Abstract
AIMS
To evaluate the effect of co-administration of rifampicin, an inducer of cytochrome P450 (CYP)3A4, on the pharmacokinetics of roflumilast and roflumilast N-oxide. Roflumilast is an oral, once-daily phosphodiesterase 4 (PDE4) inhibitor, being developed for the treatment of chronic obstructive pulmonary disease. Roflumilast is metabolized by CYP3A4 and CYP1A2, with further involvement of CYP2C19 and extrahepatic CYP1A1. In vivo, roflumilast N-oxide contributes >90% to the total PDE4 inhibitory activity.
METHODS
Sixteen healthy male subjects were enrolled in an open-label, three-period, fixed-sequence study. They received a single oral dose of roflumilast 500 µg on days 1 and 12 and repeated oral doses of rifampicin 600 mg once daily on days 5–15. Plasma concentrations of roflumilast and roflumilast N-oxide were measured for up to 96 h. Test/Reference ratios and 90% confidence intervals (CIs) of geometric means for AUC and Cmax of roflumilast and roflumilast N-oxide and for oral apparent clearance (CL/F) of roflumilast were estimated.
RESULTS
During the steady-state of rifampicin, the AUC0–∞ of roflumilast decreased by 80% (point estimate 0.21; 90% CI 0.16, 0.27); Cmax by 68% (0.32; CI 0.26, 0.39); for roflumilast N-oxide, the AUC0–∞ decreased by 56% (0.44; CI 0.36, 0.55); Cmax increased by 30% (1.30; 1.15, 1.48); total PDE4 inhibitory activity decreased by 58% (0.42; 0.38, 0.48).
CONCLUSIONS
Co-administration of rifampicin and roflumilast led to a reduction in total PDE4 inhibitory activity of roflumilast by about 58%. The use of potent cytochrome P450 inducers may reduce the therapeutic effect of roflumilast.
Keywords: CYP3A induction, drug–drug interaction, PDE4 inhibitor, pharmacokinetics, rifampicin, roflumilast
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Rifampicin is an antibiotic that is used to treat pulmonary tuberculosis. It induces several cytochrome P450 (CYP) enzymes and some drug transporter proteins; its greatest effect is as an inducer of CYP3A4 in the liver and in the small intestine.
Mechanistic drug–drug interaction studies with prototypic CYP3A4 inducers provide essential information for clinical drug development of new chemical entities that are metabolized by the involved CYP450 enzymes.
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor being developed for the treatment of chronic obstructive pulmonary disease.
The pharmacological effect is based on the total PDE4 inhibitory activity, which represents the combined PDE4 inhibitory activity of roflumilast and its major active metabolite, roflumilast N-oxide.
In patients with chronic obstructive pulmonary disease, pulmonary tuberculosis can be an accompanying disease. Thus, the drug–drug interaction between rifampicin and roflumilast is of clinical relevance.
WHAT THIS STUDY ADDS
The pharmacokinetics of roflumilast and of its major pharmacologically active metabolite roflumilast N-oxide is affected by co-administration of rifampicin.
The potent induction of CYP3A4 and other CYP450 enzymes (such as CYP2C19 and extrahepatic CYP1A1) by rifampicin has led to a 58% decrease in the total PDE4 inhibitory activity of roflumilast.
Co-administration of rifampicin with roflumilast may reduce the therapeutic efficacy of roflumilast.
Introduction
The cyclic adenosine monophosphate (cAMP)-specific phosphodiesterase 4 (PDE4) represents a novel target for the treatment of chronic inflammatory airway diseases such as chronic obstructive pulmonary disease (COPD) [1]. PDE4 catalyses the hydrolysis of intracellular cAMP, an important second messenger for the function of inflammatory and airway smooth muscle cells [2]. Roflumilast is a targeted inhibitor of PDE4. Roflumilast exerts its anti-inflammatory properties by amplifying intracellular cAMP signalling, which attenuates the inflammatory response mediated by various immune-modulatory cells [3, 4]. In clinical studies, an oral, once-daily dose of roflumilast 500 µg was shown to improve lung function, reduce exacerbations, and to be safe in patients with COPD [5, 6].
Roflumilast is rapidly absorbed after oral administration. The mean absolute bioavailability of a 500-µg immediate-release tablet is 79% and the apparent terminal plasma disposition half-life (t1/2) is about 17 h [7]. In humans, the major step in the metabolism of roflumilast is N-oxidation by cytochrome P450 (CYP) isozymes 3A4 and CYP1A2 to the pharmacologically active metabolite roflumilast N-oxide, which in vivo has a similar phosphodiesterase selectivity profile and intrinsic PDE4 inhibitory activity as the parent drug roflumilast [4]. The t1/2 of roflumilast N-oxide is about 27 h and its total exposure, i.e. area under the plasma concentration curve (AUC) exceeds that of the parent drug by about 10-fold [7]. Roflumilast and roflumilast N-oxide follow linear pharmacokinetics and show a predictable and dose-proportional, intraindividual pharmacokinetics under steady-state conditions [8]. Roflumilast N-oxide is the major contributor to the overall PDE4 inhibitory activity of roflumilast. After intake of roflumilast, the mean peak plasma concentration (Cmax) of roflumilast is reached in <1 h; the Cmax of roflumilast N-oxide is reached in about 4 h. In vitro data suggest that roflumilast N-oxide is metabolized by CYP3A4 with a contribution from CYP2C19 and extrahepatic CYP1A1. From previous studies, it is known that only very small amounts (<1%) of roflumilast and roflumilast N-oxide are excreted unchanged via the renal route.
