early postoperative ambulation. These strategies are attractive because they are noninvasive and they avoid exacerbating postoperative bleeding. Therefore, they may be an attractive option for the patient with a high risk of postoperative bleeding.
Classic teaching encourages patients to get out of bed and ambulate as soon as physically possible after surgery. The benefits of this activity (which are often cited on surgical teaching rounds) are the avoidance of DVTs, the prevention of atelectasis/pneumonia, and the encouragement of early return of bowel function. Even though the idea that early mobilization promotes venous return and avoids the potential of DVT formation makes perfectly good physiologic sense, the literature is sparse with regards to reduction in VTE and early ambulation. However, this surgical dogma remains as a common prophylactic strategy for the low-risk patient.
GCS are frequently utilized in low-risk patients. The exact mechanism of action is unknown. It is believed that by exerting a graded circumferential compression from distal to the proximal limb, venous blood is shifted from the superficial venous system to the deep venous system via perforating veins. This increase in deep venous blood volume increases venous velocity to help prevent stasis and therefore venous thrombosis.19
Sixteen randomized control trials examining GCS were recently reviewed in a Cochrane Review (2008). It was found that GCS significantly reduced the occurrence of DVTs in hospitalized postoperative patients. This analysis predominantly looked at above-the-knee stocking and their effectiveness seemed to be greater in conjunction with other prophylactic measures. The authors' conclusions were that GCS clearly lowers the risk of DVT in postoperative patients and in the higher risk patient, GCS should be combined with a second method of DVT prophylaxis.20
IPCD and VFP work via similar mechanisms. It is believed that these devices not only enhance venous return, much like GCS, but they also stimulate the endogenous fibrinolytic system. This is important because it has been demonstrated that during the postoperative period there is a transient drop in fibrinolytic activity.21 It is thought that by activating the vascular endothelium, IPCDs and VFPs cause a release in tissue plasminogen activator (tPA), helping to reduce the postoperative drop in fibrinolytic activity.22
Despite the appeal to minimize escalating bleeding risks in postoperative patients, there are several potential limitations to the use of mechanical agents as the sole method of VTE prophylaxis. Mechanical agents have not been studied as extensively as pharmacologic methods, there is no standardization to the size or pressure administered, there has never been a proven decrease in PE rate or death with their use, and patient compliance is variable.
PHARMACOLOGIC PROPHYLAXIS
The American College of Chest Physicians has recommended that moderate-risk general surgery patients who undergo a major procedure for a benign disease should receive low-molecular-weight heparin (LMWH), low-dose unfractionated heparin (LDUH), or fondaparinux (Table 5).23 Several studies have shown that the use of LMWH or LDUH in general surgery patients reduce the risk of asymptomatic DVT by up to 72% when compared with no thromboprophylaxis.24,25,26 Countless studies as well as meta-analyses compare LMWH to LDUH. However, no study, including a Cochrane Review of colorectal patients, has shown a significant difference in the rates of symptomatic VTE in patients treated prophylactically with LMWH versus LDUH.27
Table 5.
Venous Thromboembolism Prophylaxis Recommendation
| GCS, graded compression stockings; IPCD, intermittent pneumatic compression device; LMWH, low-molecular-weight heparin; LDUH, low-dose unfractionated heparin. | |
| Modified from Geerts et al.10,23 | |
| Low risk | Early ambulation |
| Moderate risk | LMWH, LDUH, or fondaparinux |
| High risk | LMWH, LDUH, or fondaparinux |
| Highest risk | LMWH, LDUH, or fondaparinux + GCS and/or IPCD |
Bleeding complications associated with VTE prophylaxis remain somewhat controversial. There are several studies that demonstrate LMWH is associated with a lower bleeding rate,28,29 whereas other studies suggest that LMWH has a greater rate of bleeding complications.30,31 Others have found bleeding complications to be dose dependent, particularly with regard to LMWH. Higher doses of LMWH have been associated with a greater rate of bleeding complications with no associated improvement in VTE prophylaxis when compared with LDUH.32 In summary, it seems that when dosed appropriately, LDUH and LMWH are equivalent in VTE prophylaxis as well as the rate of bleeding.
