Abstract
AIMS
(i) To compare the effects of intra-arterial administration of urotensin II in patients with CVD with healthy volunteers, and (ii) to study the haemodynamic effects of intra-arterial infusion of the urotensin II receptor antagonist, urantide.
METHODS
Ten healthy volunteers and 10 patients with CVD received a dose-ramped brachial artery infusion of urotensin II. A further six healthy male volunteers received a prolonged urotensin II infusion and 11 healthy male volunteers received a dose-ramped infusion of urantide. Forearm blood flow (FBF) was measured every 20 min and blood pressure and heart rate were assessed every 20 min.
RESULTS
In healthy volunteers and patients with CVD, intra-arterial infusion of urotensin II had no effect on FBF ratio. A dose-ramped infusion of urantide similarly had no effect on FBF ratio. During dose-ramped infusions of urotensin II and urantide, systolic and mean arterial blood pressure increased significantly. In healthy volunteers, urotensin II and urantide, respectively, increased systolic blood pressure from 133 ± 6 to 137 ± 5 mmHg (P < 0.01) and from 113 ± 4 to 120 ± 4 mmHg (P < 0.01). In patients with CVD, heart rate also significantly increased during dose-ramped infusion of urotensin II from 59 ± 3 to 62 ± 4 bpm (P < 0.05).
CONCLUSIONS
We have shown no in vivo effect of urotensin II or urantide on human forearm resistance vessels. Previous discrepancies do not seem to relate to either the age or CVD status of subjects. Changes in systemic cardiovascular haemodynamics during the dose-ramped infusion studies are unlikely to be caused by urotensin II receptor modulation.
Keywords: forearm blood flow, haemodynamics, urantide, urotensin II
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
In vitro studies have shown that urotensin II is a potent arterial vasoconstrictor.
Previous studies investigating the in vivo cardiovascular actions of intra-arterial administration of urotensin II in humans have provided conflicting results.
The cardiovascular actions of intra-arterial administration of the urotensin II receptor antagonist, urantide, are unknown.
WHAT THIS STUDY ADDS
We have shown no in vivo effect of urotensin II or urantide on human forearm resistance vessels.
Previous study discrepancies do not seem to relate to either the age or cardiovascular disease (CVD) status of subjects.
Introduction
Human urotensin II is an 11 amino acid vasoactive peptide originally isolated from the central nervous system of teleost fish [1]. In teleosts, urotensin II produces smooth muscle contraction, vasoconstriction, inhibits prolactin secretion and has a role in osmoregulation [2]. Receptors for urotensin II are present in rats, amphibians, nonhuman primates and in human cells [3–6]. Human urotensin II has now been found to be the endogenous ligand of the mammalian orphan receptor GPR14/SENR [7, 8]. Messenger RNA encoding this receptor has been found in human brain, heart, aorta, endothelium and smooth muscle cell lines [6].
In vitro studies suggest that urotensin II is one of the most potent arterial vasoconstrictors discovered to date with a potency 10–20-fold greater than endothelin-1. However, responses differ between mammalian species and between vascular beds within a species, possibly due to variation in receptor expression [9].
In humans, there has been much speculation as to the role of urotensin II in the cardiovascular system in both health and disease, with much controversy between studies. In vitro studies have shown that urotensin II vasoconstricts human coronary, mammary and radial arteries as well as saphenous and umbilical veins [10]. A vasodilator action is seen in pulmonary vessels [11]. In vivo studies have so far been more limited, although vasoconstriction of intradermal vessels has been observed at high urotensin II concentrations in the human skin microcirculation [12]. More recent in vivo human studies in the forearm skin microcirculation have produced disparate results, with urotensin II causing vasodilation in normal subjects, vasoconstriction in cirrhotic patients and mixed responses in hypertensive patients [13–15].
Two previous studies have investigated the effects of urotensin II on forearm blood flow (FBF) in human volunteers using venous occlusion plethysmography. Bohm and Pernow showed that intra-arterial infusion of urotensin II in nine healthy volunteers evoked dose-dependent vasoconstriction across infusion rates of 1–300 pmol min−1[16]. A threshold response was obtained at 1 pmol min−1, and the highest dose of urotensin II reduced FBF by 31%. FBF returned to baseline values within 30 min. In a similar study, we found that urotensin II had no effect on arterial or venous tone, and did not alter systemic haemodynamics despite demonstrable increases in the plasma concentration of urotensin II [17]. The reason for this contradiction was not clear, but it was noted that the two cohorts of volunteers were of different ages and the urotensin II stock and dosing regimen were different.