Results of drug–drug interaction studies with CYP3A4 inhibitors ketoconazole and erythromycin showed that the AUC of the parent compound roflumilast changed almost twofold, whereas the AUC of the metabolite roflumilast N-oxide remained relatively unchanged [9, 10]. In a study with fluvoxamine (which is a more specific CYP1A2 inhibitor and an in vivo inhibitor of CYP2C19, 40-fold more potent than in vitro[11]), a 2.5-fold increase in the AUC of the parent compound and only 50% increase of the metabolite were observed [12]. Collectively, it appears that the disposition of roflumilast and roflumilast N-oxide is susceptible to alteration by induction or inhibition of several metabolizing enzymes.
Rifampicin is an antibiotic used frequently for the treatment of pulmonary tuberculosis. Rifampicin is a potent inducer of several CYP isoforms, including CYP3A4, CYP2B6, CYP2C8, CYP2C9 and CYP2C19, and is a weak inducer of CYP1A2 [13]. It has its greatest effects on the expression of CYP3A4 in both the intestinal wall and the liver [13–17]. Furthermore, in humans rifampicin can induce the expression of some efflux transporters (e.g. P-glycoprotein and ABCB1) as well as the glucuronidation of certain drugs, such as S-oxazepam and rofecoxib [17–21]. The mechanism of rifampicin-mediated induction of enzymes and membrane transporters is primarily through the binding to the nuclear pregnane X receptor among other nuclear receptors [22]. Due to these properties, rifampicin exhibits substantial effects on the pharmacokinetics of drugs that undergo extensive CYP-mediated oxidative metabolism and/or are subject to P-glycoprotein-mediated transport [17, 23]. Overall, the effects of rifampicin on drug metabolism and transport are broad and have an established clinical significance. Given the complex time course of enzyme/transporter induction by rifampicin, potential drug–drug interactions should be considered when starting or discontinuing treatment with rifampicin.
The aim of the present study was to assess the effect of steady-state rifampicin (repeated oral dose of 600 mg for 10 days) on the pharmacokinetics of roflumilast and roflumilast N-oxide, after a single oral dose of roflumilast 500 µg.
Materials and methods
Subjects and study design
The study was approved by the responsible ethics committee (Chamber of Physicians Baden-Württemberg, Germany) and was performed at the clinical unit of AAI Pharma Deutschland GmbH & Co. KG (Neu-Ulm, Germany). The study conformed to the Declaration of Helsinki (Somerset West Amendment, 1996), Note for Guidance on Good Clinical Practice (CGMP/ICH/135/95) and the German Drug Law (AMG). Before the study start, each subject gave written informed consent to participate.
A total of 16 healthy white male subjects were enrolled. The study was performed according to an open-label, fixed-sequence design, consisting of three time periods: period 1, administration of a single oral dose of roflumilast 500 µg alone in the morning of study day 1, and a wash-out period (3 days); period 2, repeated administration of oral rifampicin 600 mg once daily alone for 7 days in the morning of days 5–11; period 3, repeated (continuous) once-daily, oral dose of rifampicin 600 mg for 4 days in the morning of days 12–15 and co-administration of a single oral dose of roflumilast 500 µg in the morning of day 12.
The selected dose of rifampicin was based on the reported use of the drug in clinical interaction studies [17] and on the recommendation by the Food and Drug Administration [24]. The selected dose of roflumilast 500 µg once daily has been shown to be safe and well tolerated in a series of clinical studies and it represents the anticipated recommended therapeutic dose [1, 25].
Roflumilast tablets were manufactured by Nycomed GmbH (Konstanz, Germany; formerly ALTANA Pharma Oranienburg GmbH, Germany). Rifampicin (Eremfat® 600) was purchased from FATOL Arzneimittel GmbH (Schiffweiler, Germany). Study medication was administered orally in the morning of day 1 after an overnight fast of at least 10 h (period 1) and days 5–15 (periods 2 and 3) between 07.00 and 09.00 h. The tablets were swallowed with 240 ml water.
