Abstract
AIMS
To assess the impact of the UK Medicines and Healthcare products Regulatory Authority (MHRA) warning in December 2003 not to prescribe selective serotonin reuptake inhibitor (SSRI) antidepressants, except fluoxetine, to under-18-year-olds.
METHODS
Interrupted time series analysis of prescriptions (UK) and general hospital presentations for nonfatal self-poisoning (three centres in England) for 2000–2006.
RESULTS
Following the MHRA warning in December 2003 there were significant decreases in prescribing of SSRI antidepressants (conservative estimate 51%) to young people aged 12–19 years. Surprisingly, this decrease also affected fluoxetine (conservative estimate 20%) and tricyclics (conservative estimate 27%). Nonfatal self-poisoning in this age group following the warning also declined significantly for SSRIs (conservative estimate 44%), but not for fluoxetine, tricyclic antidepressants, or all drugs and other substances. Rates of nonfatal self-harm did not change significantly over the study period.
CONCLUSIONS
The reduction in both prescribing and self-poisoning with SSRI antidepressants (except fluoxetine) following the MHRA warning is in keeping with reduced availability of these drugs. There was some evidence of substitution from other SSRIs to fluoxetine for use in self-poisoning. Importantly, overall rates of nonfatal self-harm and self-poisoning did not change, indicating no substitution of method or increases in self-injury.
Keywords: adolescents, selective serotonin reuptake inhibitors, self-poisoning, time trends
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Ecological studies have shown conflicting evidence in relation to associations between trends in selective serotonin reuptake inhibitor (SSRI) prescription rates and suicide rates in adolescents.
After regulatory warnings in the UK against SSRI use in children and adolescents, prescribing of antidepressants in general declined in this group; there were no related changes in rates of suicide or hospital admissions for self-harm.
WHAT THIS STUDY ADDS
Based on all presentations to general hospitals, nonfatal self-poisoning with SSRI antidepressants (but not fluoxetine) declined in 12–19-year-olds in three centres in England in line with UK prescribing trends.
There was some evidence of a possible small substitution effect from use of other SSRIs for nonfatal self-poisoning to use of fluoxetine
Overall rates of nonfatal self-harm in 12–19-year-olds in three centres in England were stable, indicating no major substitution of method to self-injury or overall adverse impact of the Medicines and Healthcare products Regulatory Authority warning.
Introduction
In recent years attention has focused on possible risks of induction of suicidality relating to selective serotonin reuptake inhibitor (SSRI) antidepressant use, particularly in children and adolescents. In June 2003 the UK Medicines and Healthcare products Regulatory Authority (MHRA) first advised against the use of paroxetine in young people following a review of clinical trial data by the Committee for Safety of Medicines (CSM) indicating that paroxetine was not efficacious in the treatment of depressive illness in this age group, and that the risk of self-harm and potentially suicidal behaviour was 1.5–3.2 times that of placebo [1]. In December 2003 the MHRA and CSM further advised against use of all SSRI antidepressants, except fluoxetine, for the treatment of major depressive disorder in under-18-year-olds because of absence of evidence of effectiveness from placebo-controlled trials, and some indication of increases in suicidal ideation and nonfatal self-harm associated with all SSRIs except fluoxetine [2]. This was soon followed by a ‘black box’ warning from the US Food and Drug Administration (FDA). An updated review by the FDA in 2006 including results of unpublished trials showed a twofold increased risk of suicidal ideation in youths prescribed SSRIs compared with placebo [3]. The UK National Institute for Health and Clinical Excellence (NICE) also issued advice in September 2005 that antidepressants should not be used in the treatment of moderate to severe depression in young people except in conjunction with psychological treatment, and not in mild depression [4].
Investigations have shown that, in general, the regulatory warnings were associated with reductions in the prescribing of all antidepressants [5], but especially SSRIs, to children and adolescents in the Netherlands [6], the USA [7] and Australia [8].
Investigations of the relationship between antidepressant prescribing and suicide in adolescents have generated conflicting results. Using ecological data for the USA, Olfson et al. [9] found an inverse relationship between regional changes in antidepressant prescribing and suicide rates. Likewise, a panel data analysis of SSRI sales in 27 countries indicated that overall suicide rates fell fastest in countries with the greatest growth in SSRI sales, although the finding was indeterminate for under-15-year-olds [10]. Recent studies in the USA and the Netherlands have suggested substantial increases in suicide rates associated with recent reductions in prescribing of SSRIs in 5–19-year-olds [11]. However, this was not found for either suicide or hospital admissions for nonfatal self-harm in England and Wales [12].
We have investigated changes in prescribing trends following the MHRA warning in December 2003 against use of SSRIs (except fluoxetine) in under-18-year-olds, and used data from the Multicentre Monitoring of Self-harm Project [13] to investigate the impact this has had on rates of self-poisoning in young people. We examined all presentations for self-poisoning rather than admissions alone, as many patients who present to the Emergency Department (ED) with self-harm are not admitted to a general hospital bed [12, 14], and this proportion has probably increased following the 4-h maximum stay rule in EDs in the UK [15]. We considered three groups of drugs: SSRIs (except fluoxetine); fluoxetine alone; and tricyclic antidepressants. We included the tricyclics as a comparison group as these drugs were not covered by the MHRA warning. Our hypotheses were that: (i) rates of prescribing of and self-poisoning with SSRIs (except fluoxetine) would decrease following the MHRA warning; (ii) rates of prescribing of and self-poisoning with fluoxetine would increase following the MHRA warning; and (iii) rates of prescribing of and self-poisoning with tricyclics would be unaffected by the MHRA warning.