In order to clarify the haemodynamic effects of urotensin II in the human cardiovascular system, we have investigated the action of intra-arterial infusion of urotensin II on blood pressure, heart rate and FBF in healthy volunteers and patients with cardiovascular disease (CVD). We tested the hypothesis that previous contradictions between studies resulted from differences in age and cardiovascular health between patient cohorts.
We have also investigated for the first time the haemodynamic effects of intra-arterial infusion of the urotensin II receptor antagonist urantide. Urantide is a potent inhibitor of human urotensin II-induced contraction in the rat isolated thoracic aorta and displaces urotensin II from specific binding at recombinant human urotensin II receptors [18]. Its in vivo effects in humans are unknown.
Methods
Study population
Studies were performed in the Clinical Pharmacology Unit, Cambridge, UK. Two dosing regimens were employed: a dose ramping protocol as used by Bohm and Pernow [16], and a prolonged infusion protocol. Ten healthy, nonsmoking men (aged 27 ± 2 years) and 10 male patients with CVD (defined as patients with a history of angina, myocardial infarction, stroke, transient ischaemic attack or peripheral vascular disease) aged 65 ± 5 years were recruited into the urotensin II dose ramping study. Six healthy male volunteers (aged 25 ± 7 years) were recruited to the prolonged urotensin II infusion study. A further 11 healthy, nonsmoking men (aged 24 ± 2 years) were recruited into the urantide dose ramping study. Patients on anticoagulants, with uncontrolled hypertension (>160/100) or those currently involved in other studies were excluded. All subjects were asked to abstain from alcohol and caffeine over the preceding 24 h. The study was approved by the Cambridge Local Research Ethics Committee and written informed consent was obtained from each subject.
Haemodynamic measurements
Subjects had a 12-lead ECG recorded at baseline and at the end of each study. Blood pressure and heart rate were measured in the brachial artery of the dominant, non-infused arm every 20 min using a validated oscillometric machine (Omron HEM-705CP; Omron Corp., Tokyo, Japan) [19].
Forearm blood flow
All studies were conducted in a quiet, temperature-controlled (22–24 °C) clinical laboratory. The brachial artery of the nondominant arm was cannulated with a 27-G steel needle (Cooper's Needle Works, Sheffield, UK) under local anaesthesia (1% lignocaine; Hameln Pharmaceuticals, Gloucester, UK). This was connected to an IVAC P6000 syringe pump via a fine 16-G epidural catheter (Portex, Watford, UK). FBF was measured at the end of each 20-min infusion over a period of 3 min using venous occlusion plethysmography (Hokanson Inc., Bellevue, WA, USA). This allows blood flow to be measured simultaneously in both arms allowing comparison of blood flow in the infused arm vs. the non-infused or control arm [20]. Wrist circulation was excluded by inflating wrist cuffs to a pressure above the systolic blood pressure (SBF). Upper arm cuffs were repeatedly inflated and then deflated for periods of 10 and 5 s, respectively, to cause intermittent block in venous return, allowing FBF measurements to be made using mercury-in-silastic gauges. The last five cycles of each 3-min period were used in the analysis of data.
Drugs
All drugs were prepared aseptically and diluted in sterile saline (0.9%; Baxter Healthcare, Thetford, UK). Urotensin II was obtained from Clinalfa AG (Laufelfingen, Switzerland), the same supplier used by Bohm and Pernow [16]. Urantide was obtained from Bachem GmbH (Weil am Rhein, Germany). Stock vials of urotensin II and urantide were diluted on receipt and stored at −80 °C. Further dilutions were made on the day of the study using 0.9% saline.
Initially, each subject was infused with 0.9% saline at a rate of 1 ml min−1 for 20 min to establish a baseline. All subsequent drug infusions were performed at this infusion rate. In dose ramping studies, urotensin II was infused for 20 min at a dose rate of 0.1 pmol min−1. This was increased every 20 min to 1.0, 10, 100 and finally 300 pmol min−1 as per the Bohm and Pernow protocol. An identical protocol was used when infusing urantide, albeit the maximum dose rate did not exceed 100 pmol min−1.
