ABSTRACT
Malignancies of the anal margin and perianal skin are relatively uncommon lesions, comprising 3 to 4% of all anorectal malignancies. Commonly included in this group of cancers are Bowen's disease (intraepithelial squamous cell cancer), perianal Paget's disease (intraepithelial adenocarcinoma), invasive squamous cell cancer, basal cell cancer, and malignant melanoma. Buschke-Lowenstein tumor, or giant condyloma acuminatum, is not always included because this lesion is technically benign, although it displays aggressive local invasive behavior that makes it difficult to manage.
Complaints are usually nonspecific, such as itching or burning, bleeding, pain, drainage, or a mass. Proper diagnosis requires a high index of suspicion on the part of the surgeon. Innocent local irritations will resolve in a short time with appropriate therapy; those that persist must be biopsied for tissue diagnosis.
Wide local excision is the mainstay of treatment for early stage tumors as it preserves continence and obtains adequate local control. Adjunct therapies have been utilized in more advanced or recurrent lesions, including radiotherapy, photodynamic therapy, and imiquimod. All have met with a fair amount of success in controlling local disease; however, the number of patients treated in each instance is small, making it difficult to design an evidence-based treatment strategy. Invasion and metastasis are relatively rare in this group of neoplasms; perianal Paget's disease has the highest risk of associated underlying neoplasm. The most important consideration in developing a treatment strategy is which strategy would achieve the best clinical result with the least morbidity to the patient.
Keywords: Anal margin cancer, diagnosis, treatment options, local excision, radiation therapy
COMMON CONCERNS IN MALIGNANCIES OF THE ANAL MARGIN AND PERIANAL SKIN
Malignancies of the anal margin and perianal skin are relatively uncommon lesions, comprising 3 to 4% of all anorectal malignancies. Commonly included in this group of cancers are Bowen's disease (intraepithelial squamous cell cancer), perianal Paget's disease (intraepithelial adenocarcinoma), invasive squamous cell cancer, basal cell cancer, and malignant melanoma. This article will discuss all of these (excepting malignant melanoma, which is discussed elsewhere in this journal) as well as Buschke-Lowenstein tumor or verrucous carcinoma.
Patients with these lesions often present with common perianal complaints, such as itching or burning, bleeding, pain, drainage, or a mass. Proper diagnosis requires a high index of suspicion on the part of the surgeon. Any person with persistent complaints of a local rash or chronic irritation can be considered at risk until proven otherwise. Less concerning are symptoms of relatively short duration and symmetrical rashes; however, all patients should be scheduled for follow-up visit within 4 to 6 weeks. Innocent local irritations will resolve in that time with appropriate therapy; those that persist must be biopsied for tissue diagnosis.
SQUAMOUS CELL CARCINOMA
Epidemiology
Anal margin cancers are less common than carcinomas of the anal canal and have a more favorable prognosis.1,2,3,4 Squamous cell carcinoma (SCCA) of the anal margin represents one-fourth to one-third of all SCCA of the anus and is the most common of the anal margin tumors.2,5 Most patients present between 65 to 75 years of age; however, there is a wide range with no clear predominance for either sex.1,2
Anatomy and Histology
The distinction between SCCA of the anal margin and anal canal has important implications for prognosis and management.5 The anal margin is described as the region beginning at the margin of the hair-bearing perianal skin and extending onto the perianal skin for a 5-cm radius; the anal canal lies superior to this, from the mucocutaneous junction to the beginning of the rectal mucosa. Squamous cell cancers in the anal margin and perianal skin are generally treated as SCCA elsewhere in the body.6,7 A majority of these lesions are well or moderately differentiated keratinizing SCCA.1,7 The arterial supply consists of the middle and inferior rectal arteries with systemic drainage via the inferior rectal vein. Tumors of the anal margin drain primarily to the inguinal nodes leading to external and common iliac nodal regions.
Presentation and Diagnosis
There is often a delay in diagnosis of anal margin cancers due to the location and nonspecific quality of symptoms. The duration of symptoms prior to diagnosis ranges from 2 to 60 months.1 Anal cancers progress slowly and are commonly mistaken for benign processes such as hemorrhoids.1 Nearly one-third of patients are misdiagnosed at their first physician visit. Presenting symptoms can include a painful mass, bleeding, pruritus, tenesmus, discharge, or a change in bowel habits.2,6 Patients' medical history may include a history of receptive anal intercourse, human papillomavirus (HPV) infection, human immunodeficiency virus (HIV) seropositivity, previous anal conditions, and a smoking history.
Physical examination should include visual inspection, digital exam, anoscopy, and examination of inguinal lymph nodes. The SCCA lesion usually has rolled, everted edges with an ulcerated center. There may be a palpable component within the subcutaneous tissues. The anal canal may become involved late in the disease, although the sphincter complex is rarely invaded.1,6 Any patient with a suspicious lesion should undergo incisional or excisional biopsy for definitive diagnosis. Excisional biopsies, however, should only be performed when it is clear that adequate margins can be achieved.1
Staging
Although these tumors are typically well differentiated and slow growing, pretreatment evaluation of a patient with SCCA of the anal margin should include a full staging workup. Staging of anal margin SCCA is based on the size of the primary tumor and lymph node involvement.1,6 The staging system applied has been set forth by the American Joint Committee on Cancer (AJCC) and is described in Table 1.6,8
Table 1.