On the days before the pharmacokinetic profiling, subjects reported to the clinical ward in the evening and were confined until completion of the 24-h blood sampling. In the morning of the study days for pharmacokinetic profiling, an indwelling cannula was inserted into a forearm vein to allow repeated blood sampling. Two hours after the study drug administration, decarbonated water and fruit tea were allowed and the subjects remained fasted until 4 h post dose. Subjects received standardized meals at specified times. During the study, the subjects were asked to avoid strenuous physical exercise. Known dietary CYP inhibitors and inducers such as grapefruit/pomelo, poppy seeds, Brussels sprouts, or broccoli were not allowed from 7 days before the first drug administration until the last blood sampling of the study [26–28]. It is known that smoking induces the enzyme CYP1A2. According to the study protocol, subjects smoking <10 cigarettes per day were allowed to enter the study. There were eight smokers enrolled and they were asked to maintain their smoking habits throughout the study.
Blood sampling and determination of drug concentrations
On the pharmacokinetic profiling days, serial blood samples for measuring plasma concentrations of roflumilast and N-oxide metabolite were collected during periods 1 and 3: prior to dosing and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 14, 24, 30, 48, 54, 72 and 96 h post administration. Predose plasma concentrations of rifampicin were determined from blood samples collected on days 5, 10 and 11 during period 2. On day 12, blood samples for rifampicin were taken predose, 0.5, 1, 1.5, 2 and 3 h post administration. These selected time points were around the time of the expected Cmax and were used to check for adequate rifampicin exposure during induction (period 3). For the analysis of roflumilast and N-oxide metabolite, blood samples were collected for up to 96 h (time points shown for periods 1 and 3 above). Blood samples were collected into monovettes containing lithium-heparinate. The samples were centrifuged within 20–30 min at 1600 g, 15 min, 4°C. Plasma was transferred into polypropylene plastic tubes within 60 min after collection and frozen at –20°C or below. The samples were kept frozen until analysis.
Plasma concentrations of roflumilast and roflumilast N-oxide were determined using a validated high-performance liquid chromatography-mass spectrometry/mass spectrometry (HPLC-MS/MS) method. Roflumilast and roflumilast N-oxide were determined in the positive ion mode using turbo ion spray HPLC-MS/MS. The assay was linear in the range of 0.1 µg l−1 and 50 µg l−1. The selected precursor- and product-ions for roflumilast were at m/z 403.2 and m/z 187.2, respectively. The selected precursor- and product-ions for the internal standard (ISTD) (D5-roflumilast) were at m/z 408.1 and m/z 190.3, respectively. The selected precursor- and product-ions for roflumilast N-oxide were at m/z 419.1 and m/z 187.4, respectively. The selected precursor- and product-ions for the ISTD (D5-roflumilast N-oxide) were at m/z 424.1 and m/z 190.3, respectively. The lower limit of quantification for both compounds was 0.1 µg l−1. For roflumilast, the interday precision (between-day coefficient of variation) ranged between 6.6 and 7.7%. Interday accuracy ranged between 98.0 and 100.6%. For roflumilast N-oxide, the interday precision ranged between 5.4 and 7.3% and interday accuracy ranged between 91.2 and 101.8%. Rifampicin did not interfere with the quantification of roflumilast or roflumilast N-oxide; this was determined before the sample analysis.
Plasma levels of rifampicin were assayed using a validated HPLC method with ultraviolet detection. The assay was linear in the range of 0.1 mg l−1 and 10 mg l−1. Diazepam was used as internal standard. Rifampicin was detected at a wavelength of 337 nm, the internal standard at 250 nm. The interday precision ranged between 4.8 and 7.6% and interday accuracy ranged from 105.1 to 108.4%. The lower limit of quantification of rifampicin was 0.1 mg l−1.
Safety investigations
Safety investigations included monitoring of clinical laboratory evaluation (blood chemistry, haematology, urine analysis), physical examination, vital signs (blood pressure, pulse rate, and aural body temperature) and resting standard 12-lead electrocardiogram (ECG) at the screening and post-study examinations. Adverse events (AEs) were monitored throughout the study.
Data analysis
Pharmacokinetic parameter estimates of roflumilast and roflumilast N-oxide following oral administrations were obtained with a noncompartmental analysis approach using WinNonLin, version 4.0.1 (Pharsight, Mountain View, CA, USA).
To compare the primary pharmacokinetic parameter estimates (AUC, Cmax and CL) of the analytes between treatments, an analysis of variance (anova) using the 90% confidence interval (CI) for the ratios of the least-square means was performed with WinNonLin (version 4.0.1) linear mixed effects modelling (bioequivalence wizard). Test/Reference ratios of geometric means for CL/F, AUC0–∞ and Cmax of roflumilast, and AUC0–∞ and Cmax of roflumilast N-oxide for induced phase (period 3, Test) and pre-induction (period 1, Reference) were calculated.
Lack of a relevant pharmacokinetic alteration of rifampicin on roflumilast and roflumilast N-oxide exposure was concluded if the 90% CIs of the point estimates were within the standard equivalence acceptance range of 0.80 and 1.25.