Methods
The three drug groups investigated were: (i) SSRI antidepressants (except fluoxetine), which included citalopram, escitalopram, fluvoxamine, paroxetine, and sertraline; (ii) fluoxetine alone; and (iii) tricyclic antidepressants, which included amitriptyline, clomipramine, dosulepin, doxepin, imipramine, lofepramine, nortriptyline and trimipramine.
Prescriptions
Data on antidepressant prescriptions dispensed in the community in the form of quarterly estimates for years 2000–2006 for the UK were obtained from the Medical Data Index and supplied by IMS Health Inc. (Norwalk, CT, USA) [16]. Data for the 12–19 years age group were used, the nearest available to the under-18-years age group of interest in this study. Mid-year population estimates for the UK by single year of age for 12–19-year-olds for years 2000–2006 were obtained from the Office for National Statistics (ONS; Newport, UK) [17]. Rates of prescribing were calculated per 100 000 population.
Self-poisonings and self-harm
Self-poisoning data for 12–19-year-olds came from three centres currently involved in the Multicentre Monitoring of Self-harm project (see [13] for a description of the first phase of this project). Data were collected on all patients who presented with self-harm to EDs at general hospitals in Oxford (one hospital), Manchester (three hospitals) and Derby (two hospitals) for the 7-year period 1 January 2000 to 31 December 2006. (See Appendix A for a description of self-harm registers in the three centres.)
Self-harm is defined as intentional self-poisoning or self-injury, irrespective of motivation [18]. Self-poisoning includes the intentional ingestion of more than the prescribed amount of any drug, or other non-ingestible substance, whether or not there is evidence that the act was intended to result in death. Data collected included sex, age, date of self-harm, and method of self-harm, including which drug(s) were ingested in self-poisoning. For this time trend study, aggregated data on all episodes of self-poisoning were included and proportions calculated of those where the patient ingested one of the antidepressants of interest, either alone or in combination with other substances, or in combination with self-injury.
During the 7-year study period there were 7854 episodes of self-harm by 5762 individual persons aged 12–19 years in the three centres combined. More than three-quarters of the young people (78.5%, n= 4521) presented with self-harm on one occasion only. Our analyses were based on all episodes in the study period rather than all persons, as the main focus of the study was the availability of specific drugs and their use for self-poisoning, rather than the personal or clinical characteristics of young people taking the drugs.
Rates of self-harm
Rates per 100 000 (based on person-years) were calculated for each centre. Numerators were calculated by identifying the first episode in each year for each person; denominators were populations of 12–19-year-olds in the catchment areas for each centre taken from mid-year population estimates for years 2000–2006 provided by the ONS [17]. Rates in Manchester were adjusted by a factor of 1.42 for the period first quarter 2000 to third quarter 2002 to account for missing data on non-assessed patients [13]. (See Appendix A for description of centres and catchment areas.) Trends in rates of self-harm over the period 2000–2006 were estimated using the first two terms of the linear regression model in Equation A1 (Appendix B).
Statistical analyses
Prescription rates (number of prescriptions per 100 000 population) for 12–19-year-olds for each quarter 2000–2006 were calculated for each drug group from UK prescription and population data. Percentages of all self-poisoning episodes for each quarter 2000–2006 were calculated for the three drug groups using data from the three self-harm monitoring centres. The denominator was the total number of episodes of self-poisoning, and the numerator was the number of episodes using the drug(s) of interest. Percentages of all self-harm episodes using self-poisoning with all drugs and other substances were also calculated on the same basis.
Analyses were conducted using Stata version 10.0 (StataCorp LP, College Station, TX, USA) [19]. We used interrupted time series analysis to estimate changes in levels and trends in prescribing and self-poisoning following the MHRA warning in December 2003 [20]. This method controls for baseline level and trend when estimating expected changes in prescribing (or self-poisoning) due to the warning. Specifically, segmented regression analysis [21] was used to estimate the rate of prescribing and percent of self-poisoning that might have occurred without the MHRA warning, and that actually occurred with the warning. The end of 2003 was the point at which the MHRA warning occurred. Thus our data comprised 28 quarters in the pre-intervention segment and 12 quarters in the post-intervention segment. We determined the absolute effect of the intervention in two ways [22]: (i) slope and level regression coefficients were used to estimate the average quarterly absolute differences at the midpoint of the post-intervention period (midway between quarter 2 and quarter 3 of 2005); (ii) a more conservative estimate of the effect, which assumed no increase after the warning, was calculated as the difference between the outcome expected at the last point of the pre-warning period, and the midpoint of the post-warning period (see Appendix B and Figure A1). This was calculated only where there was an increasing trend (positive slope) in the pre-warning period. If the trend was decreasing (negative slope) the estimate using method (i) is already conservative.