| Primary carcinoma (T) | |||
| Tis | Carcinoma in situ | ||
| T1 | Carcinoma 2 cm or less | ||
| T2 | Carcinoma more than 2 cm but not more than 5 cm | ||
| T3 | Carcinoma more than 5 cm | ||
| T4 | Carcinoma invades deep extradermal structures. | ||
| Regional lymph node(s) (N) | |||
| N0 | No lymph node metastasis | ||
| N1 | Regional lymph node metastasis | ||
| NX | Regional lymph nodes cannot be assessed. | ||
| Distant metastasis (M) | |||
| M0 | No distant metastasis | ||
| M1 | Distant metastasis | ||
| MX | Presence of distant metastasis cannot be assessed. | ||
| Stage grouping | |||
| Stage 0 | Tis | N0 | M0 |
| Stage I | T1 | N0 | M0 |
| Stage II | T2–4 | N0 | M0 |
| Stage III | T4 | N0 | M0 |
| Any T | N1 | M0 | |
| Stage IV | Any T | Any N | M1 |
Physical examination is the key component in evaluating the primary tumor. The tumor size, differentiation, and invasion of extradermal structures are all important for staging and prognosis. Studies have correlated tumor size with prognosis and found lymph node involvement to be an adverse prognostic factor.1 Lymph node involvement is associated with the size of the primary tumor. One study reported an incidence of inguinal lymph node metastasis to be 0% with a tumor size less than 2 cm, 24% with a 2 to 5 cm tumor, and 67% with primary tumors greater than 5 cm.1 Although rare, the presence of distant metastases should be evaluated with a computed tomography (CT) scan of the abdomen and pelvis to identify regional and distant lymph node metastases or liver involvement. A chest x-ray should also be performed to rule out metastatic disease to the lung.1,6
Treatment Options
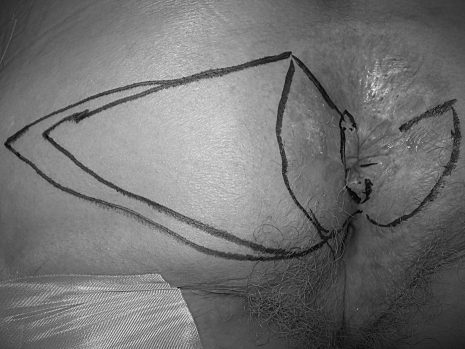
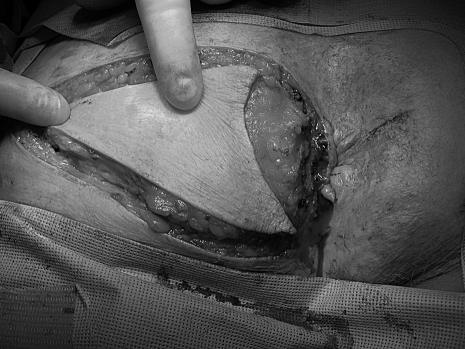
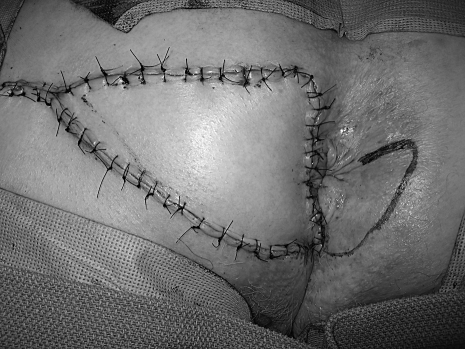
The goal of all treatment options for anal margin SCCA is to cure the patient while achieving the best functional result. The choice of treatment depends on several factors including the stage of the tumor, the anticipated functional result, and the risk of complications.6 Surgical resection with wide local excision for small tumors or abdominoperineal resection (APR) for larger, invasive tumors has been the traditional method of treatment.4 However, local excision can result in high local recurrence rates (18 to 63%) and should be reserved for tumors that can be excised with 1 cm margins, are less than 2 cm in greatest dimension, and do not involve the sphincter.1,6 The defect is closed primarily if possible with or without the use of various flaps; however, a skin graft may be needed for large surgical defects. A V-Y advancement flap encompassing skin and subcutaneous fat can generally be used by the colorectal surgeon with good results (Figs. 1–3). Larger defects may require the assistance of a plastic surgeon for closure. Inguinal node dissection is performed only for clinically positive nodes.
Figure 1.
Perianal squamous cell carcinoma—outline of area of resection with v-y advancement flap for closure.
Figure 2.
Excision of lesion with margins and dissection of flap.
Figure 3.
Closure of v-y advancement flap.