Total PDE4 inhibitory activity
To estimate the combined PDE4 inhibition of roflumilast and roflumilast N-oxide in humans following administration of roflumilast, the parameter termed ‘total PDE4 inhibitory activity’ (tPDE4i) has been established [9, 29, 30]. This parameter represents the sum of overall PDE4 inhibitory activity after exposure to roflumilast and roflumilast N-oxide, accounting for the differences in the intrinsic activity (IC50), free fraction (protein binding), and in vivo exposure (AUC values) of both compounds. The tPDE4i values are used to evaluate the pharmacokinetics of roflumilast in drug–drug interaction and in special population studies, where potentially pharmacokinetic alterations may differ greatly between roflumilast and roflumilast N-oxide [9, 10, 12, 30].
Results
Subjects
A total of 16 healthy white male subjects were enrolled in the study (age range 20–44 years, median 38 years; body weight 61–88 kg, median 75 kg). One subject discontinued the study for personal reasons during period 2. Thus, 15 subjects completed the study according to protocol.
Pharmacokinetics
After a single dose of roflumilast 500 µg alone (Reference), the plasma concentration–time course (geometric mean and 68% range) for roflumilast and roflumilast N-oxide was consistently higher over the 24-h period than that after concomitant administration of roflumilast and rifampicin (Test) in period 3 (Figure 1a,b).
Figure 1.
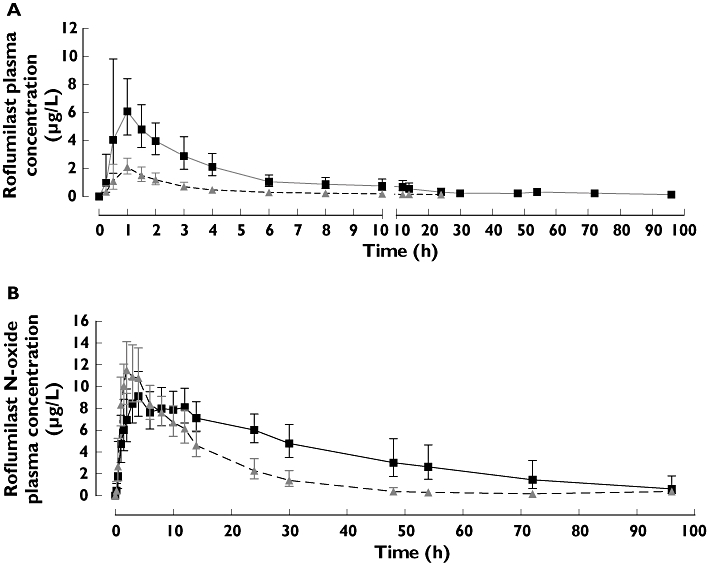
Plasma concentration of roflumilast (a) and roflumilast N-oxide (b) after a single oral dose of roflumilast 500 µg administered alone or concomitantly with rifampicin. Shown are the geometric mean and 68% range. Roflumilast alone ( ); Roflumilast with Rifampicin (
); Roflumilast with Rifampicin ( )
)
After administration of 600 mg rifampicin on day 12, all subjects had clearly detectable plasma levels of rifampicin. The plasma concentration profile for all subjects and the geometric mean are shown in Figure 2. Rifampicin levels were not detectable 24 h after administration. Our observations are consistent with data reported in other pharmacokinetic studies with rifampicin [31, 32].
Figure 2.
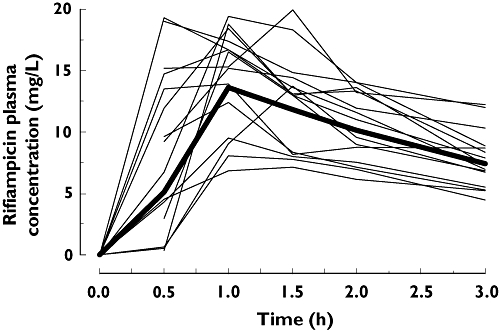
Plasma concentration levels of rifampicin on day 12 after the administration of 600 mg rifampicin once daily for 7 days (from day 5 to day 12). Shown are the geometric mean (Geo Mean) and the individual levels of the healthy male subjects (n= 16). Geometric Mean shown as ( )
)
The values for roflumilast AUC and Cmax were lower (about 80% and 68%, respectively) following co-administration of rifampicin (Table 1). The AUC for roflumilast N-oxide AUC was also decreased (by about 56%). In contrast to roflumilast, roflumilast N-oxide Cmax was about 30% higher and its tmax occurred distinctly earlier with rifampicin co-administration (shift from 6.5 to 2.6 h; Table 1); for roflumilast, the tmax remained almost unchanged whether administered alone or with rifampicin (shift from 0.9 to 1.0 h, Table 1). The AUCrofNO/AUCrof ratio for roflumilast N-oxide increased about twofold from 10.8 (roflumilast alone) to 23.1 (roflumilast plus rifampicin; Table 1). The combined effect of rifampicin co-administration on roflumilast and roflumilast N-oxide exposures led to a 58% decrease in tPDE4i (Table 2).
Table 1.
