Abstract
AIM
The aim was to investigate the impact of a disease and medicine management programme, focusing on self-management in patients with chronic obstructive pulmonary disease (COPD).
METHODS
One hundred and seventy-three patients (mean age 67 years; 54% female) were recruited; 86 patients were randomly assigned to an intervention group and 87 to a usual care (control) group. Intervention patients received education on disease state, medications and breathing techniques. Patients were given booklets and a customized action plan (antibiotic and oral steroid to be initiated promptly by patients for exacerbations). Patients were followed up at 6 and 12 months during a scheduled visit. The St George's Respiratory Questionnaire (SGRQ), COPD Knowledge and Morisky adherence questionnaires were administered to all patients at baseline, 6 and 12 months. Outcome measures included hospital admissions, emergency department (ED) visits, health-related quality of life (HRQoL) and medication adherence.
RESULTS
Over the 12-month period in the intervention group, ED visits decreased by 50% (P= 0.02) and hospitalization by approximately 60% (P= 0.01). On the SGRQ, differences reached statistical significance on the symptom (−7.5; P= 0.04) and impact (−7.4; P= 0.03) subscales but not on the physical activity subscale. There was a significant difference between the intervention and usual care groups regarding knowledge scores (75.0 vs. 59.3; P= 0.001) and good adherence to medication (77.8% vs. 60.0%, P= 0.019). There was no significant difference regarding smoking between study groups.
CONCLUSIONS
The clinical pharmacy-led management programme can improve adherence, reduce the need for hospital care in patients with COPD and improve aspects of their HRQoL.
Keywords: chronic obstructive pulmonary disease, clinical pharmacy, disease management, quality of life, self-management
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
The concept of self-management plans for patients with chronic obstructive pulmonary disease (COPD) is derived from their success in asthma management.
It is believed that selected people with COPD may benefit from the early intervention that comes from following self-management plans, which may prevent a crisis and possibly the need for hospital admission.
There is little published information on clinical pharmacist-led disease and patient self-management in the area of COPD care.
WHAT THIS STUDY ADDS
A structured education programme led by a clinical pharmacist for patients with COPD was associated with a reduction in both hospitalization and emergency department visits and an improvement in patients' adherence to treatment regimens and health-related quality of life (excluding physical activity).
The intervention programme was tailored and individualized based on a preliminary assessment of individual patient needs.
Education about self-management in COPD patients should be explicit, tailored to individual needs, and on a continuous basis.
Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease with some significant extrapulmonary effects that may contribute to the severity in individual patients. It is characterized by airflow limitation that is not fully reversible. The airflow limitation is usually progressive and associated with an abnormal inflammatory response of the lung to noxious particles or gases [1]. COPD is a progressive chronic condition; in the UK it is already the fifth commonest cause of death [2]. It is the only major common cause of death for which the incidence is increasing [3] and is expected to be the third leading cause of death worldwide by 2020 (exceeded only by heart disease and stroke) [4]. Despite the burden of COPD, there is a lack of recognition of the illness among the general public, coupled with underdiagnosis and inadequate management [5]. With appropriate treatment and support it is possible to put quality back into the lives of sufferers [6].
The concept of self-management plans for patients with COPD is derived from their success in asthma management. It is believed that selected people with COPD may benefit from the early intervention that comes from following self-management plans that may prevent a crisis and possibly the need for hospital admission [7]. Central to COPD exacerbation self-management is early antibiotic and/or oral steroid treatment. The action plans can include useful contact details such as telephone numbers for the local hospital, the general practitioner (GP) surgery (including contact details of the practice nurse) and other details such as contact information for respiratory specialist nurses and oxygen suppliers.
There have been few randomized controlled trials evaluating the benefit of education of COPD patients on their condition and its management as distinct from other rehabilitation components. In asthma, patient education, together with an individualized management plan, has been found to improve outcomes for children and adults [8–10]. General education, without a link to individualized instruction, has not been found to be effective in improving outcomes.
An individualized management plan is a structured plan giving clear instructions to patients on when to take their medications when they are well, and how to vary their medications when symptoms worsen [11]. Although asthma patients often do not follow their plans completely, studies have consistently shown that patients who receive simple written guidelines have less frequent exacerbations and better health-related quality of life (HRQoL) than patients without management plans. However, it is not automatic that medication management plans will be as effective in COPD as they are in asthma.
The aim of the present study was to examine the impact of a pharmacy-led disease and medicine management programme (with a strong focus on self-management) in patients with COPD on clinical and humanistic outcomes.
Methods
Study design
The study was a randomized, controlled, longitudinal, prospective clinical trial. Ethical approval to carry out the study was obtained from the Office of Research Ethical Committees for Northern Ireland (ORECNI; 06/NIR02/23). All participants were recruited from the outpatient COPD clinic at the Mater Hospital, Belfast, Northern Ireland.
Sample size
The primary pre-specified outcomes were hospital admissions and HRQoL measured by St George's Respiratory Questionnaire (SGRQ). Using published data [12–14] it was estimated that to show a four-point improvement (which is considered a minimum clinically significant difference) [15, 16] in the total SGRQ scores over 12 months, a sample of 80 patients per group would be required. This sample size would provide 80% power for a two-sided α of 0.05. Based on these data, and to take account of potential drop-outs, a target sample size of 180 patients (90 control and 90 intervention) was selected for the present study.
Study patients
The study started in October 2006 and continued until May 2008. The entrance criteria were as follows: confirmed diagnosis of COPD (by the hospital consultant) for at least 1 year, having a forced expiratory volume in 1 s (FEV1) of 30–80% of the predicted normal value and >45 years old. The exclusion criteria were: having congestive heart failure, moderate to severe learning difficulties (as judged by hospital consultant), attended a pulmonary rehabilitation programme in the last 6 months, and severe mobility problems or terminal illness. All COPD patients who attended the outpatient clinic at the Mater Hospital, having fulfilled the entrance criteria, and having no exclusion criteria present, were invited to participate. A research pharmacist informed patients verbally about the study. Patients who were interested in participating were provided with additional written information about the study and, if still interested in participating, were asked to sign an informed consent form. If patients were unable to sign the consent form for themselves, their next of kin or their carer was asked to sign on their behalf. Recruited patients were randomly assigned to one of two groups: the intervention group and the usual care (control group). Both groups were matched as closely as possible for the following parameters: severity of COPD (measured by FEV1), age, gender and other concomitant illness. The randomization was carried out using the minimization method described by Gore [17]. The software used in the minimization process can be accessed on: http://www-users.york.ac.uk/~mb55/guide/minim.htm[18].