In the prolonged infusion study, urotensin II was infused at a dose rate of 0.1 pmol min−1 to volunteers for a total of 60 min. In both protocols, drug infusion was followed by a 20-min wash-out period with saline. Administration of all drugs was unblinded.
Data analysis
FBF was calculated as the mean of the last five curves of blood flow in each 3-min period. This was measured as a ratio of infused arm to control arm and expressed as a percentage change from baseline. FBF values are expressed as ml min−1 per 100-ml forearm tissue volume. Data are presented as means ± standard error of the mean (SEM). Repeated measures anova and independent samples t-tests were used to determine statistical significance. A probability value of P < 0.05 was considered significant and P < 0.01 was considered highly significant. The data were blinded and analysed using SPSS for Windows v12.0 (SPSS Inc., Chicago, IL, USA). Previous FBF studies in our department had 90% power to detect a 10% change in FBF ratio in 10 volunteers at a two-sided significance level of 0.05.
Results
All trial subjects tolerated urotensin II and urantide administration well. There were no withdrawals as a result of side-effects. All subjects were documented to be in sinus rhythm and without ischaemia on their 12-lead ECGs prior to and after the administration of urotensin II or urantide. No adverse events were reported throughout the study.
Urotensin II dose ramping study
Absolute FBF for the healthy volunteers and CVD patient groups were similar at baseline (respectively, 3.08 ± 0.31 vs. 2.56 ± 0.22 ml min−1 per 100 ml, P= 0.17), as was the FBF ratio (1.04 ± 0.05 vs. 1.06 ± 0.06, P= 0.2). Neither absolute FBF nor FBF ratio changed significantly with urotensin II infusion in either group. The data are summarized in Table 1.
Table 1.
Dose-ramped infusions of urotensin II in healthy volunteers (HV) and patients with cardiovascular disease (CVD)
| Variable | Group | Baseline | Urotensin 0.1 pmol min−1 | Urotensin 1.0 pmol min−1 | Urotensin 10 pmol min−1 | Urotensin 100 pmol min−1 | Urotensin 300 pmol min−1 | Significance of trend | Significance between groups |
|---|---|---|---|---|---|---|---|---|---|
| Systolic BP (mmHg) | HV | 133 ± 6 | 127 ± 5 | 129 ± 6 | 132 ± 6 | 134 ± 7 | 137 ± 5 | <0.01 | 0.105 |
| CVD | 140 ± 5 | 140 ± 5 | 143 ± 5 | 146 ± 5 | 148 ± 4.6 | 152 ± 5 | <0.0001 | ||
| Diastolic BP (mmHg) | HV | 71 ± 3 | 67 ± 4 | 70 ± 3 | 71 ± 3 | 73 ± 3 | 72 ± 4 | 0.180 | 0.650 |
| CVD | 78 ± 5 | 80 ± 2 | 82 ± 2 | 82 ± 3 | 83 ± 3 | 84 ± 2 | 0.108 | ||
| Mean arterial pressure (mmHg) | HV | 91 ± 3 | 87 ± 2 | 90 ± 2 | 92 ± 2 | 94 ± 2 | 94 ± 3 | <0.05 | 0.382 |
| CVD | 99 ± 3 | 100 ± 3 | 102 ± 3 | 103 ± 3 | 105 ± 3 | 107 ± 3 | <0.0001 | ||
| Heart rate (bpm) | HV | 54 ± 3 | 55 ± 2 | 55 ± 2 | 54 ± 2 | 54 ± 2 | 57 ± 4 | 0.313 | 0.250 |
| CVD | 59 ± 3 | 58 ± 3 | 57 ± 2 | 60 ± 3 | 60 ± 4 | 62 ± 4 | <0.05 | ||
| Absolute FBF in infused arm (ml 100 ml−1 min−1) | HV | 3.08 ± 0.31 | 3.02 ± 0.34 | 3.05 ± 0.40 | 3.19 ± 0.36 | 2.78 ± 0.31 | 3.13 ± 0.48 | 0.560 | 0.416 |
| CVD | 2.56 ± 0.22 | 2.55 ± 0.28 | 2.87 ± 0.22 | 2.69 ± 0.20 | 2.77 ± 0.20 | 2.70 ± 0.20 | 0.273 | ||
| Ratio (infused/control arm) | HV | 1.04 ± 0.05 | 1.01 ± 0.04 | 1.09 ± 0.06 | 1.06 ± 0.07 | 1.00 ± 0.06 | 0.95 ± 0.03 | 0.236 | 0.164 |
| CVD | 1.06 ± 0.06 | 1.10 ± 0.75 | 1.14 ± 0.08 | 1.05 ± 0.09 | 1.23 ± 0.15 | 1.16 ± 0.13 | 0.512 | ||
| Change in FBF ratio (%) | HV | −1.8 ± 5.1 | 5.9 ± 5.6 | 2.8 ± 6.6 | −2.5 ± 6.8 | −7.4 ± 6.0 | 0.204 | 0.399 | |
| CVD | 2.4 ± 6.3 | 5.4 ± 4.7 | −3.4 ± 6.2 | 14.6 ± 8.6 | 9.2 ± 9.9 | 0.691 |
Data are presented as means ± SEM. BP, blood pressure; FBF, forearm blood flow.