Heparin-induced thrombocytopenia (HIT) is a well-known and feared complication of heparin thromboprophylaxis. It occurs in up to 5% of patients receiving heparin therapy. Typically when appropriately recognized, the heparin is stopped and the syndrome resolves with platelet counts returning to normal ranges. However, in a small subset of patients, paradoxic thrombotic complications can result in myocardial infarction, stroke, or limb ischemia.33 HIT is caused by heparin-dependent IgG antibodies which activate platelets via their Fc receptors.34 Warkentin et al looked at postoperative hip patients in a randomized controlled trial; they found LDUH was associated with HIT in 2.7% of patients. There were zero patients in the LMWH group (333 patients) who developed HIT. It was also found that the development of HIT was strongly associated with thrombotic events, called heparin-induced thrombic thrombocytopenia (HITT).35 These results were confirmed by a recent meta-analysis published by the American Society of Hematology, which concluded that the absolute risk for HIT with LDUH was 2.6% and with LMWH was 0.2%.36 HIT is considerably more common with LDUH than LWMH.
Fondaparinux, a newer agent, is a synthetic pentasaccharide, which acts as a selective Xa-inhibitor that prevents thrombin formation. Because it is a synthetic compound, its pharmacokinetics are very predictable with near complete bioavailability after subcutaneous injection.37 Fondaparinux was initially studied in orthopedic surgery. The results of these studies demonstrated that postoperative administration of fondaparinux reduced the rate of VTE by at least 50% when compared with LMWH, without increasing the risk of bleeding complications.38,39,40 A follow-up randomized study including nearly 3000 patients undergoing major abdominal surgery under general anesthesia compared preoperative LMWH to postoperative fondaparinux administration. There were no significant differences with regards to VTE formation, major bleeding, or death.41 In another randomized controlled trial, Turpie et al42 looked at patients undergoing abdominal surgery and compared IPCD alone to IPCD along with fondaparinux. This multicenter study included 1300 patients and found a 69% reduction in VTE in the later group, with comparable bleeding risks.
LENGTH OF PROPHYLAXIS
The risk of VTE is greatest within the first 1 to 2 weeks after surgery. Many physicians feel this justifies the tradition of stopping VTE prophylaxis when a patient is discharged. However, it is known that the risk of VTE is not zero when the patient leaves the hospital. In a study from Switzerland of nearly 30,000 patients admitted for gastrointestinal surgery, a 30%43 increase in PEs was noted when patients were followed for 30 days after discharge. In a randomized controlled trial comparing postoperative administration of low-molecular-weight heparin for 4 weeks compared with 1 week in patients undergoing major abdominal surgery, there was a trend toward decreasing the incidence of DVT. However, this trend was not significant in this study of 118 patients.44 In a larger multicenter study, ENOXACAN II, a significant reduction in the incidence of DVT was found in patients treated for 28 days after abdominal or pelvic cancer surgery compared with 9 days. Of note, there were only two symptomatic VTE in the 9-day group and only one in the 28-day treated group, and there were no significant differences in the rates of bleeding or other complications.45 These data were confirmed in a more recent randomized controlled study involving 427 patients where the cumulative incidence of VTE was reduced from 16.3% to 7.3% with prolonged thromboprophylaxis with low-molecular-weight heparin.46 The length of prophylaxis becomes a particularly interesting question in this era of cost containment and shorter hospitalizations. The National Institute for Health and Clinical Excellence from England (NICE) performed an extensive cost analysis of postoperative VTE thromboprophylaxis. Concerning abdominal surgery, NICE did not find it to be cost effective to treat nonorthopedic surgical patients longer than 7 days after surgery.47 It appears that prolonged prophylaxis does decrease the risk of asymptomatic VTE in major abdominal surgery; however, the clinical significance as well as the cost effectiveness of this strategy remains unclear.
SUMMARY
VTE is a common problem. Surgical patients, particularly colorectal patients, are prone to the development of a venous thrombus and its potential catastrophic consequences. Therefore, it is critical to be vigilant in identifying an appropriate prophylactic measure for the patient and the planned procedure. Often, the strategy will include a combination of modalities: for example, IPCD, LMWH, and early ambulation may be used in a patient undergoing an abdominoperineal resection for a low rectal cancer. However, this plan may change if significant presacral venous bleeding was encountered intraoperatively. No single regimen is suited for all circumstances, or even for all patients undergoing the same procedure. A certain level of clinical judgment is critical for the safe and effective battle against VTE. The potential risks of prophylaxis are clearly diminutive in comparison to the risks, and potential sequelae, of VTE in our high-risk patient population.