Baseline measurements and assessments
Baseline measurements were performed by the research pharmacist. All patients who agreed to take part in the study were interviewed using a custom-designed questionnaire, prepared and developed for this study based on previous work [19]. The questionnaire was used to collect data on prescribed and nonprescribed medications (name, doses and frequencies), demographic information (marital status, living arrangements and education) and healthcare utilization, i.e. emergency department (ED) visits, GP visits and hospital admissions. The patients were also asked to self-complete a range of questionnaires as follows: Morisky adherence scale [20], COPD knowledge questionnaire [21], and disease-specific health-related quality of life (SGRQ) [15, 16]. Smoking was treated as a secondary exploratory end-point, as only approximately 20% of the patients were current smokers.
Patients who had difficulty self-completing questionnaires, e.g. forgot reading glasses, had the questionnaires read to them. If this occurred, a strict protocol was followed, i.e. the questions were read to the patients and their answers sought without any interpretation of the questions being given. This helped minimize potential bias related to the fact that, for operational reasons, the researcher could not be blinded to the group to which the patient belonged.
In addition to data collected by questionnaire, patients' charts and computerized hospital records were consulted to obtain information on: ED visits within the last year, hospital admissions within the last year, FEV1, medication and medication regimen, body weight and other concomitant illness.
Follow-up assessments
The assessments (except for demographic data) were repeated at 6 and 12 months. FEV1 was measured only at baseline and at 12 months.
Study questionnaires
St George Respiratory Questionnaire
The SGRQ is a supervised, self-administered measure, designed specifically for patients with chronic airways disease. The SGRQ has been shown to be a valid measure of health impairment in patients with chronic airflow limitation and to be responsive to change as a result of therapy [16, 22]. It is a 76-item survey from which scores are calculated for three components: symptoms, activity and impact. The total score calculated for all items provides a global view of the patient's respiratory health. The scoring range for each component is from 0 to 100, with the highest scores indicating the poorest level of health. Researchers have determined that a clinically significant threshold score can be determined for the SGRQ [23]. They used criteria for clinical significance that were not based upon patients' or clinician judgment, and this was the first true test of the validity of the clinically significant threshold. A change in the mean total score of 4 units has been validated as a clinically significant threshold [24].
Self-reported adherence (Morisky scale)
The Morisky adherence scale, which measures adherence through four Yes/No response items, reflects the number of ways medication omission can occur: forgetting, carelessness, stopping when feeling better and stopping when feeling worse. On scoring of the questionnaire, each ‘yes’ response is given a score of 1 and each ‘no’ response is given a score of 0. Adherence scores can therefore range between 0 and 4. In the present study, two adherence classifications were used, i.e. high adherence (scores of 0–1) and low adherence (scores of 2–4). The Morisky self-report adherence scale has been shown to have good validity (α reliability = 0.61) [20].
COPD knowledge questionnaire
This instrument was developed by Scherer et al. [21] to assess the effectiveness of patient education in helping persons with COPD. The COPD knowledge scale consists of 16 true/false items relating to pathophysiology of COPD, breathing and exercise, energy conservation, medications, relaxation and stress control. Correct responses are scored 1 and incorrect responses are scored 0, with unsure responses receiving no score. The range of possible scores is 0–16; the higher the score, the greater the knowledge level. The instrument has been shown to be valid, with a Cronbach α for internal consistency of 0.89 [21].
Pharmacist intervention
Preliminary assessment
The intervention programme included a preliminary assessment for intervention patients to determine their individual needs. Some extra data were collected only in the intervention patients as part of this preliminary assessment, i.e. self-efficacy and exercise habits. These data, together with pertinent data from the baseline questionnaires, were forwarded by the researcher to the clinical pharmacist. This included data on:
disease knowledge
smoking status
medication adherence
self-efficacy in managing breathing difficulty
exercise and diet habit.
In the light of the above information, the interventions were individualized and tailored for each patient by the clinical pharmacist.
Intervention
The intervention in the present research was a complex intervention, as described by the Medical Research Council [25]. It was delivered by one clinical pharmacist in all cases. After the baseline assessment had been performed by the researcher, discussions between the clinical pharmacist and the consultant regarding drug therapy were initiated if rationalization of a dosage regimen was deemed appropriate. Intervention patients were educated individually by the clinical pharmacist (in a structured fashion) on COPD, their prescribed medication, the importance of adherence, inhaler technique (written information was provided) and the management of COPD symptoms. The clinical pharmacist ensured that the patient knew the indications and doses of each medicine, and was able and willing to use the inhaler devices prescribed. The clinical pharmacist also discussed with the intervention patients the importance of simple exercises that patients can do at home (e.g. upper and lower limb exercises and relaxation techniques) [26], symptom control (pursed lip technique) and the technique for expectoration (huff and puff technique) [27]. The pharmacist demonstrated these techniques and then asked the patients to carry out the techniques to ensure that they fully understood how to perform them. A booklet on these techniques [28] was prepared to assist in the education session and the patients were given a copy to take home with them. Advice, using the motivational interviewing technique, was provided to the patients who still smoked and referral to a special smoking cessation programme run within the hospital was made. A customized action plan for acute exacerbations, including advice to GPs to provide a prescription for an antibiotic (amoxicillin/clavulanic acid) and an oral corticosteroid to be initiated promptly by patients for exacerbations, was developed for each patient. The clinical pharmacist through motivational interviewing attempted to increase the intervention patients' self-efficacy to manage or avoid breathing difficulty while participating in certain activities. The interventions were tailored according to the preliminary assessment, i.e. checklists of patients' needs were prepared by the research pharmacist and forwarded to the clinical pharmacist to discuss with the patients. The initial intervention lasted for approximately 1 h for nonsmoker patients and slightly longer for patients who currently smoked.