In addition to the basic regression model for prescription data, models for self-poisoning included extra terms to control for differences in level of and slope of outcome by centre. Analyses were conducted for SSRIs, fluoxetine, and tricyclics where the outcomes were (i) rate of prescriptions; and (ii) percent of all self-poisoning episodes (i.e. six analyses). In addition, we analysed the change in level and trend in self-poisoning with all drugs or other substances (as a percentage of all self-harm episodes). Preliminary analyses indicated some autocorrelation in the data, therefore the Cochrane–Orcutt autoregression procedure was used (rather than ordinary linear regression) to correct for first order serially correlated errors. The Durbin Watson statistic of all final models was close to the preferred value of 2, indicating that no serious autocorrelation remained. (See Appendix B for details of the analytical method.)
Results
Of the 7854 episodes of self-harm in the study period, 6476 (82.5%) were for self-poisoning: 3691 before the MHRA warning and 2785 after. Overall, 333 episodes (5.1%) involved the use of SSRIs except fluoxetine, a higher proportion before the warning than after [220 (6.0%) vs. 113 (4.1%), χ2= 10.66, P= 0.001]. Fluoxetine was used in 129 episodes (3.5%) before the warning and 115 (4.1%) after (χ2= 1.63, P= 0.201); and tricyclics were used in 108 episodes (2.9%) before the warning and 56 (2.0%) after (χ2= 5.13, P= 0.024).
Interpretation of model parameters in Table 1a,b
Table 1a.
Interrupted time series segmented regression analysis of rate of prescribing in the UK in 12–19-year-olds, 2000–2006
| Segmented regression models* | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| SSRIs§ | Fluoxetine | Tricyclics | |||||||
| Coefficient | Robust SE | P | Coefficient | Robust SE | P | Coefficient | Robust SE | P | |
| Base level (β0) | 612.881 | 28.734 | <0.001 | 377.593 | 36.582 | <0.001 | 346.963 | 25.493 | <0.001 |
| Base trend (β1) | 26.267 | 2.899 | <0.001 | 16.079 | 3.482 | <0.001 | 3.756 | 3.617 | 0.310 |
| Post-warning change in level (β2)‡ | −400.233 | 43.590 | <0.001 | −92.542 | 42.629 | 0.041 | −57.447 | 42.265 | 0.187 |
| Post-warning change in trend (β3)‡ | −45.344 | 6.984 | <0.001 | −21.062 | 5.922 | 0.002 | −11.960 | 4.347 | 0.011 |
Table 1b.
Interrupted time series segmented regression analysis of self-poisoning with specific drugs in 12–19-year-olds in three centres in England, 2000–2006
| Segmented regression models† | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SSRIs§ | Fluoxetine | Tricyclics | All drugs and other substances | |||||||||
| Coefficient | Robust SE | P | Coefficient | Robust SE | P | Coefficient | Robust SE | P | Coefficient | Robust SE | P | |
| Base level (β0) | 7.873 | 1.079 | <0.001 | 4.978 | 1.870 | 0.010 | 3.517 | 0.795 | <0.001 | 80.060 | 2.556 | <0.001 |
| Base trend (β1) | 0.080 | 0.0996 | 0.482 | −0.003 | 0.128 | 0.983 | −0.083 | 0.068 | 0.225 | 0.129 | 0.189 | 0.495 |
| Post-warning change in level (β2)‡ | −4.670 | 1.220 | <0.001 | 1.765 | 1.497 | 0.242 | 0.839 | 0.748 | 0.266 | 2.139 | 1.887 | 0.261 |
| Post-warning change in trend (β3)‡ | 0.023 | 0.127 | 0.856 | −0.044 | 0.185 | 0.814 | −0.013 | 0.090 | 0.889 | −0.285 | 0.281 | 0.313 |
| Centre B (β4)¶ | −6.535 | 1.148 | <0.001 | −2.333 | 1.943 | 0.234 | 0.478 | 0.859 | 0.580 | 6.203 | 2.968 | 0.040 |
| Centre C (β4)¶ | −1.610 | 1.410 | 0.257 | −0.373 | 1.911 | 0.846 | 0.590 | 1.051 | 0.576 | 3.084 | 3.019 | 0.310 |
| Centre B × base trend (β5)†† | 0.227 | 0.060 | <0.001 | −0.039 | 0.103 | 0.705 | −0.048 | 0.051 | 0.351 | −0.407 | 0.189 | 0.035 |
| Centre C × base trend (β5)†† | −0.023 | 0.072 | 0.752 | −0.093 | 0.109 | 0.400 | −0.054 | 0.059 | 0.358 | −0.127 | 0.193 | 0.515 |
*Regression based on Equation A1[21] (see Appendix B).
Regression based on Equation A1[21] (see Appendix B). Outcome is percent of all self-poisoning episodes using the specified drug (group) or percent of all self-harm episodes using all substances (drugs and other non-ingestible substances).