The percentage change in FBF ratio also did not differ between groups, measuring, at the highest infusion rate of urotensin II, −7.4 ± 6.0% in healthy volunteers and 9.2 ± 9.9% in patients with CVD (P= 0.4).
In healthy volunteers, heart rate measured during the highest dose rate of urotensin II infusion was 57 ± 4 compared with 54 ± 3 bpm at baseline (P= 0.3). Mean arterial pressure in healthy volunteers significantly increased during the dose-ramped infusion from 91 ± 3 at baseline to 94 ± 3 mmHg (P < 0.05). In patients with CVD, there was a significant increase in both heart rate (from 59 ± 3 to 62 ± 4 bpm, P < 0.05) and mean arterial pressure (from 99 ± 3 to 107 ± 3 mmHg, P < 0.0001) during the dose-ramped infusion. SBP increased in both groups during dose ramping (healthy volunteers from 133 ± 6 to 137 ± 5 mmHg, P < 0.01; patients with CVD from 140 ± 5 to 152 ± 5 mmHg, P < 0.0001). Although there was a trend for SBP to increase more in patients with CVD compared with healthy volunteers, this was not significant (P= 0.1). There was no difference in diastolic blood pressure (DBP) from baseline to highest dose rate urotensin II in either group.
Urotensin II prolonged infusion study
Baseline data were similar to that of healthy volunteers in the dose ramping study. There was no significant change in heart rate, blood pressure (SBP, DBP or mean) or FBF (absolute, ratio or percentage change in ratio) with 0.1 pmol min−1 urotensin II infusion over 60 min (see Table 2).
Table 2.
Prolonged infusions of urotensin II at a dose rate of 0.1 pmol min−1
| Variable | Baseline | 15 min | 30 min | 45 min | 60 min | Significance of trend |
|---|---|---|---|---|---|---|
| Systolic BP (mmHg) | 123 ± 3 | 124 ± 2 | 125 ± 3 | 125 ± 4 | 126 ± 3 | 0.47 |
| Diastolic BP (mmHg) | 70 ± 2 | 71 ± 3 | 72 ± 3 | 72 ± 3 | 71 ± 3 | 0.65 |
| Mean arterial pressure (mmHg) | 88 ± 2 | 88 ± 2 | 89 ± 3 | 90 ± 2 | 89 ± 3 | 0.40 |
| Heart rate (bpm) | 55 ± 5 | 55 ± 4 | 53 ± 4 | 54 ± 4 | 55 ± 4 | 0.49 |
| Absolute FBF (ml 100 ml−1 min−1) | 2.60 ± 0.37 | 2.64 ± 0.44 | 2.87 ± 0.47 | 2.50 ± 0.38 | 2.57 ± 0.31 | 0.37 |
| Ratio (infused/control arm) | 1.02 ± 0.07 | 1.06 ± 0.11 | 1.18 ± 0.15 | 1.05 ± 0.12 | 1.06 ± 0.09 | 0.18 |
| Change in FBF ratio (%) | 1.9 ± 4.0 | 12.5 ± 8.1 | 1.0 ± 6.0 | 3.9 ± 6.4 | 0.27 |
Data are presented as mean ± SEM. BP, blood pressure; FBF, forearm blood flow.