At each outpatient clinic visit (every 6 months arranged by the hospital consultant), intervention group patients received reinforcement of the education on COPD and its treatment from the clinical pharmacist. In addition, follow-up telephone calls by the clinical pharmacist to reinforce the education and motivate the patients to achieve their goals were made at 3 and 9 months, i.e. between outpatient clinic appointments.
Control patients
Control patients received usual hospital outpatient care from medical and nursing staff, but did not receive the structured intervention by the clinical pharmacist referred to above.
Data analysis
Statistical analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). A per-protocol analysis was used. All tests of significance were two-sided. Both standard deviation and confidence intervals (CIs) were used for normally distributed data, and median and interquartile ranges for non-normally distributed data. A comparison of the proportions of hospital admissions or ED and GP visits was carried out using the χ2 test. For SGRQ scores, differences between study groups and 95% CIs were assessed using Student's t-test. For disease knowledge the nonparametric Mann–Whitney U-test was used. A P-value <0.05 was considered statistically significant (two-sided test) in all cases.
Results
During the study period, 173 COPD patients (86 intervention, 87 control) were recruited from the outpatient clinic for inclusion in the study. During the study period, three patients from the intervention group and five from the control group died and a total of 22 patients withdrew from the study; 12 patients from the intervention group and 10 from the control group (Figure 1). Therefore, the total number of patients lost to follow-up over the complete study was 30, leaving 72 patients in the control group and 71 participants in the intervention group at the end of the study.
Figure 1.
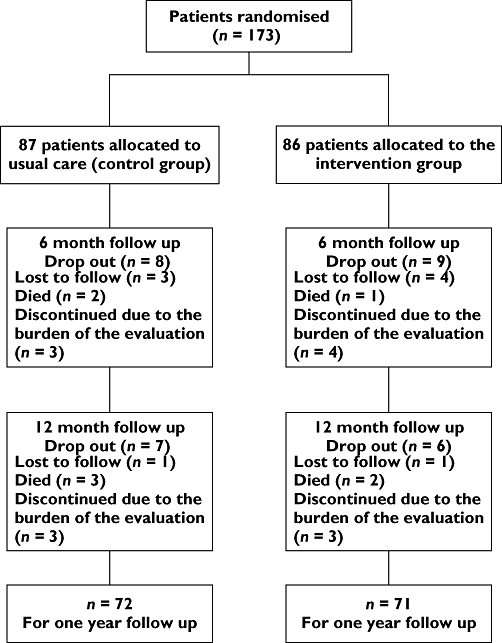
Flow chart indicating patient numbers at different stages of the study
Baseline characteristics were similar across sociodemographic, clinical and functional variables. Most patients were elderly, not highly educated and had moderate to severe COPD reflected by a mean FEV1 of approximately 50% of the predicted normal value. About 50% of participants reported having comorbidities, which included arthritis, osteoporosis, diabetes and various cardiac conditions. The use of respiratory medications was similar between the study groups. The remaining patients were ex-smokers; however, 21.8% of participants in the control group and 20.9% of the intervention group still smoked. There were no differences in the disease severity (FEV1) or health service utilization (ED visits and hospital admissions in the last year) between the two study groups at the baseline assessment point (P > 0.05; Table 1).
Table 1.
Baseline characteristics of study patients
| Control group | Intervention group | ||
|---|---|---|---|
| Characteristics | (n= 87) | (n= 86) | P-value |
| Age (mean, SD, year) | 67.3 (9.2) | 65.63 (10.1) | 0.20* |
| Female % | 56.3 | 55.8 | 0.91† |
| FEV1 (mean, SD), | |||
| l | 1.1 (0.50) | 0.95 (0.48) | 0.21* |
| % predicted | 52 (17.8) | 52.0 (15.9) | |
| FEV1/ FVC | 57 (10.5) | 56.3 (9.50) | |
| Education, n (%) | |||
| Primary | 25 (28.7) | 24 (27.9) | 0.86† |
| Secondary/tertiary | 62 (71.3) | 62 (72.1) | |
| Smoking status, n (%) | 0.09† | ||
| Ex-smokers | 53 (60.9) | 60 (69.7) | |
| Current smokers | 19 (21.8) | 18 (20.9) | |
| Never smoked | 15 (17.2) | 8 (9.3) | |
| Occupational level, n (%) | 0.01† | ||
| Low | 60 (69.0) | 50 (58.1) | |
| Moderate | 12 (13.8) | 26 (30.2) | |
| High | 15 (17.2) | 10 (11.6) | |
| No. of medications (mean, SD) | 8.0 (3.8) | 8.3 (2.9) | 0.57* |
| Years of diagnosis (mean, SD) | 6.4 (4.2) | 6.5 (5.0) | 0.89* |
| Medications at baseline, n (%) | 0.58† | ||
| Short-acting β2-agonist | 81 (93.1) | 82 (95.3) | |
| Long-acting β2-agonist | 68 (78.2) | 69 (81.2) | |
| Long acting anticholinergic | 64 (73.6) | 66 (76.7) | |
| Inhaled steroids | 60 (69.0) | 54 (62.8) | |
| Oral steroids | 9 (10.3) | 7 (8.1) | |
| Comorbid conditions, n (%) | 44 (50.5) | 41 (47.7) | 0.62† |
| No. ED visits (last year) | 79 | 75 | 0.53† |
| No. hospital admissions (last year) | 66 | 61 | 0.59† |
t-test.
χ2 test. FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Health resources utilization
At the 6-month follow-up, patients in the intervention group had a significant reduction in both hospital admissions (P= 0.01) and ED visits (P= 0.02) for acute exacerbation of COPD. Unscheduled GP visits were significantly higher in the usual care group (P= 0.01) over the initial 6-month follow-up period (Table 2). This desired trend in outcomes was also evident at the end of the 1-year follow-up period. The intervention group had a significant reduction in both hospital admissions (P= 0.01) and ED visits (P= 0.02) for acute exacerbations of COPD. The overall length of hospital stay was significantly lower in the intervention group (Table 3). There was evidence of increased frequency of COPD exacerbations in patients with increased disease severity in both the intervention and control groups (Table 4).