Intervention point is the end of 2003: the Medicines & Healthcare products Regulatory Authority (MHRA) warning in December 2003 not to prescribe SSRI antidepressants except fluoxetine to under-18-year-olds.
SSRIs except fluoxetine.
Term to control for differences in centres: dummy variable comparing this centre (B or C) with centre A.
Interaction term controlling for different base trends in different centres, comparing this centre (B or C) with centre A. SE, standard error.
The base level parameter gives the rate of prescribing (or self-poisoning) at the beginning of the pre-warning period. The base trend shows how this changes during the pre-warning period (e.g. a positive, significant coefficient indicates an increasing rate). The post-warning change in level parameter indicates the rate of prescribing (or self-poisoning) immediately after the warning [e.g. a negative, significant coefficient tells us there has been a sudden decrease in rate of prescribing (or self-poisoning)]. The post-warning change in trend tells us whether the rate of prescribing (or self-poisoning) has changed in the post-warning period [e.g. a positive, significant coefficient would tell us there was an increase in rate of prescribing (or self-poisoning) in the post-warning period].
Prescribing
Trends in SSRI prescribing to 12–19-year-olds in the UK showed a steady increase prior to the MHRA warning, followed by a steep decline immediately after. Rates then appeared to level off during 2005 and increase in the last three quarters of 2006 (Figure 1). Steady increases in prescribing prior to the warning, and decreases after were also apparent for fluoxetine (Figure 1). The trend in prescribing of tricyclics was similar, but of smaller magnitude (Figure 1).
Figure 1.
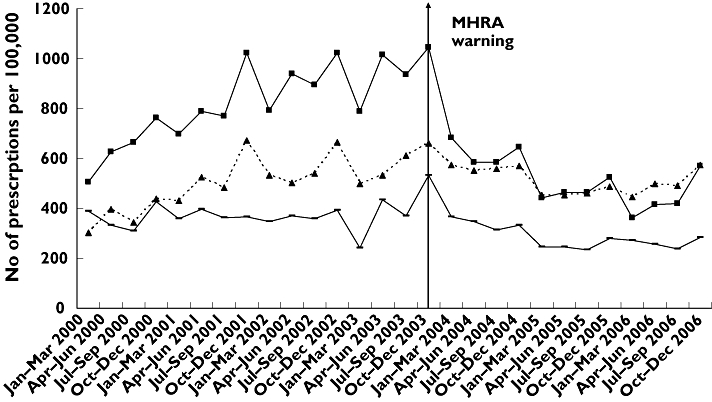
Rates of antidepressant prescribing in the UK for the 12–19-year-old age group, 2000–2006. SSRIs except fluoxetine ( ); fluoxetine (
); fluoxetine ( ); tricyclics (
); tricyclics ( )
)
Regression analyses indicated a significant decrease in the post-warning period in both level (β2=−400.233, P < 0.001) and slope (β3=−45.344, P < 0.001) in the prescribing of SSRIs (Table 1a), such that the rate of prescribing (per 100 000 population) decreased by an average of 695 [95% confidence interval (CI) 602, 788] per quarter in the post-warning period (Table 2). This equated to an overall decrease of approximately 58% in the period 2004–2006. The conservative estimate of the change in prescribing of SSRIs (per 100 000) was −524 (95% CI −461, −588) per quarter, which equated to a decrease of approximately 51% during 2004–2006 (Table 2).
Table 2.
Changes in prescribing in the UK and self-poisoning in three centres in England in 12–19-year-olds, 2000–2006, associated with the Medicines and Healthcare products Regulatory Authority (MHRA) warning in December 2003
| Estimation of the absolute effect during 2004–2006 of the MHRA warning* | |||||
|---|---|---|---|---|---|
| Mean quarterly estimated number with the MHRA warning† | Mean quarterly estimated number without the MHRA warning† | Conservative mean quarterly estimated number without the MHRA warning† | Mean quarterly change during 2004–2006‡ (95% CIs)¶ | Conservative mean quarterly change during 2004–2006§(95% CIs)¶ | |
| Prescription rate (per 100 000) | |||||
| SSRIs** | 508.925 | 1203.893 | 1033.156 | −695 (−602, −788) | −524 (−461, −588) |
| Fluoxetine | 509.920 | 739.366 | 634.854 | −230 (−125, −334) | −125 (−56, −193) |
| Tricyclics | 296.283 | 431.473 | 407.056 | −135 (−18, −252) | −111 (−38, −183) |
| Percent of all self-poisoning episodes using specific drugs | |||||
| SSRIs** | 5.2 | 9.7 | 9.2 | −4.5 (−1.5, −7.5) | −4.0 (−1.9, −6.1) |
| Fluoxetine | 6.4 | 4.9 | NA | 1.5 (−1.2, 4.2) | NA |
| Tricyclics | 2.4 | 1.6 | NA | 0.8 (−1.1, 2.6) | NA |
| Percent of all self-harm episodes using self-poisoning | |||||
| All drugs and other substances | 83.3 | 83.0 | NA | 0.3 (−3.9, 4.5) | NA |
Using Interrupted Time Series Segmented Regression Analysis [21].
Medicines & Healthcare products Regulatory Authority (MHRA) warning in December 2003 not to prescribe SSRI antidepressants except fluoxetine to under-18-year-olds.