Urantide dose ramping study
During the dose-ramped infusion of urantide in healthy volunteers (Table 3), absolute FBF increased from 2.60 ± 0.37 to 3.14 ± 0.38 ml min−1 per 100 ml (P < 0.01). However, as absolute FBF also increased in the control arm, no significant changes in FBF ratio or percentage change in FBF ratio were observed (Figure 1). SBP increased significantly during dose ramping from 113 ± 4 to 120 ± 4 mmHg (P < 0.01). This was accompanied by a significant increase in mean arterial blood pressure from 82 ± 3 to 87 ± 3 mmHg (P < 0.05). DBP and heart rate did not change significantly during dose ramping.
Table 3.
Dose-ramped infusions of urantide
| Variable | Baseline | Urantide 0.1 pmol min−1 | Urantide 1.0 pmol min−1 | Urantide 10 pmol min−1 | Urantide 100 pmol min−1 | Significance of trend |
|---|---|---|---|---|---|---|
| Systolic BP (mmHg) | 113 ± 4 | 116 ± 5 | 119 ± 5 | 120 ± 5 | 120 ± 4 | 0.002 |
| Diastolic BP (mmHg) | 66 ± 3 | 68 ± 3 | 69 ± 3 | 70 ± 3 | 70 ± 3 | 0.308 |
| Mean arterial pressure (mmHg) | 82 ± 3 | 84 ± 3 | 86 ± 3 | 87 ± 3 | 87 ± 3 | 0.022 |
| Heart rate (bpm) | 48 ± 2 | 50 ± 2 | 49 ± 2 | 49 ± 1 | 50 ± 1 | 0.112 |
| Absolute FBF in infused arm (ml 100 ml−1 min−1) | 2.60 ± 0.37 | 2.65 ± 0.35 | 2.80 ± 0.37 | 3.02 ± 0.42 | 3.14 ± 0.38 | 0.002 |
| Ratio (infused/control arm) | 1.27 ± 0.14 | 1.24 ± 0.11 | 1.21 ± 0.10 | 1.16 ± 0.10 | 1.16 ± 0.11 | 0.214 |
| Change in FBF ratio (%) | 0.4 ± 5.4 | −1.0 ± 7.1 | −6.6 ± 6.6 | −10.7 ± 6.6 | 0.390 |
Data are presented as means ± SEM. BP, blood pressure; FBF, forearm blood flow.
Figure 1.
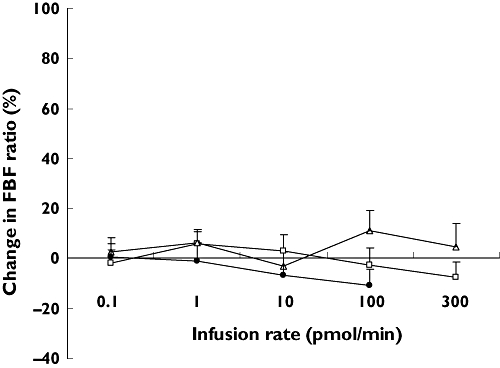
The effects of dose-ramped infusion of urotensin II and urantide on the percentage change in forearm blood flow (FBF) ratio. Urotensin II was infused at 0.1–300 pmol min−1 in healthy volunteers (□) and patients with cardiovascular disease (Δ). Urantide was infused at 0.1–100 pmol min−1 in healthy volunteers (•). Data are presented as means + SEM
Discussion
In this study we have shown no effect of urotensin II on FBF, suggesting an absence of urotensin II-mediated arterial vasoconstriction under the conditions we have studied. Our protocol and healthy volunteer cohort closely resemble those of the experiments of Bohm and Pernow [16]. The results of this study are therefore in disagreement with those of Bohm and Pernow, but concur with our previous FBF study [17], in which we showed no role for urotensin II in the regulation of vascular tone in healthy volunteers. Moreover, we have found no evidence that previous study discrepancies relate to either the age or CVD status of the trial subjects, as no differences in FBF were observed between the younger healthy volunteers and older patients with established CVD.