Table 2.
Hospital admission, emergency department (ED) visits and general practitioner (GP) visits during the initial 6-month follow-up period
| Control group | Intervention group | ||
|---|---|---|---|
| Variable | n= 79 | n= 77 | P-value |
| GP visit (scheduled) | 158 | 188 | 0.52* |
| GP visit (unscheduled) | 89 | 53 | 0.01* |
| ED visit for COPD | 42 | 20 | 0.02† |
| Hospital admission for acute exacerbation | 34 | 15 | 0.01† |
| Hospital days total | 237 | 79 | 0.03* |
Mann–Whitney test.
χ2 test. COPD, chronic obstructive pulmonary disease.
Table 3.
Emergency department (ED) visits and hospital admissions during the 1-year study period
| Control group | Intervention group | ||
|---|---|---|---|
| Variable | n= 72 | n= 71 | P-value† |
| ED visits for exacerbation during 1-year follow-up | 80 | 40 | 0.02 |
| Hospital admissions for acute exacerbation during 1-year follow-up | 64 | 26 | 0.01 |
| Total of hospital days during 1-year follow-up | 466 | 164 | 0.02* |
Mann–Whitney test.
χ2 test.
Table 4.
Health resource utilization over the 12-month period by patients who completed the study (according to their disease severity)
| GP visit (unscheduled) | ED visits | Hospital admission | |
|---|---|---|---|
| Control group (n= 72) | |||
| Mild (n= 11) | 5 (45.5%) | 4 (36.4%) | 3 (27.3%) |
| Moderate (n= 34) | 23 (67.6%) | 19 (55.9%) | 15 (44.1%) |
| Severe (n= 27) | 19 (70.4%) | 17 (63.0%) | 14 (51.9%) |
| Intervention group (n= 71) | |||
| Mild (n= 13) | 4 (30.8%) | 4 (30.8%) | 2 (15.4%) |
| Moderate (n= 37) | 12 (32.4%) | 10 (27.0%) | 10 (27.0%) |
| Severe (n= 21) | 12 (57.1%) | 11 (52.4%) | 6 (28.6%) |
GP, general practitioner; ED, emergency department.
Health-related quality of life-disease specific (SGRQ)
The HRQoL scores using the SGRQ were comparable between control and intervention groups (subscales and total scores) at the baseline assessment (P > 0.05; Table 5). Symptoms and impact subscales and total scores were significantly improved at the 6-month follow-up point in the intervention group. The differences between the intervention and control groups reached statistical significance on the symptom domain (P= 0.01), impact domain (P= 01) and total subscale (P= 0.04), the latter also reaching clinically significant improvement in more than four units. The lowest improvement was noted in the physical activity subscale (Table 5). At the12-month assessment, the differences between intervention and control groups in the symptoms (P= 0.04) and impact (P= 0.03) subscales remained statistically significant. The differences in activity (P= 0.51) and total (P= 0.17) scores, however, were not statistically significant (Table 5). Furthermore, the total score at 12 months failed to reach the clinically significant threshold of four units improvement.
Table 5.
St George's Respiratory Questionnaire (SGRQ) scores at baseline, 6- and 12-month assessment points
| Control group | Intervention group | Unit differences | ||
|---|---|---|---|---|
| SGRQ | Mean (95% CI) | Mean (95% CI) | (95% CI) | P-value* |
| Baseline | ||||
| Symptoms | 69.6 (65.1, 74.0) | 68.8 (64.3, 73.3) | −0.7 (−6.9, 5.5) | 0.81 |
| Activity | 75.2 (70.6, 79.8) | 73.9 (68.8, 77.9) | −1.2 (−7.4, 4.6) | 0.65 |
| Impact | 56.9 (52.6, 61.3) | 55.1 (50.8, 59.0) | −1.8 (−7.8, 3.8) | 0.57 |
| Total | 64.2 (60.5, 67.9) | 63.6 (59.8, 66.6) | −0.9 (−6.0, 4.0) | 0.69 |
| Six-month assessment point | ||||
| Symptoms | 71.1 (66.8, 75.4) | 63.5 (58.8, 68.2) | −7.8 (−14.3, 1.0) | 0.01 |
| Activity | 71.6 (66.9, 76.1) | 69.6 (65.6, 73.4) | −1.8 (−7.8, 4.2) | 0.53 |
| Impact | 58.4 (53.9, 62.8) | 50.5 (46.4, 54.6) | −7.5 (−13, 1.5) | 0.01 |
| Total | 64.2 (60.8, 68.2) | 59.2 (55.6, 62.8) | −5.2 (−10, 1.0) | 0.04 |
| Twelve-month assessment point | ||||
| Symptoms | 72.0 (66.6, 77.5) | 65.1 (59.7, 70.5) | −7.5 (−14.1, 0.1) | 0.04 |
| Activity | 76.2 (71.9, 80.4) | 74.5 (71.0, 77.9) | −1.8 (−7.0, 3.0) | 0.51 |
| Impact | 57.1 (52.1, 62.1) | 50.4 (45.6, 55.2) | −7.4 (−14, 0.6) | 0.03 |
| Total | 65.3 (61.0, 69.6) | 61.8 (57.9, 65.6) | −3.8 (−9.5, 2.0) | 0.17 |
t-test.
Forced expiratory volume in 1 s
FEV1 was recorded at baseline and the 12-month assessment point for both patient groups. Statistical analysis revealed no significant differences between the two groups at baseline and the 12-month assessment point (P= 0.68 and 0.13, respectively; Table 6).
Table 6.