Difference between column 2 and column 3. See Appendix B for calculation, method ‘a’ using Equation A4.
Difference between column 2 and column 4. See Appendix B for calculation, method ‘b’ using Equation A6.
95% Confidence intervals (CI) calculated according to Zhang et al. [28].
SSRIs except fluoxetine. NA, not applicable where trend is decreasing in pre-warning period.
There were also significant, although smaller, changes in both level and slope for fluoxetine in the post-warning period (Table 1a), such that the rate of prescribing (per 100 000 population) decreased by an average of 230 (95% CI 125, 334) per quarter, equating to an overall decrease of approximately 31% in the period 2004–2006 (Table 2). The conservative estimate of change in prescribing of fluoxetine was almost half the other estimate (Table 2), and equated to a decrease of 20% during 2004–2006.
For tricylics, there was a significant change in the post-warning slope such that the rate of prescribing (per 100 000 population) decreased by an average of 135 (95% CI 18, 252) per quarter, or approximately 31% in the period 2004–2006 (Table 2). The conservative estimate of change in prescribing for tricyclics remained significant, although of smaller magnitude (Table 2), and equated to a 27% decrease during 2004–2006.
Self-poisoning
Trends in self-poisoning showed more fluctuation than found for prescribing (Figure 2A–C). However, a decline in self-poisoning with SSRIs was evident immediately after the MHRA warning (Figure 2A). For fluoxetine, there appeared to be a decline earlier than the MHRA warning following a peak in October–December 2001, and an apparent increase after the warning (Figure 2B). The rate of self-poisoning with tricyclics appeared to decline steadily over the whole period (Figure 2C).
Figure 2.
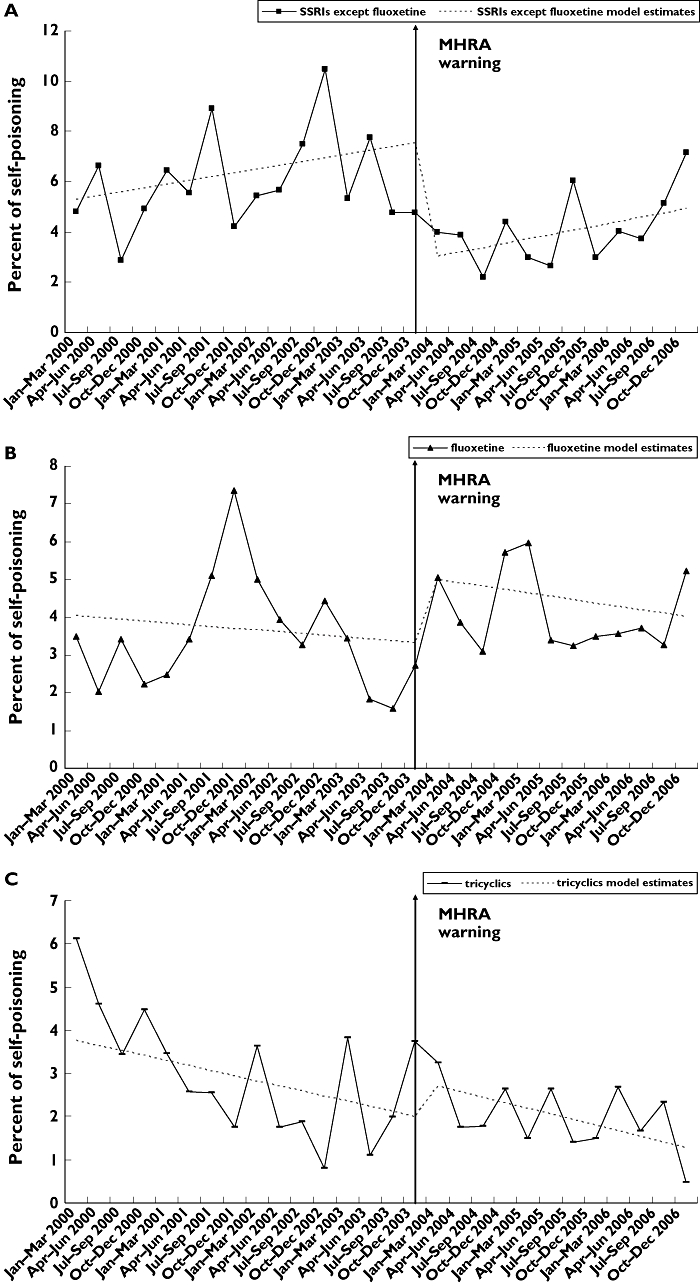
Percent of all self-poisoning episodes using (A) selective serotonin reuptake inhibitor (SSRI) antidepressants except fluoxetine; (B) fluoxetine; (C) tricyclic antidepressants; in three centres in England, for the 12–19-year-old age group, 2000–2006
Regression analyses indicated a significant decrease in level (β2=−4.670, P < 0.001) in self-poisoning with SSRIs (Table 1b), such that the percent of self-poisoning decreased by an average of 4.5 (95% CI 1.5, 7.5) per quarter in the post-warning period (Table 2). This equated to an overall decrease of approximately 47% in the period 2004–2006. The conservative estimate was similar, an average of −4.0% (95% CI −1.9, −6.1) per quarter, equating to a 44% decrease during 2004–2006.