Introduced in the early 1970s, radiation therapy has become the mainstay of treatment for SCCA of the anal canal. It is also currently being applied to SCCA of the anal margin with favorable results. Patients with T1 and early T2 tumors may be treated with either radiation therapy or primary surgical treatment achieving similar local control rates.1 Superficial, well-differentiated T1 and early T2 lesions can be radiated through a perineal field alone; however, inguinal nodes are often irradiated as well due to the significant incidence of nodal disease even with small tumors.1,3 More advanced lesions can be effectively treated with chemoradiation. Perineal and inguinal fields are often employed, even in the absence of clinically positive groin nodes. Pelvic lymph nodes are also treated for T3/T4 lesions and those presenting with inguinal node metastases.1,6 Local control rates for radiation therapy by T stage are 50 to 100% for T1, 60 to 100% for T2, and 37 to 100% for T3.6 One study reported 100% (19 patients) local control after radiation or radiation and chemotherapy in patients with T1–3, N0 disease. Elective inguinal node radiation was performed in 18 of 19 patients, and the patient not receiving elective groin radiation subsequently died with regional and distant disease.1 Chapet et al reported a local control rate of 33% for T2 and T3–4 lesions using adjuvant radiation after inadequate local excision. However, with radiation alone, local control was reached in 63.6% of T2 and 100% of T3 tumors, thereby reinforcing the idea that local excision should only be performed for small lesions when clear margins can be achieved.4 Excluding T1 lesions treated by local excision, Gerhard et al found that patients with anal margin cancer had a less favorable outcome than those with anal canal cancer. The 5-year-overall and colostomy-free survival rates were 54% and 69%, respectively.9 Local excision or APR can be used to treat persistent disease after chemoradiation with a salvage rate of ~50%. Patients who develop recurrence after radiation therapy or chemoradiation may also undergo salvage surgery for cure.1,6
Weighing the effectiveness of a treatment option with its risks to each individual patient is an important factor in determining the most appropriate plan for treatment. For T1 tumors that do not involve the sphincters, wide local excision may be a better choice than radiation due to the decreased morbidity and time spent in treatment even though radiation provides similar control and survival. Due to the significant risk of recurrence and lymph node metastases, radiation to the primary lesion and inguinal fields decreases the morbidity to the patient while achieving similar control rates compared with surgical therapy for T2 lesions. T3 and T4 tumors should be treated with radiation to the primary lesion as well as inguinal and pelvic nodal basins. Due to its increased morbidity and necessitating a permanent colostomy, APR should be reserved for the treatment of patients with persistent disease after unsuccessful radiation/chemoradiation or those with recurrent disease not amenable to local excision.1,2,4,6,7
Due to the risk of local recurrence as well as distant disease, it is essential that patients be closely followed for several years. It is recommended that a full anorectal and nodal examination be performed every 3 months for the first 2 years after treatment and then every 6 months until year 5.
Specific follow-up recommendations vary among surgeons, dermatologists, and primary care physicians; however, routine physical examination for 2 years after treatment is generally accepted. Some physicians may recommend regular follow-up for 5 years although the risk of local recurrence is greatest within 2 years.
BASAL CELL CARCINOMA
Basal cell carcinoma (BCC) accounts for 65 to 80% of reported nonmelanoma skin cancer.10 However, BCC of the anus comprises only 0.1% of all BCCs diagnosed and less than 1% of all anorectal neoplasms,6,10,11 rarer even than anorectal melanoma. The average age at presentation is 65 to 75 years with 60 to 80% occurring in men of whom approximately one-third have a history of BCC at other skin sites.6 The average lesion size at presentation is 1 to 2 cm, although lesions as large as 10 cm with extension into the anal canal have been reported.6,11 BCCs arise in the basal layer of the epidermis and pilosebaceous follicle units.10 Preexisting skin conditions such as basal cell nevus syndrome, xeroderma pigmentosum, and immunodeficiency contribute to the etiology of BCC of both sun-exposed skin as well as the perianal region. Radiation, chronic irritation or infection, trauma, or burns may play a particular role in the development of perianal BCC,6,12 which has been known to arise in longstanding anal fistula tracts.
On examination, BCCs generally have raised edges with a central ulceration and are typically superficial and mobile with little metastatic potential.6,10,11 In contrast to previously reported experiences, Manstein et al found no correlation between tumor size and metastatic spread when evaluating a small number of giant BCCs.10 They are histologically similar to BCCs of other areas of the body and should be differentiated from the basaloid variant of squamous cell carcinoma, which carries a worse prognosis.6,11 BCCs of the perianal region do not contain HPV.6
Treatment depends on the size of the tumor and the extent of invasion. Lesions <2 cm can be treated with wide local excision ensuring adequate 1 cm margins. Larger lesions that do not extend into the anal canal may require primary excision in combination with skin grafting or flap to aid with closure.6,10 Mohs microsurgery is another option to preserve as much uninvolved tissue as possible. Large lesions extending into the anal canal may need to be treated with radiation and/or APR. Local excision has a recurrence rate ranging from 0 to 29%.6 One review of 19 cases of BCC of the anal margin reported that all patients were free of recurrence at a mean follow-up of 72 months following local excision.11 If feasible, recurrences can be treated with re-excision; however, radiation or APR may be needed.6,11
PAGET'S DISEASE
Diagnosis
Perianal Paget's is quite uncommon, with only 195 cases reported in the literature from 1963 to 1995.13 It is an intraepithelial adenocarcinoma arising from the dermal apocrine sweat glands. It is most commonly found in older patients (average age 66 years), shows a preponderance for women, and is often initially confused with benign conditions, which can lead to a delay in diagnosis. Perianal Paget's should be considered in patients who present with perianal itching or rash refractory to local therapy. There may also be drainage, bleeding, or pain. Lesions are usually erythematous and crusty, eczematoid, or scaly-appearing. Differential diagnosis includes leukoplakia, Bowen's disease, melanoma, basal and squamous cell carcinoma, condylomata acuminata, dermatitis, eczema, and psoriasis.14,15
Full-thickness biopsies of the affected anal margin skin must be obtained. Perianal mapping biopsies should be obtained; the accepted method is described in the section on Bowen's disease. Microscopic examination of perianal skin affected by Paget's disease will show classic Paget cells, which appear as large rounded cells with pale vacuolated cytoplasm and hyperchromatic eccentric nuclei.16 There may be hyperkeratosis, parakeratosis, and acanthosis of the epidermal cells. Periodic acid-Schiff stain (PAS) may identify sialomucin, whereas Bowen's disease does not display positive PAS staining. A correct diagnosis is important for treatment and prognosis; though progression to invasive cancer occurs in ~5% of Bowen's cases, invasive cancer has been reported in up to 40% of patients with untreated Paget's disease.