Pharmacokinetic parameter estimates of roflumilast and roflumilast N-oxide after administration of a single oral dose of roflumilast 500 µg alone and after co-administration with steady-state rifampicin
| Compound | Roflumilast | Roflumilast N-oxide | ||||
|---|---|---|---|---|---|---|
| Parameter | Roflumilast alone (n= 16) | Roflumilast and rifampicin (n= 15) | Ratio test : reference (90% CI) | Roflumilast alone (n= 16) | Roflumilast and rifampicin (n= 15) | Ratio test : reference (90% CI) |
| AUC0–∞ (h µg l−1)* | 38.5 (24.1–61.5) | 7.8 (5.4–11.4) | 0.21 (0.16, 0.27)‡ | 414.1 (275.6–622.2) | 180.2 (141.0–230.2) | 0.44 (0.36, 0.55)‡ |
| AUC0–last (h µg l−1)* | 35.1 (21.7–56.7) | 6.5 (4.5–9.4) | 0.19 (0.15, 0.25)‡ | 368 (279.5–484.3) | 177.0 (138.5–226.3) | 0.49 (0.41, 0.57)‡ |
| CL/F (l h−1)* | 13.0 (8.1–20.8) | 64.1 (44.0–93.4) | 4.84 (3.70, 6.32)‡ | NA | NA | NA |
| Cmax (µg l−1)* | 6.9 (4.9–9.7) | 2.2 (1.7–2.8) | 0.32 (0.26, 0.39)‡ | 9.5 (7.6–11.7) | 12.2 (10.1–14.8) | 1.30 (1.15, 1.48)‡ |
| tmax (h)† | 0.9 (0.6–1.5) | 1.0 (0.7–1.3) | NA | 6.5 (3.5–12.2) | 2.6 (1.7–4.0) | NA |
| t1/2 (h)* | 15.7 (9.4–26.3) | 6.0 (3.2–11.2) | 0.33 (0.23, 0.47) | 24.0 (15.3–37.5) | 9.9 (8.2–12.0) | 0.41 (0.33, 0.51) |
| AUCrofNO/AUCrof | NA | NA | NA | 10.8 | 23.1 | NA |
Geometric mean (68% range).
tmax: median (min.–max.). NA, not applicable.
Point estimate and 90% CI exceeding the predefined equivalence acceptance range of 0.8 to 1.25; P > 0.05 (two one-sided t-tests).
Table 2.
Total PDE4 inhibitory (tPDE4i) activity of roflumilast after administration of a single oral dose of roflumilast 500 µg alone and after co-administration with steady-state rifampicin
| Compound | Roflumilast and roflumilast N-oxide | ||
|---|---|---|---|
| Parameter | Roflumilast alone (n= 16) | Roflumilast and rifampicin (n= 15) | Ratio test: reference (90% CI) |
| tPDE4i* | 0.79 | 0.34 | 0.42 (0.38, 0.48)† |
Total PDE4 inhibitory activity (tPDE4i) is expressed as the sum of the total exposure resulting from roflumilast and roflumilast N–oxide.
Point estimate and 90% CI exceeding the predefined equivalence acceptance range of 0.8 to 1.25; P > 0.05 (two one-sided t-tests).
Safety and tolerability
During the three study periods, 14 (88%) of the 16 subjects reported 40 AEs. The intensity of nearly all reported AEs (39/40) was mild or moderate. In most cases, the symptoms subsided spontaneously after a short duration and all AEs resolved completely. The most frequent AE was headache, which occurred in eight subjects. None of the AEs led to study discontinuation and no serious or unexpected AEs were reported. Laboratory values and physical examination parameters, including electrocardiogram and vital signs, showed no clinically relevant changes between the screening and post-study examination. Co-administration of oral roflumilast 500 µg and rifampicin 600 mg once daily was well tolerated.
Discussion
The present study is part of a drug–drug interaction programme to evaluate the sources of the pharmacokinetic variability of the PDE4 inhibitor roflumilast and to determine its proper clinical use and safety, especially for patients who may be treated with both roflumilast and rifampicin.
After steady-state co-administration of rifampicin, a decrease (about 80%) in total roflumilast exposure was observed. Co-administration of rifampicin also led to a decrease in the roflumilast Cmax (about 68%), whereas correspondingly roflumilast N-oxide Cmax increased (about 30%). In line with this observed effect of rifampicin, the AUCrofNO/AUCrof ratio increased by about twofold from 10.8 (roflumilast alone) to 23.1 (roflumilast co-administered with rifampicin). This result is in agreement with an increase in the formation rate of roflumilast N-oxide from roflumilast (CL/F), as reflected by the increase in roflumilast N-oxide Cmax and a shift in roflumilast N-oxide tmax from 6.5 to 2.6 h (Table 1). Moreover, such an increase in the formation rate of roflumilast N-oxide is consistent with the observed marked rifampicin-mediated induction of CYP3A4.