DISCLAIMER
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government.
REFERENCES
- 1.Horlander K T, Mannino D M, Leeper K V. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711–1717. doi: 10.1001/archinte.163.14.1711. [DOI] [PubMed] [Google Scholar]
- 2.Anderson F A, Jr, Wheeler H B, Goldberg R J, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151(5):933–938. [PubMed] [Google Scholar]
- 3.Silverstein M D, Heit J A, Mohr D N, Petterson T M, O'Fallon W M, Melton L J., III Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158(6):585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 4.Sandler D A, Martin J F. Autopsy proven pulmonary embolism in hospital patients: are we detecting enough deep vein thrombosis? J R Soc Med. 1989;82:203–205. doi: 10.1177/014107688908200407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seddighzadeh A, Zurawska U, Shetty R, Goldhaber S Z. Venous thromboembolism in patients undergoing surgery: low rates of prophylaxis and high rates of filter insertion. Thromb Haemost. 2007;98(6):1220–1225. doi: 10.1160/th07-06-0405. [DOI] [PubMed] [Google Scholar]
- 6.Yu H T, Dylan M L, Lin J, et al. Hospitals' compliance with prophylaxis guidelines for venous thromboembolism. Am J Health Syst Pharm. 2007;64:69–76. doi: 10.2146/ajhp060115. [DOI] [PubMed] [Google Scholar]
- 7.Virchow R LK. Translation in Matzdorff AC, Bell WR, trans, editor. Thrombosis and embolie (1846–1856) Canton, MA: Science History Publications; 1998. Originally published in German as “Thrombose und Embolie. Gefässentzündung und septische Infektion,” Gesammelte Abhandlungen zur wissenschaftlichen Medicin. Frankfurt am Main: Von Meidinger & Sohn; 1856:219–732.
- 8.Anning S T. The historical aspects of venous thrombosis. Med Hist. 1957;1:28–37. doi: 10.1017/s0025727300020743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kikura M, Takada T, Sato S. Age- and sex-specific incidence, risk, and latency period of a perioperative acute thromboembolism syndrome (PATS) Thromb Haemost. 2004;91:725–732. doi: 10.1160/TH03-10-0613. [DOI] [PubMed] [Google Scholar]
- 10.Geerts W H, Graham F P, Heit J A, et al. Prevention of venous thromboembolism: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:338S–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 11.Geerts W H, Heit J A, Clagett G P, Pineo G F, Colwell C W, Anderson F A, Wheeler B. Prevention of venous thromboembolism. Chest. 2001;119:132S–175S. doi: 10.1378/chest.119.1_suppl.132s. [DOI] [PubMed] [Google Scholar]
- 12.Iversen L H, Thorlacius-Ussing O. Relationship of coagulation test abnormalities to tomour burden and postoperative DVT in resected colorectal cancer. Thromb Haemost. 2002;87:402–408. [PubMed] [Google Scholar]
- 13.Stender M T, Nielsen T S, Frøkjaer J B, Larsen T B, Lundbye-Christensen S, Thorlacius-Ussing O. High preoperative prevalence of deep venous thrombosis in patients with colorectal cancer. Br J Surg. 2007;94(9):1100–1103. doi: 10.1002/bjs.5754. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein C N, Blanchard J F, Houston D S, et al. The incidence of deep venous thrombosis and pulmonary embolism among patients with inflammatory bowel disease: A population-based cohort study. Thromb Haemost. 2001;85:430–434. [PubMed] [Google Scholar]
- 15.Solem C A, Loftus E V, Tremaine W J, Sandborn W J. Venous thromboembolism in inflammatory bowel disease. American Journal of Gastroenterology. 2004;99(1):97–101. doi: 10.1046/j.1572-0241.2003.04026.x. [DOI] [PubMed] [Google Scholar]
- 16.Anderson F A, Spencer F A. Risk factors for venous thromboembolism. Circulation. 2003;107(Suppl 1):I9–I16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen N T, Cronan M, Braley S, Rivers R, Wolfe B M. Duplex ultrasound assessment of femoral venous flow during laparoscopic and open gastric bypass. Surg Endosc. 2003;17(2):285–290. doi: 10.1007/s00464-002-8812-z. [DOI] [PubMed] [Google Scholar]