It is possible that the absence of an effect of urotensin II on FBF results from an inadequate local concentration of drug. In previous studies infusing 300 pmol min−1 of urotensin II intravenously, an increase in circulating plasma urotensin II immunoreactivity, sufficient to account for biological activity, was measured [21]. However, a similar biological activity cannot be extrapolated to this study in view of the different route of administration. Another explanation for the absence of an effect of urotensin II is receptor tachyphylaxis. Urotensin II-mediated calcium mobilization in human endothelial cells and urotensin II-mediated contraction of vascular smooth muscle in the frog are both subject to desensitization [22, 23].
We have found that during dose-ramped infusion of urotensin II, significant systemic haemodynamic changes occurred in both healthy volunteers and CVD patients, a finding that we have not seen previously in our plethysmography protocols. The most significant of these was a rise in systolic and mean arterial blood pressure. This was associated with, and may be partly explained by, a mild elevation in heart rate that was significant in the patients with CVD. However, as similar increases in systolic and mean arterial blood pressure occurred during dose-ramped infusion of urantide, it is likely that they relate to time rather than to drug. A time-control arm to the study, in which volunteers received saline infusion throughout, would have provided a definitive answer but was not included on the ethical grounds that another healthy volunteer and patient cohort would have required arterial puncture. All volunteers had to lie still for the 140-min duration of the study, which is arduous for even the most relaxed participant. The study duration in our other plethysmography protocols is in the order of 90 min. The significant age difference between the healthy volunteer group and patient population may explain some of the differences in systemic haemodynamic trends between the two groups.
A much less likely explanation is that urotensin II has a positive inotropic action in humans that leads to a significant rise in SBP and mean arterial pressure. Watson et al. have shown positive inotropic and chronotropic effects of intravenous and intracerebroventricular administration of urotensin II in sheep [24]. Mean arterial pressure started to rise within 5 min of a bolus injection, peaking at 30 min and returning to control levels at 1 h. An inotropic role for urotensin II has also been suggested in various CVD states [25, 26]. For example, the levels of urotensin II and its receptor are abundant in the cardiac tissue of patients with dilated or ischaemic cardiomyopathy [26]. One could speculate that upregulation of urotensin II receptors in CVD explains the more pronounced systemic haemodynamic effects of urotensin II in the CVD group compared with healthy volunteers.
For the first time, this study has described the in vivo actions of intra-arterial infusion of the urotensin II receptor antagonist, urantide, in humans. During dose-ramped infusion of urantide, no significant changes in FBF ratio or percentage change in FBF ratio were observed. This suggests that the absence of urotensin II-mediated arterial vasoconstriction that we have observed is not the result of tonic receptor activation. However, we acknowledge that this study is not powered to detect a small dilator or pressor response to urantide. Given the absence of any pharmacokinetic data on urantide administration to humans, we cannot be confident that the infusion rate of drug was optimal. In vitro experiments suggest that urantide should actually be considered a low efficacy partial agonist, since it mimics urotensin II-induced calcium mobilization in cells expressing recombinant urotensin II receptors [27]. Until pure nonpeptide antagonists become commercially available, inconsistencies in our understanding of urotensin II receptor activation will remain.
At present, the role of urotensin II in the human cardiovascular system in health and disease is poorly understood. Our results suggest that urotensin II is a much less important mediator of vascular tone in human brachial artery in vivo than in many vascular beds studied in vitro. Further studies are required to determine conclusively if urotensin II receptor modulation affects heart rate, stroke volume and peripheral vascular resistance in humans.
Competing interests
J.C.'s current work is partly related to/funded by GlaxoSmithKline. This research had no funding, support or use of GlaxoSmithKline's resources.