Management
After exclusion of other potential perianal diseases and proper diagnosis of perianal Paget's disease on a histologic basis, treatment is essentially surgical in nature. However, prior to proceeding with local treatment exclusion of an associated underlying malignancy is obligatory. Mammary Paget's is generally associated with underlying ductal carcinoma. In contrast, extramammary disease is associated with an underlying neoplasm in a significant percentage of cases.17 Association with tuboovarian adenocarcinoma is seen in 7 to 24% of cases and gastrointestinal carcinoma in 12 to 14% of cases.18,19 Appropriate imaging studies and fiberoptic endoscopy are recommended preoperatively to rule out other associated malignancies, the presence of which may alter treatment recommendations.20
If the disease appears to be locally confined on preoperative workup and is noninvasive on biopsy, wide local excision is the treatment of choice.15,18,20,21 Because Paget's disease can extend horizontally in the dermis well beyond the boundary of clinically evident disease, it is imperative to perform perianal mapping biopsies prior to formal wide excision. As traditional frozen section without histochemical staining may show falsely negative results, perianal mapping biopsies should be done several days before definitive treatment.15 Groin lymph node dissection should be performed if the patient presents with clinically positive nodes and should be considered if Paget's cells are seen throughout the dermis on histologic inspection of the resected specimen. If an associated malignancy of the anorectum is detected on preoperative workup, an APR is the procedure of choice to treat the anorectal cancer with the addition of wide local excision to treat the cutaneous Paget's disease20 (Table 2). More advanced tumors may benefit from preoperative radiation or chemoradiation therapy; however the use of these modalities in the treatment of perianal Paget's remains controversial.
Table 2.
Staging and Treatment for Perianal Paget's Disease
| Stage | Description | Therapy |
|---|---|---|
| I | Paget's cells found in | Wide local excision |
| perianal epidermis and | ||
| adnexa without primary | ||
| carcinoma | ||
| IIA | Cutaneous Paget's disease | Wide local excision |
| with associated adnexal | ||
| carcinoma | ||
| IIB | Cutaneous Paget's disease | Abdominoperineal resection |
| with associated anorectal | ||
| carcinoma | ||
| III | Paget's disease in which | Inguinal node dissection |
| associated carcinoma | Abdominoperineal | |
| has spread to regional | resection | |
| nodes | ||
| IV | Paget's disease with | Chemotherapy, radiotherapy |
| distant metastases of | local palliative treatment | |
| associated carcinoma |
Although primary closure of the resulting defect after wide local excision is often possible, several methods have been described to provide coverage for defects too large for primary closure including myocutaneous flaps, rotational or advancement skin flaps, as well as skin grafting.15,22 Although fecal diversion is not mandatory for all flap closures, larger defects requiring flap or graft closure may benefit from proximal fecal diversion to prevent wound infection and subsequent flap failure. In general, defects involving more than half the circumference of the anus or those with a radius of more than 3 cm should be considered for diversion.23 We feel that absolute size of the defect is less important than the amount of the circumference of anal margin skin that is involved. Proximal diversion can lower rates of wound infection, which may result in higher incidences of dehiscence, prolonged recovery, and ultimately poor functional outcome, especially for larger flaps.20
Several noninvasive modalities have been proposed as well for the treatment of perianal Paget's disease including radiation therapy, chemotherapy, photodynamic therapy, and topical imiquimod.24,25,26 Patients who have multifocal widespread cutaneous disease may benefit most from these therapies, either alone or in conjunction with wide local excision of disease that is clinically evident on physical examination. Unfortunately, because the number of patients in these reports is quite small, it is difficult to objectively compare these modalities, and they are often reserved for medically high-risk patients or those who refuse to undergo more radical therapy.
Patients who present with a more advanced stage of perianal Paget's disease tend to have a worse prognosis than patients in whom disease is confined to the epidermis.15,20,21 The largest series of patients in the literature is from McCarter et al consisting of 27 patients treated at Memorial Sloan-Kettering Cancer Center between 1950 and 2000. The overall disease-free 5-year survival rate of patients without an invasive component was 64% compared with 59% in those with an invasive component.21
Local recurrence has been reported to be as high as 60% in some series, and may be higher in patients with more advanced or multifocal disease.15,27 Although there is some variation with regard to recommendations for follow-up of these patients, most authors agree that annual physical examination and random perianal skin biopsies is appropriate.20 Fiberoptic endoscopy is recommended every 2 to 3 years as well because of the association with underlying gastrointestinal malignancy. Wolfgang et al also recommend a CT scan every 1 to 2 years.20 Obviously, any of these tests may be performed earlier if warranted clinically.