- 18.Lindberg F, Bergqvist D, Rasmussen I. Incidence of thromboembolic complications after laparoscopic cholecystectomy: review of the literature. Surg Laparosc Endosc. 1997;7:324–331. [PubMed] [Google Scholar]
- 19.Benko T, Cooke E A, McNally M A, Mollan R AB. Graduated compression stockings: knee length or thigh length. Clin Orthop Relat Res. 2001;383:197–203. [PubMed] [Google Scholar]
- 20.Amaragiri S V, Lees T A. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database Sys Rev. 2008;(1) doi: 10.1002/14651858.CD001484. CD001484. [DOI] [PubMed] [Google Scholar]
- 21.Prins M H, Hirsh J. A critical review of the evidence supporting a relationship between impaired fibrinolytic activity and venous thromboembolism. Arch Intern Med. 1991;151:1721–1731. [PubMed] [Google Scholar]
- 22.Ginzburg E, Cohn S M, Lopez J, Jackowski J, Brown M, Hameed S M, Miami Deep Vein Thrombosis Study Group Randomized clinical trial of intermittent pneumatic compression and low molecular weight heparin in trauma. Br J Surg. 2003;90(11):1338–1344. doi: 10.1002/bjs.4309. [DOI] [PubMed] [Google Scholar]
- 23.Geerts W H, Berqvist D, Pineo G F, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed.) Chest. 2008;133(suppl):381s–453s. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 24.Collins R, Scrimgeor A, Yusuf S. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin: overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162–1173. doi: 10.1056/NEJM198805053181805. [DOI] [PubMed] [Google Scholar]
- 25.Clagett G P, Reisch J S. Prevention of venous thromboembolism in general surgical patients: results of meta-analysis. Ann Surg. 1988;208:227–240. doi: 10.1097/00000658-198808000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mismetti P, Laporte S, Darmon J Y, et al. Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg. 2001;88:913–930. doi: 10.1046/j.0007-1323.2001.01800.x. [DOI] [PubMed] [Google Scholar]
- 27.Wille-Jorgensen P, Rasmussen M D, Anderson B R, et al. Heparins and mechanical methods for thromboprophylaxis in colorectal surgery. Cochrane Database Syst Rev. 2004;(1) doi: 10.1002/14651858.CD001217. CD001217. [DOI] [PubMed] [Google Scholar]
- 28.Kakkar V V, Cohen A T, Edmonson R A, et al. Low molecular weight versus standard heparin for prevention of venous thromboembolism after major abdominal surgery. Lancet. 1993;341:259–265. doi: 10.1016/0140-6736(93)92614-y. [DOI] [PubMed] [Google Scholar]
- 29.Nurmohamed M T, Verhaeghe R, Haas S, et al. A comparative trial of low molecular weight heparin(enoxaparin) versus standard heparin for the prophylaxis of postoperative deep vein thrombosis in general surgery. Am J Surg. 1995;169:567–571. doi: 10.1016/s0002-9610(99)80222-0. [DOI] [PubMed] [Google Scholar]
- 30.Bergqvist D, Matzsch T, Burmark U S, et al. Low molecular weight heparin given the evening before surgery compared with conventional low-dose heparin in prevention of thrombosis. Br J Surg. 1988;75:888–891. doi: 10.1002/bjs.1800750920. [DOI] [PubMed] [Google Scholar]
- 31.McLeod R S, Geerts W H, Sniderman K W, et al. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian Colorectal DVT Prophylaxis Trial: a randomized, double-blind trial. Ann Surg. 2001;233:438–444. doi: 10.1097/00000658-200103000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koch A, Bouges S, Ziegler S, et al. Low molecular weight heparin and unfractionated heparin in thrombosis prophylaxis after major surgical intervention: update of previous meta-analysis. Br J Surg. 1997;84:750–759. [PubMed] [Google Scholar]
- 33.King D J, Kelton J G. Heparin-associated thrombocytopenia. Ann Intern Med. 1984;100:535–540. doi: 10.7326/0003-4819-100-4-535. [DOI] [PubMed] [Google Scholar]