REFERENCES
- 1.Pearson D, Shively JE, Clark BR, Geschwind II, Barkley M, Nishioka RS, Bern HA. Urotensin II: a somatostatin-like peptide in the caudal neurosecretory system of fishes. Proc Natl Acad Sci USA. 1980;77:5021–4. doi: 10.1073/pnas.77.8.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bern HA, Pearson D, Larson BA, Nishioka RS. Neurohormones from fish tails: the caudal neurosecretory system. I. ‘Urophysiology’ and the caudal neurosecretory system of fishes. Recent Prog Horm Res. 1985;41:533–52. doi: 10.1016/b978-0-12-571141-8.50016-0. [DOI] [PubMed] [Google Scholar]
- 3.Itoh H, Itoh Y, Rivier J, Lederis K. Contraction of major artery segments of rat by fish neuropeptide urotensin II. Am J Physiol. 1987;252:R361–6. doi: 10.1152/ajpregu.1987.252.2.R361. [DOI] [PubMed] [Google Scholar]
- 4.Coulouarn Y, Lihrmann I, Jegou S, Anouar Y, Tostivint H, Beauvillain JC, Conlon JM, Bern HA, Vaudry H. Cloning of the cDNA encoding the urotensin II precursor in frog and human reveals intense expression of the urotensin II gene in motoneurons of the spinal cord. Proc Natl Acad Sci USA. 1998;95:15803–8. doi: 10.1073/pnas.95.26.15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elshourbagy NA, Douglas SA, Shabon U, Harrison S, Duddy G, Sechler JL, Ao Z, Maleeff BE, Naselsky D, Disa J, Aiyar NV. Molecular and pharmacological characterization of genes encoding urotensin-II peptides and their cognate G-protein-coupled receptors from the mouse and monkey. Br J Pharmacol. 2002;136:9–22. doi: 10.1038/sj.bjp.0704671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ames RS, Sarau HM, Chambers JK, Willette RN, Aiyar NV, Romanic AM, Louden CS, Foley JJ, Sauermelch CF, Coatney RW, Ao Z, Disa J, Holmes SD, Stadel JM, Martin JD, Liu WS, Glover GI, Wilson S, McNulty DE, Ellis CE, Elshourbagy NA, Shabon U, Trill JJ, Hay DW, Ohlstein EH, Bergsma DJ, Douglas SA. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282–6. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- 7.Marchese A, Heiber M, Nguyen T, Heng HH, Saldivia VR, Cheng R, Murphy PM, Tsui LC, Shi X, Gregor P. Cloning and chromosomal mapping of three novel genes, GPR9, GPR10, and GPR14, encoding receptors related to interleukin 8, neuropeptide Y, and somatostatin receptors. Genomics. 1995;29:335–44. doi: 10.1006/geno.1995.9996. [DOI] [PubMed] [Google Scholar]
- 8.Tal M, Ammar DA, Karpuj M, Krizhanovsky V, Naim M, Thompson DA. A novel putative neuropeptide receptor expressed in neural tissue, including sensory epithelia. Biochem Biophys Res Commun. 1995;209:752–9. doi: 10.1006/bbrc.1995.1563. [DOI] [PubMed] [Google Scholar]
- 9.Maguire JJ, Davenport AP. Is urotensin-II the new endothelin? Br J Pharmacol. 2002;137:579–88. doi: 10.1038/sj.bjp.0704924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maguire JJ, Kuc RE, Davenport AP. Orphan-receptor ligand human urotensin II: receptor localization in human tissues and comparison of vasoconstrictor responses with endothelin-1. Br J Pharmacol. 2000;131:441–6. doi: 10.1038/sj.bjp.0703601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stirrat A, Gallagher M, Douglas SA, Ohlstein EH, Berry C, Kirk A, Richardson M, MacLean MR. Potent vasodilator responses to human urotensin-II in human pulmonary and abdominal resistance arteries. Am J Physiol Heart Circ Physiol. 2001;280:H925–8. doi: 10.1152/ajpheart.2001.280.2.H925. [DOI] [PubMed] [Google Scholar]
- 12.Leslie SJ, Denvir M, Webb DJ. Human urotensin II causes vasoconstriction in the human skin microcirculation. Circulation. 2001;102:542. [Google Scholar]
- 13.Sondermeijer B, Kompa A, Komesaroff P, Krum H. Effect of exogenous urotensin-II on vascular tone in skin microcirculation of patients with essential hypertension. Am J Hypertens. 2005;18:1195–9. doi: 10.1016/j.amjhyper.2005.03.748. [DOI] [PubMed] [Google Scholar]