BOWEN'S DISEASE
Diagnosis
Bowen's disease (BD) is a nonkeratinizing, intraepithelial squamous-cell carcinoma of the perianal skin. It is characterized by marked epidermal hyperplasia and thickening of the rete ridges. The entire thickness of the squamous epithelium is disorganized with hyperchromatic large squamous cells extending from the base to the surface of the epithelium.28 Perianal BD has been associated with human papillomavirus (HPV) types 16 and 18 infection28,29,30 and needs to be differentiated from other perianal dermatologic conditions such as Paget's disease, Bowenoid papulosis, and melanoma.28
BD in the perianal region can be asymptomatic or symptomatic at presentation. Signs and symptoms are usually nonspecific and include pruritus, bleeding, discharge, and presence or sensation of a perianal lump or lesion.28,29,30,31 Clinically, it appears as a reddish well-defined, scaly erythematous plaque-like area.28 It is not uncommon to diagnose perianal BD incidentally during histologic examination of perianal tissue removed during another anorectal surgery such as a hemorrhoidectomy or a biopsy of the perianal skin for nonspecific complaints.28,31 Historically, BD used to be associated with visceral malignancies, although more recent studies have contradicted this association.29,30,31
The relationship between anal intraepithelial neoplasia (AIN) is not clearly defined in the literature.28 While histologically BD is a high-grade form of AIN and similar to AIN III in pathologic characteristics, clinically we consider them different disease entities. Unlike BD which is more common in females (66 to 79%) in the fifth decade of life, AIN is more common in men and immunocompromised individuals.28,29,30,31 The natural history of BD is relatively benign and progress to an invasive adenocarcinoma has only been seen in 2 to 5% of the cases.28 Although the risk of AIN progressing to invasive cancer is not reliably known, we believe it is likely to be higher than BD.
Management
Since its original description by John T. Bowen in 1912, over 200 cases have been reported in the literature with the largest reported series being of 47 patients from the Cleveland Clinic managed between 1972 and 1993.29,30 As a result, evidence-based recommendations for the diagnosis, management, and follow up of patients with perianal BD have been difficult to develop.32,33 Several treatment options including cryosurgery, radiation, argon laser therapy and photodynamic therapy have been reported to be effective in treating perianal BD. However wide surgical excision of all involved perianal skin and subcutaneous tissue is currently considered the standard surgical treatment.28,29,32,33
Wide local excision is performed on the basis of intraoperative, systematic, four-quadrant biopsy specimens that are sent for immediate frozen section evaluation.29 Despite these mapping techniques, high recurrence rates for wide local excision of perianal BD have been reported, suggesting the presence of undetected dysplastic epithelium in the adjacent perianal region.29,30,31 Another option for perianal mapping reported by Marchesa et al29 includes preoperative mapping under local anesthetic and sampling the anal canal including the dentate line, anal verge, and perianal skin (2 cm from the anal verge) at the major (4) and the minor (4) points of the compass. This procedure has been described as being usually done in the office using a minimal amount of local anesthetic and a skin punch or fine scalpel blade. The pathologist then reviews the specimens preoperatively thus reducing the operative time and provides a more accurate pathologic assessment. Any particular area that is of concern on the preoperative biopsies on permanent section can then also be evaluated with frozen section biopsy and resected at the time of definitive surgery.29
Depending upon the extent of microscopic and macroscopic disease the resulting perianal defect following wide local excision can be of varying sizes. Smaller defects can be closed primarily or allowed to heal by secondary intention. However, larger defects pose a significant challenge and various techniques including split thickness skin grafts, subcutaneous flaps, and myocutaneous flaps have been described to gain adequate tissue coverage.32,33,34,35
The main pitfall in the surgical management of perianal BD has been the high recurrence rates despite apparent adequate surgical therapy. Marchesa et al29 from the Cleveland Clinic reported a local recurrence rate of 23% (6 patients out of 26) after wide local excision with microscopic clearance of resected margins. In a series of 19 patients from the Mayo Clinic managed between 1970 and 1994, Sarmiento et al31 reported a 1-year and 5-year recurrence of 16% and 31% after wide local excision of perianal BD. Margenthaler et al30 from Washington University reported on a series of 25 patients managed between 1978 and 2001 and found a 12% local recurrence rate after wide local excision with formal mapping.
In recent years several pharmacologic therapies, including imiquimod36 and 5-fluorouracil (5-FU),37,38 have been shown to be effective in treating BD. Imiquimod is an immune response modifier that is an agonist for the toll-like receptor 7 (TLR-7). It is also thought to induce cutaneous cytokines, thereby enhancing both innate and acquired immunity and thus having potent antiviral and antitumor activity.39 Several prospective and retrospective studies have reported the efficacy of imiquimod and 5-FU in treating BD.36,37,38,40 Based upon these promising results, several authors40,41 have suggested that pharmacologic therapies be considered as an alternative to the surgical treatment of BD. Due to the relative rarity of perianal BD, it is unlikely that controlled trials will be possible to determine the superiority of one treatment modality over the other. The current literature, however, does provide evidence-based support for pharmacologic therapy as an alternative to surgical treatment of perianal BD. In patients with extensive perianal disease, these therapies may avoid the need for radical excision with soft tissue reconstruction that can, in turn, lead to adverse functional outcomes.35 Concurrently, it is important that these pharmacologic therapies be carefully monitored for their long-term oncologic efficacy, adverse effects, as well as patient reported outcomes.