There were no statistically significant changes associated with the MHRA warning for self-poisoning with fluoxetine, tricyclic antidepressants, or all drugs and other substances (Table 1b).
There were few differences between centres. For self-poisoning with SSRIs, the baseline level for centre B was significantly lower than centre A, but with a higher initial trend (Table 1b). For self-poisoning with all drugs and other substances, the baseline level for centre B was significantly higher than centre A, but with a lower initial trend (Table 1b). There were no significant differences between centre C and centre A (Table 1b).
Rates of self-harm
Rates of self-harm per 100 000, based on person-years, in each centre appeared to be stable over the period 2000–2006 (Figure 3). Regression analysis of combined data showed that there was no significant trend over time (β1=−0.386, SE = 0.697, P= 0.635; F1,2= 0.310, P= 0.635).
Figure 3.
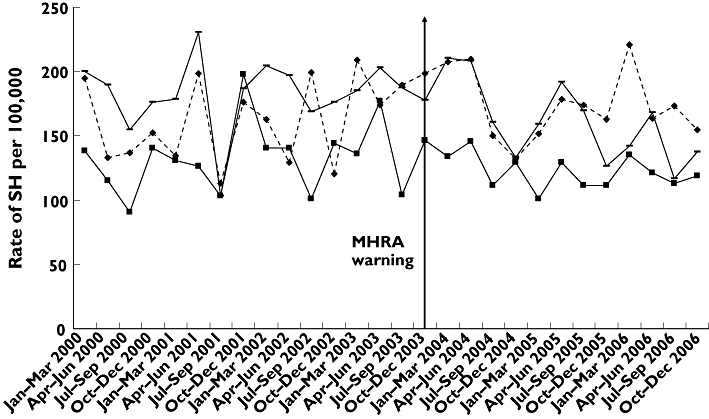
Rates of nonfatal self-harm in 12–19-year-olds in three centres in England, 2000–2006, based on first episode by each person per year. centre A ( ); centre B (
); centre B ( ); centre C (
); centre C ( )
)
Discussion
The findings of this study support our first hypothesis. Thus, UK prescription rates of SSRI antidepressants (citalopram, escitalopram, fluvoxamine, paroxetine and sertraline) declined substantially for the 12–19-year-old group in 2004–2006 following the MHRA warning not to prescribe these drugs to children and adolescents. However, rates of prescribing of fluoxetine also declined in the post-warning period. This drug was specifically recommended for use in young people with moderate to severe depression, and this finding was unexpected and contrary to our second hypothesis. Rates of prescribing of tricyclic antidepressants also declined, contrary to our third hypothesis. These drugs are unrelated to SSRIs and are associated with high toxicity in overdose [23] and are not recommended for young people [4]. Overall, these findings, which are in keeping with other recent studies [6–8, 12], appear to indicate heightened caution among clinicians as a result of the advice/warnings that affected prescriptions of all antidepressants in young people, but with a more marked effect for SSRIs.
There was much media interest and scientific controversy in the UK surrounding the use of SSRIs in general in the years preceding the December 2003 MHRA warning for under-18-year-olds, including several television programmes (e.g. Panorama in October 2002 [24]). Public debate was sufficient to prompt the MHRA to issue a response emphasizing that there was ‘no need for new concerns on the basis of this television programme’ and that ‘Tapering down the dose of SSRIs rather than abruptly stopping them is recommended to avoid withdrawal symptoms’[25]. The general controversy surrounding SSRIs, as well as the MHRA warning against their prescription to under-18-year-olds, may possibly have influenced prescribing practices by doctors and willingness of patients to receive prescriptions. These effects may have carried over to all antidepressants including tricyclics.
Rates of self-poisoning involving SSRIs (except fluoxetine) in adolescents decreased significantly after the MHRA warning, in further support of our first hypothesis. Assuming that prescribing trends in the areas where our rates of nonfatal self-poisoning were measured were similar to those in the UK, and that patients took their own prescribed drugs (a reasonable assumption for antidepressants), it seems likely that reduced availability of these specific drugs resulted in their reduced use for self-harm. This is not unexpected. In Scotland in the early 1990s, for instance, trends in prescribing were associated with trends in self-poisoning for many major drugs groups including antidepressants [26].
This inference does not hold for fluoxetine, however. Contrary to our second hypothesis, rates of prescribing of fluoxetine also decreased after the MHRA warning, but unlike other SSRIs, self-poisoning with fluoxetine did not decrease. The trend, although nonsignificant, was for a small increase. As well as the MHRA warning, NICE recommended that antidepressant therapy should be used only in cases of moderate or severe depression in young people [4]. Therefore an explanation may be the selective prescribing of fluoxetine following the warning to more severely depressed patients who might be more likely to self-poison. Thus there may have been a small substitution effect in some young people from use of other SSRIs for self-poisoning to use of fluoxetine.