BMI and FEV1 values for intervention and control group patients at each assessment point
| Control group | Intervention group | |||||
|---|---|---|---|---|---|---|
| Variables | Time | n | Mean (95%CI) | n | Mean (95%CI) | P* |
| FEV1 (l) | Baseline | 85 | 1.12 (0.98, 1.21) | 86 | 1.15 (0.99, 1.23) | 0.68 |
| 12 months | 72 | 1.05 (0.94, 1.17) | 71 | 1.19 (1.05, 1.31) | 0.13 | |
| BMI | Baseline | 85 | 27.4 (26.4, 29.0) | 86 | 28.7 (27.5, 30.0) | 0.12 |
| 6 months | 79 | 28.4 (27.2, 29.7) | 76 | 26.8 (25.6, 28.0) | 0.08 | |
| 12 months | 72 | 27.1 (25.8, 28.2) | 71 | 28.4 (27.3, 29.8) | 0.09 | |
t-test. BMI, body mass index; FEV1, forced expiratory volume in 1 s.
Body mass index
Body weight was recorded at baseline and at 6 and 12 months to calculate body mass index (BMI). There were only slight variations in the BMI values throughout the study with no clear patterns between the two groups (Table 6). There were no significant differences in BMI values between the two groups at any assessment point.
Adherence to prescribed medication
At the baseline assessment the number of patients in the intervention group and the control group who exhibited low adherence to their prescribed medications was approximately the same (P= 0.84). At the 6- and 12-month follow-up points, a higher proportion of patients in the intervention group exhibited high adherence scores (0–1) than control group patients (81% vs. 63% and 77.8% vs. 60%, respectively; Figure 2).
Figure 2.
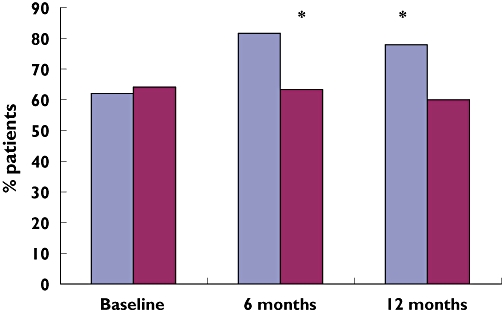
Percentage of patients who reported high adherence in intervention and control group at each assessment point. (*P= 0.019). Intervention ( ); Control (
); Control ( )
)
Knowledge of medication and disease management
The patients had fair knowledge about their medication and disease management at baseline, as measured by the COPD knowledge questionnaire. There was no significant difference in the median scores between the intervention and control groups (P= 0.32). Overall, patients scored less well in the questions ‘pursed lip breathing helps prevent collapsing of small airways’ and ‘in diaphragmatic breathing, your abdomen should pull in during inhalation’. Patient knowledge scores in the intervention group were higher at 6 and 12 months (P < 0.001). The knowledge scores improved in the intervention group, whereas there was evidence of deterioration in the control patient group (Table 7).
Table 7.
Knowledge scores for intervention and control group patients at each assessment point
| Control | Intervention | ||||
|---|---|---|---|---|---|
| Time | n | Median (IQR) | n | Median (IQR) | P* |
| Baseline | 87 | 65.5 (31.0) | 86 | 68.2 (31.0) | 0.32 |
| 6 months | 79 | 62.5 (31.0) | 77 | 81.2 (20.5) | 0.001 |
| 12 months | 72 | 59.3 (33.0) | 71 | 75.0 (32.0) | 0.001 |
Mann–Whitney U-test. IQR, interquartile range.
Using χ2 testing, no significant differences in stage of change status in relation to smoking was found at any assessment point, i.e. baseline, 6-month and 12-month follow-up points, between intervention and control current smoker patients (Table 8). However, there was 22.2% self-reported abstinence in the intervention group smokers at the 6-month follow-up. This was maintained at the 12-month follow-up and compared favourably with 5.3% and 10.5% in the control group smokers, respectively. Moreover, 22.2% of pre-contemplator intervention group smokers progressed to the next stage compared with 5.3% in the control group smokers. The results did not, however, reach statistical significance (P > 0.05).
Table 8.
Stages of change of smokers in the intervention and control group at each assessment point
| Control (n= 19) | Intervention (n= 18) | ||||
|---|---|---|---|---|---|
| Stage of changes | n | (%) | n | (%) | P* |
| Baseline | 0.63 | ||||
| Pre-contemplation | 8 | (42.1) | 7 | (38.9) | |
| Contemplation | 8 | (42.1) | 5 | (27.8) | |
| Preparation | 2 | (10.5) | 4 | (22.2) | |
| Action | 1 | (5.3) | 2 | (11.1) | |
| Maintenance | 0 | (0) | 0 | (0) | |
| Six-month follow-up | |||||
| Pre-contemplation | 7 | (36.8) | 3 | (16.7) | 0.28 |
| Contemplation | 7 | (36.8) | 4 | (22.2) | |
| Preparation | 2 | (10.5) | 4 | (22.2) | |
| Action | 2 | (10.5) | 3 | (16.7) | |
| Maintenance | 1 | (5.3) | 4 | (22.2) | |
| Twelve-month follow-up | |||||
| Pre-contemplation | 6 | (31.6) | 3 | (16.7) | 0.27 |
| Contemplation | 8 | (42.1) | 4 | (22.2) | |
| Preparation | 1 | (5.3) | 5 | (27.8) | |
| Action | 2 | (10.5) | 2 | (11.1) | |
| Maintenance | 2 | (10.5) | 4 | (22.2) | |
χ2 test.
Discussion
Although this is one of the largest single-centre studies involving COPD patients yet performed, the recruitment sample fell slightly short of the target sample size of 90 patients in each group. The study population (n= 173) was recruited over a period of approximately 7 months. All intervention approaches (e.g. COPD education booklet) and data collection instruments were found to be fit for purpose and presented no major difficulties during the research study. The action plan and booklets were received positively by patients; they all found that they helped with the introduction of disease management skills. Analysis of the results revealed that there was a trend for the outcomes for intervention patients to be improved overall when compared with control patients over the 12-month study period.
Health resource utilization
An important finding in the present study was the 50% reduction in ED visits, the 59% reduction in hospital admissions and the 39% reduction in unscheduled GP visits in the intervention group patients when compared with the control group during the 1-year follow-up. As a greater percentage of patients with moderate and severe disease were admitted to hospital, such patients should be targeted for the intervention.