GIANT CONDYLOMA ACUMINATUM
Large condyloma acuminata (GCA) that show features of local destructive invasion have been called Buschke-Lowenstein tumors, giant condylomas, and verrucous carcinomas. They arise from HPV, and may affect any portion of the anogenital region. Giant condylomas are generally slow-growing, and patient history often reveals that the lesion has been present for several years before presentation. Nevertheless, they are locally aggressive and destructive to surrounding tissue; however, distant metastases are rare. A subset of patients have identifiable immune defects, most commonly HIV, and in these patients highly active antiretroviral therapy (HAART) therapy is felt to decrease the tumor's aggressiveness.42 Whether this is a continuum of the anal intraepithelial neoplasia pathway, or a deviation, is not clear.
The microscopic appearance does not differ from that of small common condylomata acuminata; however, GCA has a malignant transformation as high as 50% and a local recurrence rate after excision of ~66%.43
Wide local excision remains the mainstay of therapy. As the tumor often involves the entire circumference of the perianal skin and extends for a variable distance onto the buttock skin, staged excision is not uncommon. Similar to wide excisions for the other neoplasms discussed here, the defects can be closed several ways. Due to the high recurrence rate after excision, some authors favor primary closure of the bulk of the defect and allow the remainder to granulate secondarily. Local tissue flaps are also common, such as the S-plasty.44
The high recurrence rate after wide local excision has prompted the employment of therapy adjuncts. Both radiation and chemotherapy have been used, with a preoperative regimen the most common.45 There are rare reports of primary radiotherapy producing complete tumor regression; long-term follow-up is not known.
CONCLUSION
Malignancies of the anal margin and perianal skin are rare, and the nonspecific symptoms often seen may be ascribed to more common perianal complaints. This can lead to inaccurate diagnoses and treatment dilemmas when the disease is recognized at an advanced stage. History and physical examination, biopsies, and a metastatic workup (when indicated) all play a role in correctly diagnosing the problem, establishing the extent of the disease, and selecting the most effective form of therapy. Tumor location and involvement of surrounding structures, especially the sphincters, are critical pieces of information that help guide treatment strategies.
Surgical treatment continues to be the standard of care for patients with amenable lesions who are medically fit to undergo surgery. Nonsurgical modalities such as radiation therapy with or without systemic chemotherapy, photodynamic therapy, or other topical agents are increasingly used as adjuncts. This may be due to many factors, including the inability of medically frail patients to tolerate a major surgical resection; a desire to preserve function; the recognition of the high incidence of local recurrence; or dissatisfaction with quality of life after resective procedures. The rarity of these neoplasms renders it nearly impossible to design and conduct randomized trials to directly compare treatment options.
REFERENCES
- 1.Newlin H E, Zlotecki R A, Morris C G, Hochwald S N, Riggs C E, Mendenhall W M. Squamous cell carcinoma of the anal margin. J Surg Oncol. 2004;86(2):55–62. discussion 63. doi: 10.1002/jso.20054. [DOI] [PubMed] [Google Scholar]
- 2.Mendenhall W M, Zlotecki R A, Vauthey J N, Copeland E M., III Squamous cell carcinoma of the anal margin. Oncology (Williston Park) 1996;10(12):1843–1848. discussion 1848, 1853–1854. [PubMed] [Google Scholar]
- 3.Khanfir K, Ozsahin M, Bieri S, Cavuto C, Mirimanoff R O, Zouhair A. Patterns of failure and outcome in patients with carcinoma of the anal margin. Ann Surg Oncol. 2008;15(4):1092–1098. doi: 10.1245/s10434-007-9794-9. [DOI] [PubMed] [Google Scholar]
- 4.Chapet O, Gerard J P, Mornex F, et al. Prognostic factors of squamous cell carcinoma of the anal margin treated by radiotherapy: the Lyon experience. Int J Colorectal Dis. 2007;22(2):191–199. doi: 10.1007/s00384-006-0114-9. [DOI] [PubMed] [Google Scholar]
- 5.Quan S. Anal cancers squamous and melanoma. Cancer. 1992;70(suppl 5):1384–1389. doi: 10.1002/1097-0142(19920901)70:3+<1384::aid-cncr2820701528>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Welton M, Varma M. In: Wolff B, Fleshman J, Beck D, Pemberton J, Wexner S, et al, editor. The ASCRS Textbook of Colon and Rectal Surgery. New York: Springer Science + Business Media; 2007. Anal cancer. pp. 482–500.
- 7.Chawla A K, Willett C G. Squamous cell carcinoma of the anal canal and anal margin. Hematol Oncol Clin North Am. 2001;15(2):321–344, vi. doi: 10.1016/s0889-8588(05)70215-x. [DOI] [PubMed] [Google Scholar]
- 8.Gordon P H, Nivatvongs S. Principles and Practice of Surgery for the Colon, Rectum, and Anus. 2nd ed. St. Louis, MO: Quality Medical Publishing Inc.; 1999. pp. 450–451.