- 34.Kelton J G, Sheridan D, Santos A, et al. Heparin-induced thrombocytopenia: laboratory studies. Blood. 1988;72:925–930. [PubMed] [Google Scholar]
- 35.Warkentin T E, Levine M N, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med. 1995;332:1330–1335. doi: 10.1056/NEJM199505183322003. [DOI] [PubMed] [Google Scholar]
- 36.Martel N, Lee J, Wells P. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylasxis: a meta-analysis. Blood. 2005;106:2710–2715. doi: 10.1182/blood-2005-04-1546. [DOI] [PubMed] [Google Scholar]
- 37.Samama M M, Gerotziafas G T. Evaluation of the pharmacological properties and clinical results of the synthetic pentasaccharide(fondaparinux) Thromb Res. 2003;109:1–11. doi: 10.1016/s0049-3848(03)00030-6. [DOI] [PubMed] [Google Scholar]
- 38.Eriksson B I, Bauer K A, Lassen M R, Turpie A GG, for the Steering Committee of the Pentasaccharide in Hip-Fracture Surgery Study Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip-fracture surgery. N Engl J Med. 2001;345:1298–1304. doi: 10.1056/NEJMoa011100. [DOI] [PubMed] [Google Scholar]
- 39.Lassen M R, Bauer K A, Eriksson B I, Turpie A GG, for the European Pentasaccharide Hip Elective Surgery Study (EPHESUS) Steering Committee Postoperative fondaparinux versus preoperative enoxaparin for prevention of venous thromboembolism in elective hip-replacement surgery: a randomised double-blind comparison. Lancet. 2002;359:1715–1720. doi: 10.1016/S0140-6736(02)08652-X. [DOI] [PubMed] [Google Scholar]
- 40.Turpie A GG, Bauer K A, Eriksson B I, Lassen M R, for the Steering Committees of the Pentasaccharide Orthopedic Prophylaxis Studies Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: a meta-analysis of 4 randomized double-blind studies. Arch Intern Med. 2002;162:1833–1840. doi: 10.1001/archinte.162.16.1833. [DOI] [PubMed] [Google Scholar]
- 41.Agnelli G, Bergqvist D, Cohen A T, Gallus A S, Gent M, and PEGASUS Investigators Randomised clinical trial of postoperative fondaparinux versus perioperative dalteparin for prevention of venous thromboembolism in high-risk abdominal surgery. Br J Surg. 2005;92:1212–1220. doi: 10.1002/bjs.5154. [DOI] [PubMed] [Google Scholar]
- 42.Turpie A G, Bauer K A, Caprini J A, Comp P C, Gent M, Muntz J E, Apollo investigators Fondaparinox combined with intermittent pneumatic compression vs. intermittent pneumatic compression alone for prevention of venous thromboembolism after abdominal surgery: a randomized double-blind comparison. J Thromb Haemost. 2007;5:1854–1861. doi: 10.1111/j.1538-7836.2007.02657.x. [DOI] [PubMed] [Google Scholar]
- 43.Huber O, Bounameaux H, Borst F, Rohner A. Postoperative pulmonary embolism after hospital discharge. An underestimated risk. Arch Surg. 1992;127:310–313. doi: 10.1001/archsurg.1992.01420030076014. [DOI] [PubMed] [Google Scholar]
- 44.Lausen I, Jensen R, Jorgensen L N, et al. Incidence and prevention of deep venous thrombosis occurring late after general surgery: randomized controlled study of prolonged thromboprophylaxis. Eur J Surg. 1998;164:657–663. doi: 10.1080/110241598750005534. [DOI] [PubMed] [Google Scholar]
- 45.Bergqvist D, Angelli G, Cohen A, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346:975–980. doi: 10.1056/NEJMoa012385. [DOI] [PubMed] [Google Scholar]
- 46.Rasmussen M S, Jorgensen L N, Wille-Jorgensen P, et al. Prolonged prophylaxis with dalteparin to prevent late thromboembolic complications in patients undergoing major abdominal surgery: a multicenter open-label study. J Thromb Haemost. 2006;4:2384–2390. doi: 10.1111/j.1538-7836.2006.02153.x. [DOI] [PubMed] [Google Scholar]
- 47.National Institute for Health and Clinical Excellence Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in inpatients undergoing sugery. NICE clinical guideline No. 46:1–160. Available at: http://www.nice.org.uk/CG046. Accessed September 28, 2008. Available at: http://www.nice.org.uk/CG046