Four studies [13, 29–31] have reported a reduction in emergency visits and hospital admissions as a result of educational and self-management interventions. Bourbeau et al. reported a 40% reduction in hospital admissions for COPD exacerbations and a 41% reduction in ED visits [13]. Tinkelman et al.[32] developed a telephonic disease management educational intervention, with availability 24 h per day, 7 days a week case management and a series of regularly scheduled follow-up calls for 349 COPD patients [32]. They reported significantly lower ED visits (57%), hospital admissions (53%), and unscheduled GP visits (67%) as a result of their programme. They also found a significant decline in utilization of healthcare facilities across all degrees of disease severity, but the education had the greatest impact on those with moderate to severe disease.
A randomized control study involving 62 COPD patients evaluated an intervention that consisted of educational group sessions administered by a trained nurse [30]. The patients allocated to the educational programme had GP visits reduced by 73% compared with the control group. In the present study the intervention group had slightly higher numbers of scheduled GP visits compared with the control group patients. Although not statistically significant, this probably reflected the effect of the intervention programme, which motivated the patients to seek the support of their GP in their disease management.
Other types of intervention have been successful in COPD management. The Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT) trial [33] was a 4-year multicentre (470 sites), multinational (37 countries), randomized, double-blind, placebo-controlled, parallel-group prospective trial involving 5993 COPD patients. Patients were randomized 1 : 1 to receive either 18 µg tiotropium or placebo (control) once daily. In both arms, patients were allowed to use all other prescribed respiratory medications, except for inhaled anticholinergics. UPLIFT showed that tiotropium produced a significant delay in time to first exacerbation by a median of 4.1 months (P < 0.001) vs. control, a significant reduction in the number of exacerbations per patient year (14%; P < 0.001). In addition, it significantly reduced the risk of exacerbations leading to hospitalisations (hazard ratio 0.86; P < 0.002).
An integral part of the comprehensive care recommended for patients with COPD is a pulmonary rehabilitation programme. Hui and Hewitt developed a simple outpatient pulmonary rehabilitation programme [34] that consisted of incremental exercise endurance training, upper- and lower-limb weight training, and endurance activities. The team involved in this programme included a physiotherapist supervised by a respiratory physician. Thirty-six patients completed the study. The number of hospital admissions was reduced significantly when the year before the study was compared with the year after completion of the programme. In addition, the average length of hospital stay was reduced significantly from 7.4 days before the programme to 3.3 days after the programme was completed.
Health-related quality of life (SGRQ)
COPD and the related symptoms greatly affect a patient's ability to perform normal daily activities, therefore affecting their quality of life (GOLD) [35]. Patients in the present study demonstrated a low HRQoL at baseline; however, intervention patients' quality of life improved clinically (four-unit change in SGRQ; P < 0.05) at 6 months when compared with control patients. This improvement diminished over time, i.e. the differences in SGRQ scores remained significant in two (symptom and impact) subscales only at the 12-month assessment (Table 5). This suggests that moving forward the clinical pharmacist intervention needs to be matured to include more robust patient follow-up. The present findings on improved HRQoL in COPD patients in response to self-management programmes have also been demonstrated by others [13, 36]. However, findings in this area are inconsistent; for example, in two studies involving self-management programmes, educational sessions and prompt initiation of an antibiotic and an oral corticosteroid for 10–14 days for an exacerbation with infective symptoms resulted in no significant improvement in SGRQ scores [11, 12]. The baseline SGRQ scores for the participants in the two latter studies were very low (37.2 and 36.8, respectively), indicating generally good health status of the participants. This may have reduced the ability to detect improvements and hence detect differences between groups. Patients in the present study had poor quality of life as measured by the SGRQ (mean score 64.2) and there was therefore a larger margin available for improvement.
FEV1 and BMI
There was very little difference between the two groups with regard to FEV1 and BMI over the study period, which is not surprising as these variables were not expected to be sensitive to the management programme. COPD is a disease characterized by irreversible damage, and hence FEV1 is difficult to change [37]. Malnutrition may be present in 25–70% of COPD patients. Weight loss is an independent prognostic factor for poor survival [38] even in groups of patients who have FEV1 values of >40% predicted. Surprisingly, the BMI values in intervention and control group patients were within the normal range, i.e. the BMI mean values were 28.7 and 27.4, respectively. In general, the results of nutritional interventions in stable COPD patients have been disappointing. Although it has been shown that effective interventions can result in weight gain and associated improvement in respiratory function, it seems very difficult to achieve this end-point [39–41].
Knowledge about medications and disease management
At the baseline measurement, patient knowledge of medications in both groups was fairly good. However, there was a lack of knowledge about symptoms management and relaxation techniques. The results of the present study demonstrated that knowledge of medications and of disease management in intervention group patients improved steadily throughout the study period (P= 0.001; Table 7), whereas that of control patients, who received no additional education, remained approximately constant throughout the study. Improved knowledge in COPD patients in response to education interventions has also been reported by others [12, 42].
Adherence to prescribed medications
An improvement in adherence can be directly beneficial to a patient's clinical condition, and medication counselling contributes to improved medication adherence to prescribed regimens [43]. Patient counselling in the present research had an obvious impact on self-reported adherence to prescribed medicines. An early study by Gallefoss and Bakke [44] randomized 78 asthmatics and 62 COPD patients during ordinary outpatient management. The intervention consisted of two 2-h group sessions and one or two individual sessions by a trained nurse and physiotherapist. They found a higher number of adhering subjects (adherence >75%) in asthmatics who received the education intervention. However, patient education did not alter adherence in the COPD group. The structured intervention in the present study, involving the clinical pharmacist detailing the need for individual treatments and demonstrating inhaler technique, was effective, although the adherence results should be treated with caution due to the simple measure used.
Stage of change (smoking cessation)
Of 18 active smokers in the intervention group only four (22.2%) reported abstinence at the 1-year follow-up. The finding of this research is consistent with that of Monninkhof et al. [45]. The participants in the latter study were COPD patients who received three 15–30-min home counselling sessions. After 9 months' follow-up, eight (12.7%) of the patients were abstinent. This was a low rate of success compared with two other behavioural smoking cessation studies [46, 47]. Several reasons might explain this. The smoking interventions in both these latter studies were more intensive and, indeed, the intensity of cessation counselling has a strong dose–response dependent relationship with effectiveness [48]. Another reason could have been the timing of the intervention. In the study of Pederson et al. [46], counselling occurred while the patients were in the hospital. Hospitalization can be an opportune time to initiate smoking interventions because patients can be more motivated to quit by the perceived vulnerability related to hospitalization for their COPD.