- 9.Grabenbauer G G, Kessler H, Matzel K E, Sauer R, Hohenberger W, Schneider I H. Tumor site predicts outcome after radiochemotherapy in squamous-cell carcinoma of the anal region: long-term results of 101 patients. Dis Colon Rectum. 2005;48(9):1742–1751. doi: 10.1007/s10350-005-0098-5. [DOI] [PubMed] [Google Scholar]
- 10.Manstein C, Gottlieb N, Manstein M, Manstein G. Giant basal cell carcinoma: a series of seven t3 tumors without metastasis. Plastics and Reconstructive Surgery. 2000;106(3):653–656. doi: 10.1097/00006534-200009030-00021. [DOI] [PubMed] [Google Scholar]
- 11.Moore H G, Guillem J G. Anal neoplasms. Surg Clin North Am. 2002;82(6):1233–1251. doi: 10.1016/s0039-6109(02)00057-9. [DOI] [PubMed] [Google Scholar]
- 12.Wang S Q, Goldberg L H. Multiple polypoid basal cell carcinomas on the perineum of a patient with basal cell nevus syndrome. J Am Acad Dermatol. 2007;57 (2, Suppl):S36–S37. doi: 10.1016/j.jaad.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 13.Beck D E. Perianal Paget's disease and Bowen's disease of the anus. Semin Colon Rectal Surgery. 1995;6:143–149. [Google Scholar]
- 14.Tjandra J. Perianal Paget's disease. Report of three cases. Dis Colon Rectum. 1988;31(6):462–466. doi: 10.1007/BF02552618. [DOI] [PubMed] [Google Scholar]
- 15.Beck D E, Fazio V W. Perianal Paget's disease. Dis Colon Rectum. 1987;30(4):263–266. doi: 10.1007/BF02556169. [DOI] [PubMed] [Google Scholar]
- 16.Goldman S, Ihre T, Lagerstedt U, Svensson C. Perianal Paget's disease: report of five cases. Int J Colorectal Dis. 1992;7(3):167–169. doi: 10.1007/BF00360360. [DOI] [PubMed] [Google Scholar]
- 17.Zollo J D, Zeitouni N C. The Roswell Park Cancer Institute experience with extramammary Paget's disease. Br J Dermatol. 2000;142(1):59–65. doi: 10.1046/j.1365-2133.2000.03242.x. [DOI] [PubMed] [Google Scholar]
- 18.Marchesa P, Fazio V W, Oliart S, Goldblum J R, Lavery I C, Milsom J W. Long-term outcome of patients with perianal Paget's disease. Ann Surg Oncol. 1997;4(6):475–480. doi: 10.1007/BF02303671. [DOI] [PubMed] [Google Scholar]
- 19.Chanda J J. Extramammary Paget's disease: prognosis and relationship to internal malignancy. J Am Acad Dermatol. 1985;13(6):1009–1014. doi: 10.1016/s0190-9622(85)70254-x. [DOI] [PubMed] [Google Scholar]
- 20.Gaertner W B, Hagerman G F, Goldberg S A, Finne C O. Perianal Paget's disease treated with wide excision and gluteal skin flap reconstruction: report of a case and review of the literature. Dis Colon Rectum. 2008;51:1842–1845. doi: 10.1007/s10350-008-9409-y. [DOI] [PubMed] [Google Scholar]
- 21.McCarter M D, Quan S H, Busam K, Paty P P, Wong D, Guilem J G. Long term outcome of perianal Paget's disease. Dis Colon Rectum. 2003;46:612–616. doi: 10.1007/s10350-004-6618-x. [DOI] [PubMed] [Google Scholar]
- 22.St Peter S D, Pera M, Smith A A, Leslie K O, Heppell J. Wide local excision and split thickness skin graft for circumferential Paget's disease. Am J Surg. 2004;187(3):413–416. doi: 10.1016/j.amjsurg.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Shutze W P, Gleysteen J J. Perianal Paget's disease. Classification and review of management: report of two cases. Dis Colon Rectum. 1990;33(6):502–507. doi: 10.1007/BF02052147. [DOI] [PubMed] [Google Scholar]
- 24.Shieh S, Dee A S, Cheney R T, Frawley N P, Zeitouni N C, Oseroff A R. Photodynamic therapy for the treatment of extramammary Paget's disease. Br J Dermatol. 2002;146:100–105. doi: 10.1046/j.1365-2133.2002.04801.x. [DOI] [PubMed] [Google Scholar]
- 25.Moreno-Arias G A, Conill C, Castells-Mas A, Arenas M, Grimalt R. Radiotherapy for genital extramammary Paget's disease in-situ. Dermatol Surg. 2001;27:587–590. doi: 10.1046/j.1524-4725.2001.00304.x. [DOI] [PubMed] [Google Scholar]
- 26.Burrows N P, Jones D H, Hudson P M, Pye R J. Treatment of extramammary Paget's disease by radiotherapy. Br J Dermatol. 1995;132:970–972. doi: 10.1111/j.1365-2133.1995.tb16957.x. [DOI] [PubMed] [Google Scholar]
- 27.Sarmiento J M, Wolff B G, Burgart L J, Frizelle F A, Ilstrup D M. Paget's disease of the perianal region – an aggressive disease? Dis Colon Rectum. 1997;40:1187–1194. doi: 10.1007/BF02055165. [DOI] [PubMed] [Google Scholar]