The metabolic clearance of roflumilast N-oxide (CLrofNO) was not directly assessed in this study. However, our data suggest that CLrofNO may have been increased upon co-administration of rifampicin, as reflected by the 56% decrease in the AUC of roflumilast N-oxide and a 2.5-fold reduction of half-life [24 h (roflumilast alone) to 9.9 h (roflumilast co-administered with rifampicin)], which occurred despite an increase in the formation rate of the metabolite, indicating an effect of rifampicin on roflumilast N-oxide formation and clearance rates. Based on data from in vitro studies, roflumilast N-oxide is formed from roflumilast through the contributions of CYP1A2 as well as CYP3A4 and is metabolized by CYP3A4, CYP2C19 and extrahepatic CYP1A1. Previous mechanistic interaction studies with prototypic and specific inhibitors of CYP3A4 [9, 10] corroborate the contribution of CYP3A4 during the formation of roflumilast N-oxide and also suggest a role of CYP3A4 during the clearance of roflumilast N-oxide. Furthermore, the results of the study with fluvoxamine, which is a potent CYP1A2 and CYP2C19 inhibitor, showed a 2.5-fold increase in the AUC of the parent and 50% of the metabolite [12], supporting the involvement of CYP1A2 in the metabolism of roflumilast and CYP2C19 in the metabolism of roflumilast N-oxide. Thus, it is unlikely that the alterations in the exposure of the roflumilast N-oxide observed in the present study can be explained solely by the induction of CYP3A4 through rifampicin. Recent in vitro data by Oscarson et al.[16] and in vivo data by Kanebratt et al.[33] show that rifampicin induces CYP2C19 to a similar extent as CYP3A4. We therefore suggest that the observed decrease in roflumilast N-oxide exposure could be attributed to the induction of CYP2C19 in addition to CYP3A4 during the elimination of the metabolite [20].
We observed that co-administration of rifampicin reduced the interindividual variability of roflumilast and roflumilast N-oxide exposure. These observations are in agreement with those of LeCluyse, who reported that although there was marked variation in the basal activity (10-fold) of hepatocyte preparations from the 17 liver donors, the extent of CYP induction was dependent on basal CYP3A4 activity [34]. These authors observed that the CYP3A4 activity at maximal induction by rifampicin tends to be quantitatively similar among individuals, suggesting a limit to which the CYP enzymes can be maximally induced [34, 35].
Roflumilast and roflumilast N-oxide have similar intrinsic PDE4 inhibitory activity in vivo. Total PDE4 inhibition (tPDE4i) in humans represents the combined effect of both roflumilast and roflumilast N-oxide, whereby the active metabolite roflumilast N-oxide accounts for about 90% of roflumilast's overall pharmacological effects [29]. Concomitant administration of rifampicin and roflumilast led to a decrease of 58% in the tPDE4i.
Based on the present results with healthy subjects, it can be postulated that co-administration of rifampicin, which is used to treat pulmonary tuberculosis, may potentially alter the total systemic exposure to roflumilast in patients with COPD. This is clinically relevant because COPD often occurs in patients who may have comorbidities, including pulmonary tuberculosis [36]. Therefore, the intake of rifampicin has to be taken into account during the management of patients with COPD who are treated with roflumilast [13, 36–38].
In conclusion, co-administration of rifampicin and roflumilast led to a decrease in systemic exposure to roflumilast and roflumilast N-oxide, translating into a decrease in tPDE4i of roflumilast. Thus, co-administration of rifampicin with roflumilast may reduce the therapeutic efficacy of roflumilast.
Competing interests
N.N., A.H., R. Herzog, K.D., K.Z. and G.L. are employees of Nycomed. O.v.R and R. Hermann were employees of ALTANA Pharma AG until 2007. M.K. is an employee of the CRO where the study was performed.
This study was sponsored by Nycomed GmbH (Konstanz, Germany; formerly ALTANA Pharma AG). The authors thank Mrs Gabriele Piskol (AAI Pharma Deutschland GmbH & Co. KG, Neu-Ulm, Germany) for organizational support during the study and pharm-analyt Labor GmbH (Baden, Austria) for determination of plasma concentrations of roflumilast and roflumilast N-oxide. The authors thank Dr Kathy B. Thomas and Dr Angela Schilling (formerly of Nycomed GmbH, Konstanz, Germany) for valuable suggestions during the preparation of the manuscript.