Conclusions
The present study was designed to measure the impact of an intervention programme led by a clinical pharmacist on a wide range of clinical and humanistic outcomes in patients with COPD. The results revealed that there was a strong trend for the outcomes in intervention patients to be improved overall when compared with control patients. The evidence suggested a drop off in the effectiveness of the programme over time, highlighting the need for more intensive clinical pharmacist follow-up with the patients. It also highlights areas in which further improvement to the current programme can be made.
Competing interests
None to declare.
The authors wish to thank Dr Chris Cardwell, Department of Epidemiology and Public Health, Queen's University Belfast, for his statistical support and Chest Heart and Stroke (N. Ireland) for financial support.
REFERENCES
- 1.Global Strategy for Diagnosis, Management, and prevention of COPD. Executive Summary. Pocket Guide. Updated 2008). Available at http://www.goldcopd.org (last accessed 1 September 2008.
- 2.National Statistics. Health Statistics Quarterly 30. Available at http://www.statistics.gov.uk/downloads/theme_health/HSQ30.pdf (last accessed 1 September 2008.
- 3.National Collaborating Centre for Chronic Conditions. Chronic obstructive pulmonary disease. National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax. 2004;59(Suppl. 1):1–232. [PMC free article] [PubMed] [Google Scholar]
- 4.European Respiratory Society. European White Lung Book. Brussels: European Respiratory Society; 2003. [Google Scholar]
- 5.Crockett A. Managing Chronic Obstructive Pulmonary Disease in Primary Care. Oxford: Blackwell Science; 2002. [Google Scholar]
- 6.Calverley PJ, Sondhi S. The burden of obstructive lung disease in the UK – COPD and asthma. Thorax. 1998;53(Suppl. 4):A38. [Google Scholar]
- 7.Lorig K, Holman H, Sobel D, Stewart AL, Brown BW, Jr, Holman HR. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care. 1999;37:5–14. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Madge P, McColl J, Paton J. Impact of a nurse-led home management training programme in children admitted to hospital with acute asthma: a randomised controlled study. Thorax. 1997;52:223–8. doi: 10.1136/thx.52.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wesseldine LJ, McCarthy P, Silverman M. Structured discharge procedure for children admitted to hospital with acute asthma: a randomised controlled trial of nursing practice. Archiv Dis Child. 1999;80:110–4. doi: 10.1136/adc.80.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cordina M, McElnay JC, Hughes CM. Assessment of a community pharmacy-based program for patients with asthma. Pharmacotherapy. 2001;21:1196–203. doi: 10.1592/phco.21.15.1196.33894. [DOI] [PubMed] [Google Scholar]
- 11.Monninkhof E, van der Valk P, van der Palen J, van Herwaarden C, Partridge MR, Zielhuis G. Self-management education for patients with chronic obstructive pulmonary disease: a systematic review. Thorax. 2003;58:394–8. doi: 10.1136/thorax.58.5.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGeoch RB, Willsman K, Dowson C, Town G, Frampton C, McCartin F, Cook J, Epton M. Self-management plans in the primary care of patients with chronic obstructive pulmonary disease. Respirology. 2006;11:611–8. doi: 10.1111/j.1440-1843.2006.00892.x. [DOI] [PubMed] [Google Scholar]
- 13.Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupre A, Begin R, Renzi P, Nault D, Borycki E, Schwartzman K, Singh R, Collet JP. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med. 2003;163:585–91. doi: 10.1001/archinte.163.5.585. [DOI] [PubMed] [Google Scholar]
- 14.Watson PB, Town GI, Holbrook N, Dwan C, Toop LJ, Drennan CJ. Evaluation of a self-management plan for chronic obstructive pulmonary disease. Eur Respir J. 1997;10:1267–71. doi: 10.1183/09031936.97.10061267. [DOI] [PubMed] [Google Scholar]
- 15.Jones PW. Quality of life measurement for patients with diseases of the airways. Thorax. 1991;46:676–82. doi: 10.1136/thx.46.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–7. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 17.Gore SM. Assessing clinical trials – restricted randomisation. BMJ. 1981;282:2114–7. doi: 10.1136/bmj.282.6282.2114. Clinical research ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans S, Royston P, Day S. Minim allocation by minimisation in clinical trials. Available at http://www-users.york.ac.uk/~mb55/guide/minim.htm (last accessed 24 September 2006.