Finally, we found that self-poisoning with all drugs and other substances, and overall rates of self-harm did not change significantly over the study period in three centres in England. SSRIs (except fluoxetine) accounted for only a small proportion (7–8%) of all self-poisoning episodes, and their decreased use in the post-warning period, together with a small although nonsignificant increase in use of fluoxetine, was not sufficient to affect the rates of self-poisoning overall. Also, there was no evidence that decreased prescribing of all antidepressants to 12–19-year-olds, probably associated with the MHRA warning, affected self-harming behaviour overall, indicating no substitution to other methods such as self-injury. This is contrary to the recent trend found in the USA and the Netherlands [11] of increased suicide with decreased antidepressant prescribing, but in keeping with other findings from the UK [12].
Strengths and limitations
This study was based on all presentations for self-harm to general hospitals in our study, not just admissions. This is important, because many self-harm patients presenting to hospital are not admitted [14]. However, the study did not include episodes of self-harm in the community where the young person did not present to the ED [27].
Our method of statistical analysis – interrupted time series regression – controlled for baseline level and trend when estimating expected changes due to the MHRA warning, and was preferable to simpler methods such as change in proportions pre- and post-warning, which do not take long-term baseline data into account. Although the estimate involved extrapolation, which was inevitably associated with some uncertainty, we utilized two methods and found good agreement between them. Model estimates (Figure 2) showed reasonable goodness-of-fit for the SSRIs and tricyclics, but less so for fluoxetine. Estimates of standard errors for the absolute mean quarterly changes were determined exactly, including the covariance of level and slope terms [28]. These calculations are often either not carried out, or are poorly reported in analyses of this type [29]. Estimates of percentage changes over the 3-year post-estimation period, however, were point estimates and were not determined with standard error calculations. Therefore caution must be advised in taking these percentages too literally.
The time frame of the analysis was not sufficient to model other temporal influences that overlapped with our study period (e.g. negative media attention to SSRIs in 2001 [30]). The steep decline in fluoxetine self-poisoning after the October–December quarter in 2001, 2 years before the MHRA warning, may have been related to adverse publicity. Similar warnings by the MHRA, e.g. in June 2003 [31] and May 2006 regarding paroxetine, and general advice by NICE in 2005 [4] were not included in our model, although they may have affected prescribing practices.
This was an ecological study and causal inferences cannot strictly be made. Our analysis used UK prescribing data as local prescribing data for the three centres were not available by age group. Also, UK prescribing data were available only for 12–19-year-olds rather than the under-18-years-old group to which the MHRA warning applied, although this is unlikely to have substantially undermined the findings.
Our analysis of self-poisoning data controlled for differences in the three centres. Some data were missing on non-assessed patients in Manchester for a short time during the study period (first quarter 2000 to third quarter 2002), and rates of self-harm were adjusted to account for these non-assessed patients. In addition, a proportion of children aged 12–15 years resident in the northern part of Manchester may have presented directly to a children's hospital in this part of the city throughout the whole study period. They would not be included in this study, leading to an underestimate of absolute numbers of self-harm episodes in this group. However, we used proportions of self-poisoning episodes with specific drug(s) as the main outcome in analyses rather than numbers. Moreover, it is unlikely that the proportion presenting to the children's hospital varied systematically by quarter. Our findings therefore are unlikely to be substantially affected by this limitation.
Research and clinical implications
It would be useful to analyse 2007–2008 data, to see if the upward trends in SSRI prescribing and self-poisoning evident in the last three quarters of 2006 have continued, and whether adolescents prescribed SSRIs after the MHRA warning were those with more severe disorders. Studies on nonfatal self-poisonings related to regulatory authority warnings have come mainly from the UK and the USA. Similar studies should be conducted in other countries.
The clinical implications of the regulatory warnings on SSRI use in young people have been far reaching. Studies in the USA have found evidence that the FDA advisory warning on risk of paediatric suicidality with SSRIs was associated with significant reductions in aggregated rates of diagnosis and treatment of paediatric depression [32], as well as a spill-over effect on the treatment of adult depression in the community [33]. Further studies in the UK are required to determine whether clinical outcomes of adolescents with depression have altered following advice on the prescribing of antidepressants from the MHRA.
Conclusion
There is evidence that in the UK, prescribing of specific SSRI antidepressants including fluoxetine declined in the 12–19-year-old age group in 2004–2006 following the MHRA warning in December 2003 not to use SSRIs other than fluoxetine for children and adolescents <18 years old. This was associated with a reduction in use of these SSRIs (except fluoxetine) for self-poisoning episodes in three centres in England. There may have been a small substitution effect in some young people from use of other SSRIs for self-poisoning to use of fluoxetine. Overall, however, since rates of nonfatal self-harm did not change in the same period, the effect of the MHRA warning as a public health measure was limited.
Acknowledgments
The authors thank Karen Smith of the Centre for Statistics in Medicine, University of Oxford, for expert statistical advice. We also thank Ben Wheeler and David Gunnell from the Department of Social Medicine, University of Bristol, and Peter Stephens from IMS Health, who kindly supplied the antidepressant prescription data. We acknowledge financial support from the Department of Health under the NHS R&D Programme. The views and opinions expressed herein do not necessarily reflect those of the Department of Health.