REFERENCES
- 1.Lipworth BJ. Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary disease. Lancet. 2005;365:167–75. doi: 10.1016/S0140-6736(05)17708-3. [DOI] [PubMed] [Google Scholar]
- 2.Mehats C, Jin SL, Wahlstrom J, Law E, Umetsu DT, Conti M. PDE4D plays a critical role in the control of airway smooth muscle contraction. FASEB J. 2003;17:1831–41. doi: 10.1096/fj.03-0274com. [DOI] [PubMed] [Google Scholar]
- 3.Bundschuh DS, Eltze M, Barsig J, Wollin L, Hatzelmann A, Beume R. In vivo efficacy in airway disease models of roflumilast, a novel orally active PDE4 inhibitor. J Pharmacol Exp Ther. 2001;297:280–90. [PubMed] [Google Scholar]
- 4.Hatzelmann A, Schudt C. Anti-inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitro. J Pharmacol Exp Ther. 2001;297:267–79. [PubMed] [Google Scholar]
- 5.Rabe KF, Bateman ED, O'Donnell D, Witte S, Bredenbroeker D, Bethke TD. Roflumilast – an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005;366:563–71. doi: 10.1016/S0140-6736(05)67100-0. [DOI] [PubMed] [Google Scholar]
- 6.Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:154–61. doi: 10.1164/rccm.200610-1563OC. [DOI] [PubMed] [Google Scholar]
- 7.David M, Zech K, Seiberling M, Weimar C, Bethke TD. Roflumilast, a novel, oral, selective PDE4 inhibitor, shows high absolute bioavailability. J Allergy Clin Immunol. 2004;113:S220–1. [Google Scholar]
- 8.Bethke TD, Böhmer GM, Hermann R, Hauns B, Fux R, Mörike K, David M, Knoerzer D, Wurst W, Gleiter CH. Dose-proportional intraindividual single- and repeated-dose pharmacokinetics of roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor. J Clin Pharmacol. 2007;47:26–36. doi: 10.1177/0091270006294529. [DOI] [PubMed] [Google Scholar]
- 9.Lahu G, Huennemeyer A, von Richter O, Hermann R, Herzog R, McCracken N, Zech K. Effect of single and repeated ketoconazole on the pharmacokinetics of roflumilast and roflumilast N-oxide. J Clin Pharmacol. 2008;48:1339–49. doi: 10.1177/0091270008321941. [DOI] [PubMed] [Google Scholar]
- 10.Lahu G, Huennemeyer A, Herzog R, et al. Effect of repeated dose of erythromycin on the pharmacokinetics of roflumilast and roflumilast N-oxide. Int J Clin Pharmacol Ther. 2009;47:236–45. doi: 10.5414/cpp47236. [DOI] [PubMed] [Google Scholar]
- 11.Yao C, Kunze KL, Trager WF, Kharasch ED, Levy RH. Comparison of in vitro and in vivo inhibition potencies of fluvoxamine toward CYP2C19. Drug Metab Dispos. 2003;31:565–71. doi: 10.1124/dmd.31.5.565. [DOI] [PubMed] [Google Scholar]
- 12.von Richter O, Lahu G, Huennemeyer A, Herzog R, Zech K, Hermann R. Effect of fluvoxamine on the pharmacokinetics of roflumilast and roflumilast N-oxide. Clin Pharmacokinet. 2007;46:613–22. doi: 10.2165/00003088-200746070-00006. [DOI] [PubMed] [Google Scholar]
- 13.Backman JT, Granfors MT, Neuvonen PJ. Rifampicin is only a weak inducer of CYP1A2-mediated presystemic and systemic metabolism: studies with tizanidine and caffeine. Eur J Clin Pharmacol. 2006;62:451–61. doi: 10.1007/s00228-006-0127-x. [DOI] [PubMed] [Google Scholar]
- 14.Kullak-Ublick GA, Becker MB. Regulation of drug and bile salt transporters in liver and intestine. Drug Metab Rev. 2003;35:305–17. doi: 10.1081/dmr-120026398. [DOI] [PubMed] [Google Scholar]
- 15.Schuetz EG. Induction of cytochromes P450. Curr Drug Metab. 2001;2:139–47. doi: 10.2174/1389200013338595. [DOI] [PubMed] [Google Scholar]
- 16.Oscarson M, Burk O, Winter S, Schwab M, Wolbold R, Dippon J, Eichelbaum M, Meyer UA. Effects of rifampicin on global gene expression in human small intestine. Pharmacogenet Genomics. 2007;17:907–18. doi: 10.1097/FPC.0b013e3280143dfc. [DOI] [PubMed] [Google Scholar]
- 17.Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivisto KT. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet. 2003;42:819–50. doi: 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- 18.Chung JY, Cho JY, Yu KS, Kim JR, Jung HR, Lim KS, Jang IJ, Shin SG. Effect of the UGT2B15 genotype on the pharmacokinetics, pharmacodynamics, and drug interactions of intravenous lorazepam in healthy volunteers. Clin Pharmacol Ther. 2005;77:486–94. doi: 10.1016/j.clpt.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Kyrklund C, Backman JT, Neuvonen M, Neuvonen PJ. Effect of rifampicin on pravastatin pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 2004;57:181–7. doi: 10.1046/j.1365-2125.2003.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niemi M, Backman JT, Neuvonen M, Neuvonen PJ. Effect of rifampicin on the pharmacokinetics and pharmacodynamics of nateglinide in healthy subjects. Br J Clin Pharmacol. 2003;56:427–32. doi: 10.1046/j.1365-2125.2003.01884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oscarson M, Zanger UM, Rifki OF, Klein K, Eichelbaum M, Meyer UA. Transcriptional profiling of genes induced in the livers of patients treated with carbamazepine. Clin Pharmacol Ther. 2006;80:440–56. doi: 10.1016/j.clpt.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Raymond K. Roles of rifampicin in drug–drug interactions: underlying molecular mechanisms involving the nuclear pregnane X receptor. Ann Clin Microbiol Antimicrob. 2006;5:3. doi: 10.1186/1476-0711-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.la Porte CJ, Colbers EP, Bertz R, Voncken DS, Wikstrom K, Boeree MJ, Koopmans PP, Hekster YA, Burger DM. Pharmacokinetics of adjusted-dose lopinavir-ritonavir combined with rifampin in healthy volunteers. Antimicrob Agents Chemother. 2004;48:1553–60. doi: 10.1128/AAC.48.5.1553-1560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research CDER. In Vivo Drug Metabolism/Drug Interaction Studies – Study Design, Data Analysis, and Recommendations for Dosing and Labeling. Available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM072119.pdf (last accessed 14 July 2009.