- 19.Varma S, McElnay JC, Hughes CM, Passmore AP, Varma M. Pharmaceutical care of patients with congestive heart failure: interventions and outcomes. Pharmacotherapy. 1999;19:860–9. doi: 10.1592/phco.19.10.860.31565. [DOI] [PubMed] [Google Scholar]
- 20.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Scherer YK, Schmieder LE, Shimmel S. The effects of education alone and in combination with pulmonary rehabilitation on self-efficacy in patients with COPD. Rehabil Nurs. 1998;23:71–7. doi: 10.1002/j.2048-7940.1998.tb02133.x. [DOI] [PubMed] [Google Scholar]
- 22.Spencer S, Calverley PM, Sherwood Burge P, Jones PW, ISOLDE Study Group Inhaled steroids in obstructive lung disease. Health status deterioration in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:122–8. doi: 10.1164/ajrccm.163.1.2005009. [DOI] [PubMed] [Google Scholar]
- 23.Osman IM, Godden DJ, Friend JA, Legge JS, Douglas JG. Quality of life and hospital re-admission in patients with chronic obstructive pulmonary disease. Thorax. 1997;52:67–71. doi: 10.1136/thx.52.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J. 2002;19:398–404. doi: 10.1183/09031936.02.00063702. [DOI] [PubMed] [Google Scholar]
- 25.Campbell NC, Murray E, Darbyshire J, Emery J, Farmer A, Griffiths F, Guthrie B, Lester H, Wilson P, Kinmonth AL. Designing and evaluating complex interventions to improve health care. BMJ. 2007;334:455–9. doi: 10.1136/bmj.39108.379965.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lolak S, Connors GL, Sheridan MJ, Wise TN. Effects of progressive muscle relaxation training on anxiety and depression in patients enrolled in an outpatient pulmonary rehabilitation program. Psychother Psychosom. 2008;77:119–25. doi: 10.1159/000112889. [DOI] [PubMed] [Google Scholar]
- 27.Ambrosino N, Di Giorgio M, Di Paco A. Strategies to improve breathlessness and exercise tolerance in chronic obstructive pulmonary disease. Respir Med. 2006;2:2–8. COPD Update. [Google Scholar]
- 28.Treasure J. Motivational interviewing. Adv Psychiatr Treat. 2004;10:331–7. [Google Scholar]
- 29.Solomon DK, Portner TS, Bass GE, Gourley DR, Gourley GA, Holt JM, Wicke WR, Braden RL, Eberle TN, Self TH, Lawrence BL. Clinical and economic outcomes in the hypertension and COPD arms of a multicenter outcomes study. J Am Pharm Assoc. 1998;38:574–85. doi: 10.1016/s1086-5802(16)30371-0. [DOI] [PubMed] [Google Scholar]
- 30.Gallefoss F, Bakke PS. Impact of patient education and self-management on morbidity in asthmatics and patients with chronic obstructive pulmonary disease. Respir Med. 2000;94:279–87. doi: 10.1053/rmed.1999.0749. [DOI] [PubMed] [Google Scholar]
- 31.Tougaard L, Krone T, Sorknaes A, Ellegaard H. Economic benefits of teaching patients with chronic obstructive pulmonary disease about their illness. The PASTMA group. Lancet. 1992;339:1517–20. doi: 10.1016/0140-6736(92)91274-c. [DOI] [PubMed] [Google Scholar]
- 32.Tinkelman D, Corsello P, McClure D, Yin M. One year outcomes from a disease management program for COPD. Dis Manage Health Outcomes. 2003;11:49–59. [Google Scholar]
- 33.Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S Decramer M for the UPLIFT Study Investigators. A 4-Year Trial of Tiotropium in Chronic Obstructive Pulmonary Disease. N Eng J Med. 2008;359:1543–4. [Google Scholar]
- 34.Hui KP, Hewitt AB. A simple pulmonary rehabilitation programme improves health outcomes and reduces hospital utilisation in patients with COPD. Chest. 2003;124:94–7. doi: 10.1378/chest.124.1.94. [DOI] [PubMed] [Google Scholar]
- 35.GOLD. Guidelines for chronic obstructive pulmonary disease treatment and issues of implementation. Proc Am Thorac Soc. 2006;3:641–4. doi: 10.1513/pats.200604-099SS. [DOI] [PubMed] [Google Scholar]
- 36.Gallefoss F, Bakke PS, Rsgaard PK. Quality of life assessment after patient education in a randomized controlled study on asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:812–7. doi: 10.1164/ajrccm.159.3.9804047. [DOI] [PubMed] [Google Scholar]
- 37.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ. 1977;1:1645–8. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson D, Rogers R, Sanders M, Anthonison N. Body weight in chronic obstructive pulmonary disease: the National Institute of Health Intermittent Positive Pressure Breathing Trial. Am Rev Respir Dis. 1989;139:1435–38. doi: 10.1164/ajrccm/139.6.1435. [DOI] [PubMed] [Google Scholar]
- 39.Schols A, Soeters P, Dingemans A, Mostert R, Frantzen P, Wouters E. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis. 1993;147:1151–6. doi: 10.1164/ajrccm/147.5.1151. [DOI] [PubMed] [Google Scholar]
- 40.Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1791–7. doi: 10.1164/ajrccm.157.6.9705017. [DOI] [PubMed] [Google Scholar]
- 41.Donahoe M, Mancino J, Costantino J, Lebow H, Rogers RM. The effect of an aggressive nutritional support regimen on body composition in patients with severe COPD and weight loss. Am J Respir Crit Care Med. 1994;149:A313. [Google Scholar]
- 42.Hesselink AE, Penninx BW, van der Windt DA, van Duin BJ, de Vries P, Twisk JW, Bouter LM, van Eijk JT. Effectiveness of an education programme by a general practice assistant for asthma and COPD patients: results from a randomised controlled trial. Patient Educ Couns. 2004;55:121–8. doi: 10.1016/j.pec.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Goodyer LI, Miskelly F, Milligan P. Does encouraging good compliance improve patients' clinical condition in heart failure? Br J Clin Pract. 1995;49:173–6. [PubMed] [Google Scholar]
- 44.Gallefoss F, Bakke PS. How does patient education and self-management among asthmatics and patients with chronic obstructive pulmonary disease affect medication? Am J Respir Crit Care Med. 1999;160:2000–5. doi: 10.1164/ajrccm.160.6.9901028. [DOI] [PubMed] [Google Scholar]
- 45.Monninkhof E, van der Valk P, van der Palen J, Mulder H, Pieterse M, van Herwaarden C, Zielhuis G. The effect of a minimal contact smoking cessation programme in out-patients with chronic obstructive pulmonary disease: a pre-post-test study. Patient Educ Couns. 2004;52:231–6. doi: 10.1016/S0738-3991(03)00096-X. [DOI] [PubMed] [Google Scholar]
- 46.Pederson LL, Wanklin JM, Lefcoe NM. The effects of counseling on smoking cessation among patients hospitalized with chronic obstructive pulmonary disease: a randomized clinical trial. Int J Addict. 1991;26:107–19. doi: 10.3109/10826089109056242. [DOI] [PubMed] [Google Scholar]
- 47.Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, Conway WA, Jr, Enright PL, Kanner RE, O'Hara P. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1 the lung health study. JAMA. 1994;272:1497–505. [PubMed] [Google Scholar]
- 48.Fiore MC. Treating tobacco use and dependence: an introduction to the US public health service clinical practice guideline. Respir Care. 2000;45:1196–9. [PubMed] [Google Scholar]