- 28.Cleary R K, Schaldenbrand J D, Fowler J J, Schuler J M, Lampman R M. Perianal Bowen's disease and anal intraepithelial neoplasia: review of the literature. Dis Colon Rectum. 1999;42(7):945–951. doi: 10.1007/BF02237107. [DOI] [PubMed] [Google Scholar]
- 29.Marchesa P, Fazio V W, Oliart S, Goldblum J R, Lavery I C. Perianal Bowen's disease: a clinicopathologic study of 47 patients. Dis Colon Rectum. 1997;40(11):1286–1293. doi: 10.1007/BF02050810. [DOI] [PubMed] [Google Scholar]
- 30.Margenthaler J A, Dietz D W, Mutch M G, Birnbaum E H, Kodner I J, Fleshman J W. Outcomes, risk of other malignancies, and need for formal mapping procedures in patients with perianal Bowen's disease. Dis Colon Rectum. 2004;47:1655–1660. discussion 1660–1651. doi: 10.1007/s10350-004-0662-4. [DOI] [PubMed] [Google Scholar]
- 31.Sarmiento J M, Wolff B G, Burgart L J, Frizelee F A, Ilstrup D M. Perianal Bowen's disease. Associated tumors, human papillomavirus, surgery and other controversies. Dis Colon Rectum. 1997;40(8): 912–918. doi: 10.1007/BF02051198. [DOI] [PubMed] [Google Scholar]
- 32.Cox N H, Eedy D J, Morton C A. Guidelines for management of Bowen's disease: 2006 update. Br J Dermatol. 2007;156:11–21. doi: 10.1111/j.1365-2133.2006.07610.x. [DOI] [PubMed] [Google Scholar]
- 33.Cleary R K, Schaldenbrand J D, Fowler J J, Schuler J M, Lampman R M. Treatment options for perianal Bowen's disease: Survey of American Society of Colon and Rectal Surgeons Members. Am Surg. 2000;66(7):686–688. [PubMed] [Google Scholar]
- 34.Hassan I, Horgan A F, Nivatvongs S. V-Y island flaps for repair of large perianal defects. Am J Surg. 2001;181(4):363–365. doi: 10.1016/s0002-9610(01)00578-5. [DOI] [PubMed] [Google Scholar]
- 35.Conklin A, Hassan I, Chua H K, et al. Long term functional and quality of life outcomes of patients after repair of large perianal skin defects for Paget's and Bowen's disease. J Gastrointest Surg. 2009 doi: 10.1007/s11605-009-0822-x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36.Patel G K, Goodwin R, Chawla M, et al. Imiquimod 5% cream monotherapy for cutaneous squamous cell carcinoma in situ (Bowen's disease): a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2006;54(6):1025–1032. doi: 10.1016/j.jaad.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 37.Bargman H, Hochman J. Topical treatment of Bowen's disease with 5-fluorouracil. J Cutan Med Surg. 2003;7(2):101–105. doi: 10.1007/s10227-002-0158-6. [DOI] [PubMed] [Google Scholar]
- 38.Graham B D, Jetmore A B, Foote J E, Arnold L K. Topical 5-fluorouracil in the management of extensive anal Bowen's disease: a preferred approach. Dis Colon Rectum. 2005;48(3):444–450. doi: 10.1007/s10350-004-0901-8. [DOI] [PubMed] [Google Scholar]
- 39.Gupta A K, Browne M, Bluhm R. Imiquimod: a review. J Cutan Med Surg. 2002;6(6):554–560. doi: 10.1007/s10227-001-0134-6. [DOI] [PubMed] [Google Scholar]
- 40.Rosen T, Harting M, Gibson M. Treatment of Bowen's disease with topical 5% imiquimod cream: retrospective study. Dermatol Surg. 2007;33:427–431. discussion 431–422. doi: 10.1111/j.1524-4725.2007.33089.x. [DOI] [PubMed] [Google Scholar]
- 41.Peris K, Micantonio T, Fargnoli M C, Lozzi G P, Chimenti S. Imiquimod 5% cream in the treatment of Bowen's disease and invasive squamous cell carcinoma. J Am Acad Dermatol. 2006;55(2):324–327. doi: 10.1016/j.jaad.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 42.De Toma G, Cavallaro G, Bitonti A, Polistena A, Onesti M G, Scuderi N. Surgical management of perianal giant condyloma acuminatum (Buschke-Löwenstein tumor). Report of three cases. Eur Surg Res. 2006;38(4):418–422. doi: 10.1159/000094979. [DOI] [PubMed] [Google Scholar]
- 43.Chu Q D, Vezeridis M P, Libbey N P, Wanebo H J. Giant condyloma acuminatum (Buschke-Lowenstein tumor) of the anorectal and perianal regions. Analysis of 42 cases. Dis Colon Rectum. 1994;37(9):950–957. doi: 10.1007/BF02052606. [DOI] [PubMed] [Google Scholar]
- 44.Cintron J R. Buschke-Lowenstein tumor of the perianal and anorectal region. Semin Colon Rectal Surg. 1995;6:135–139. [Google Scholar]
- 45.Tytherleigh M G, Birtle A J, Cohen C E, Glynne-Jones R, Livingstone J, Gilbert J. Combined surgery and chemoradiation as a treatment for the Buschke-Löwenstein tumour. Surgeon. 2006;4(6):378–383. doi: 10.1016/s1479-666x(06)80114-9. [DOI] [PubMed] [Google Scholar]