- 14.Rossi M, Magagna A, Di Maria C, Franzoni F, Taddei S, Santoro G. Skin vasodilator effect of exogenous urotensin-II in hypertensives not exposed to antihypertensive medication. Blood Press. 2008;17:18–25. doi: 10.1080/08037050701757994. [DOI] [PubMed] [Google Scholar]
- 15.Kemp W, Roberts S, Komesaroff PA, Zomer E, Krum H. Urotensin II in chronic liver disease: in vivo effect on vascular tone. Scand J Gastroenterol. 2008;43:103–9. doi: 10.1080/00365520701580009. [DOI] [PubMed] [Google Scholar]
- 16.Bohm F, Pernow J. Urotensin II evokes potent vasoconstriction in humans in vivo. Br J Pharmacol. 2002;135:25–7. doi: 10.1038/sj.bjp.0704448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkinson IB, Affolter JT, de Haas SL, Pellegrini MP, Boyd J, Winter MJ, Balment RJ, Webb DJ. High plasma concentrations of human urotensin II do not alter local or systemic hemodynamics in man. Cardiovasc Res. 2002;53:341–7. doi: 10.1016/s0008-6363(01)00485-0. [DOI] [PubMed] [Google Scholar]
- 18.Patacchini R, Santicioli P, Giuliani S, Grieco P, Novellino E, Rovero P, Maggi CA. Urantide: an ultrapotent urotensin II antagonist peptide in the rat aorta. Br J Pharmacol. 2003;140:1155–8. doi: 10.1038/sj.bjp.0705555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Brien E, Mee F, Atkins N, Thomas M. Evaluation of three devices for self-measurement of blood pressure according to the revised British Hypertension Society Protocol: the Omron HEM-705CP, Philips HP5332, and Nissei DS-175. Blood Press Monit. 1996;1:55–61. [PubMed] [Google Scholar]
- 20.Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharmacol. 2001;52:631–46. doi: 10.1046/j.1365-2125.2001.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Affolter JT, Newby DE, Wilkinson IB, Winter MJ, Balment RJ, Webb DJ. No effect on central or peripheral blood pressure of systemic urotensin II infusion in humans. Br J Clin Pharmacol. 2002;54:617–21. doi: 10.1046/j.1365-2125.2002.t01-1-01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brailoiu E, Jiang X, Brailoiu GC, Yang J, Chang JK, Wang H, Dun NJ. State-dependent calcium mobilization by urotensin-II in cultured human endothelial cells. Peptides. 2008;29:721–6. doi: 10.1016/j.peptides.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yano K, Hicks JW, Vaudry H, Conlon JM. Cardiovascular actions of frog urotensin II in the frog, Rana catesbeiana. Gen Comp Endocrinol. 1995;97:103–10. doi: 10.1006/gcen.1995.1010. [DOI] [PubMed] [Google Scholar]
- 24.Watson AM, Lambert GW, Smith KJ, May CN. Urotensin II acts centrally to increase epinephrine and ACTH release and cause potent inotropic and chronotropic actions. Hypertension. 2003;42:373–9. doi: 10.1161/01.HYP.0000084633.85427.E6. [DOI] [PubMed] [Google Scholar]
- 25.Bousette N, Pottinger J, Ramli W, Ohlstein EH, Dhanak D, Douglas SA, Giaid A. Urotensin-II receptor blockade with SB-611812 attenuates cardiac remodeling in experimental ischemic heart disease. Peptides. 2006;27:2919–26. doi: 10.1016/j.peptides.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Tzanidis A, Hannan RD, Thomas WG, Onan D, Autelitano DJ, See F, Kelly DJ, Gilbert RE, Krum H. Direct actions of urotensin II on the heart: implications for cardiac fibrosis and hypertrophy. Circ Res. 2003;93:246–53. doi: 10.1161/01.RES.0000084382.64418.BC. [DOI] [PubMed] [Google Scholar]
- 27.Camarda V, Song W, Marzola E, Spagnol M, Guerrini R, Salvadori S, Regoli D, Thompson JP, Rowbotham DJ, Behm DJ, Douglas SA, Calo G, Lambert DG. Urantide mimics urotensin-II induced calcium release in cells expressing recombinant UT receptors. Eur J Pharmacol. 2004;498:83–6. doi: 10.1016/j.ejphar.2004.07.089. [DOI] [PubMed] [Google Scholar]