- 25.Reid P. Roflumilast Altana Pharma. Curr Opin Investig Drugs. 2002;3:1165–70. [PubMed] [Google Scholar]
- 26.Goho C. Oral midazolam–grapefruit juice drug interaction. Pediatr Dent. 2001;23:365–6. [PubMed] [Google Scholar]
- 27.Kall MA, Clausen J. Dietary effect on mixed function P450 1A2 activity assayed by estimation of caffeine metabolism in man. Hum Exp Toxicol. 1995;14:801–7. doi: 10.1177/096032719501401004. [DOI] [PubMed] [Google Scholar]
- 28.Kall MA, Vang O, Clausen J. Effects of dietary broccoli on human in vivo drug metabolizing enzymes: evaluation of caffeine, oestrone and chlorzoxazone metabolism. Carcinogenesis. 1996;17:793–9. doi: 10.1093/carcin/17.4.793. [DOI] [PubMed] [Google Scholar]
- 29.Hermann R, Lahu G, Hauns B, Bethke TD, Zech K. Total PDE4 inhibitory activity: a concept for evaluating pharmacokinetic alterations of roflumilast and roflumilast N-oxide in special populations and drug–drug interactions. Eur Respir J. 2006;28:436s. [Google Scholar]
- 30.Hermann R, Nassr N, Lahu G, Péterfai E, Knoerzer D, Herzog R, Zech K, de Mey C. Steady-state PK of roflumilast and roflumilast N-oxide in patients with mild and moderate liver cirrhosis. Clin Pharmacokinet. 2007;46:403–16. doi: 10.2165/00003088-200746050-00003. [DOI] [PubMed] [Google Scholar]
- 31.Peloquin CA, Namdar R, Singleton MD, Nix DE. Pharmacokinetics of rifampin under fasting conditions, with food, and with antacids. Chest. 1999;115:12–8. doi: 10.1378/chest.115.1.12. [DOI] [PubMed] [Google Scholar]
- 32.Droste JA, Verweij-van Wissen CP, Kearney BP, Buffels R, Vanhorssen PJ, Hekster YA, Burger DM. Pharmacokinetic study of tenofovir disoproxil fumarate combined with rifampin in healthy volunteers. Antimicrob Agents Chemother. 2005;49:680–4. doi: 10.1128/AAC.49.2.680-684.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanebratt KP, Diczfalusy U, Bäckström T, Sparve E, Bredberg E, Böttiger Y, Andersson TB, Bertilsson L. Cytochrome P450 induction by rifampicin in healthy subjects: determination using the Karolinska cocktail and the endogenous CYP3A4 marker 4beta-hydroxycholesterol. Clin Pharmacol Ther. 2008;84:589–94. doi: 10.1038/clpt.2008.132. [DOI] [PubMed] [Google Scholar]
- 34.LeCluyse EL. Human hepatocyte culture systems for the in vitro evaluation of cytochrome P450 expression and regulation. Eur J Pharm Sci. 2001;13:343–68. doi: 10.1016/s0928-0987(01)00135-x. [DOI] [PubMed] [Google Scholar]
- 35.Lin JH. CYP induction-mediated drug interactions: in vitro assessment and clinical implications. Pharm Res. 2006;23:1089–116. doi: 10.1007/s11095-006-0277-7. [DOI] [PubMed] [Google Scholar]
- 36.Maliwan N, Zvetina JR. Clinical features and follow up of 302 patients with Mycobacterium kansasii pulmonary infection: a 50 year experience. Postgrad Med J. 2005;81:530–3. doi: 10.1136/pgmj.2004.026229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fromm MF, Dilger K, Busse D, Kroemer HK, Eichelbaum M, Klotz U. Gut wall metabolism of verapamil in older people: effects of rifampicin-mediated enzyme induction. Br J Clin Pharmacol. 1998;45:247–55. doi: 10.1046/j.1365-2125.1998.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohan A, Premanand R, Reddy LN, Rao MH, Sharma SK, Kamity R, Bollineni S. Clinical presentation and predictors of outcome in patients with severe acute exacerbation of chronic obstructive pulmonary disease requiring admission to intensive care unit. BMC Pulm Med. 2006;6:27. doi: 10.1186/1471-2466-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]