Appendix A
Description of self-harm registers
The Oxford Monitoring System was established in 1976 to collect information on all patients attending the general hospital for self-harm [18, 34–36]. For self-harm patients who are assessed, psychiatric staff complete a monitoring data sheet recording a wide range of sociodemographic and clinical information. For non-assessed episodes research staff record basic information from examination of medical records. This includes on-going scrutiny of the patient information system that records details of all patients presenting to the Emergency Department (ED) to identify self-harm patients not referred to the self-harm service, including screening of records where the presenting problem may not have indicated self-harm (e.g. where terms such as ‘not responsive’, ‘lacerations’, or ‘psychiatric problems’ have been used). This method of data collection has previously been investigated and found to be reliable [37]. The Oxford Monitoring System has near complete coverage of all hospital presentations for self-harm in Oxford City (<2% present to hospitals other than the general hospital), and varying proportions from other areas of the county (44% Cherwell; 72% South Oxfordshire; 91% Vale of White Horse; 84% West Oxfordshire).
The Manchester Self-Harm Project is a city-wide collaboration between the University of Manchester and three local hospitals, established in 1997 [38]. It is supported by the Manchester Mental Health and Social Care Trust, and three acute hospital Trusts. Self-harm attendances are identified via examination of computerized ED records. Information is collected on two assessment forms. Initial psychosocial assessments are performed by ED doctors, and subsequently for many cases more detailed psychiatric assessments are performed by psychiatric staff. Previously information was collected only for those episodes for which either type of assessment was carried out. Since September 2002, however, some information on non-assessed episodes has also been collected (e.g. age, gender, area of residence, date and time of presentation, method of self-harm). Six hospitals with EDs in Greater Manchester (Stepping Hill, Trafford, Hope, Fairfield, Oldham and Tameside) are within 10 miles of central Manchester. Although it is possible that due to the proximity of other hospitals some Manchester residents attend EDs outside the study area, a recent audit estimated that this was not a major problem for those age ≥16 years. However, a proportion of children aged 12–15 years resident in the northern half of Manchester may have presented directly to a children's hospital in this part of the city. They would not be included in the current study.
In Derby, data are collected on patients presenting with self-harm to the EDs of the two district general hospitals by the specialist self-harm team [39] that was established in 1989. Patients presenting with self-harm may be referred to the team for psychiatric assessment. Where patients are not assessed or do not attend appointments, data are extracted from other sources, including case notes. Team members also visit wards and monitor records to identify all patients presenting with self-harm [39]. A small proportion of patients may present to other EDs due to their closer proximity to patients' homes.
Appendix B
Segmented regression analysis
We used the method of Wagner et al. [21] in this analysis. Segmented regression analysis is a method of estimating changes in levels and trends in an outcome (prescribing and self-poisoning in this study) associated with an intervention (MHRA warning). The time series regression equation for this model is
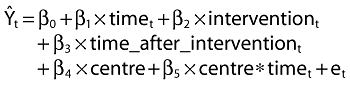 |
(1) |
Ŷt is the outcome (rate of prescription or percent of self-poisoning episodes per quarter); time indicates the number of quarters from the start of the series (1.28); intervention is a dummy variable taking the values 0 in the pre-intervention segment and 1 in the post-intervention segment; time_after_intervention is 0 in the pre-intervention segment and counts the quarters in the post-intervention segment at time t (1.12); centre is a categorical variable indicating each of the three monitoring centres (two dummy variables are constructed comparing centre B and centre C with centre A). The two terms involving centre are not included when the outcome is rate of prescriptions because national data were used for this. The coefficient β0 estimates the base level of the outcome at the beginning of the series; β1 estimates the base trend, i.e. the change in outcome per quarter in the pre-intervention segment; β2 estimates the change in level of the outcome in the post-intervention segment; β3 estimates the change in slope in outcome in the post-intervention segment; et estimates the error; β4 estimates the change in level of self-poisoning, and β5 estimates the change in slope of self-poisoning, for each centre relative to centre A.
Coefficients and errors from full models including all terms in Equation A1 are given in Table 1a,b. Nonsignificant terms were included as there may be correlation between slope and level terms that should be accounted for.
Absolute effect of the intervention
The model was used to estimate the absolute effect of the intervention in two ways [22], both of which we used.
(a) First, we calculated the difference between the estimated outcome at a certain time after the intervention and the outcome at that time if the intervention had not taken place. For example, to estimate the effect of the intervention at the midpoint of the post-intervention period (when time= 22.5 and time_after_intervention= 6.5), where the outcome is the rate of prescribing, we have
| (2) |
| (3) |
thus, the absolute effect of the intervention is
| (4) |
Thus the absolute effect of the intervention was calculated from Equation A4, and this corresponds to the distance p to r in Figure A1. The standard errors were calculated according to the method of Zhang et al. [28]. The expression for the standard error included the covariance between β2 and β3, which for our calculation was obtained from an autoregression post-estimation command (estat vce) in Stata v10.0 [19]. Results are presented in Table 2.
Figure A1.
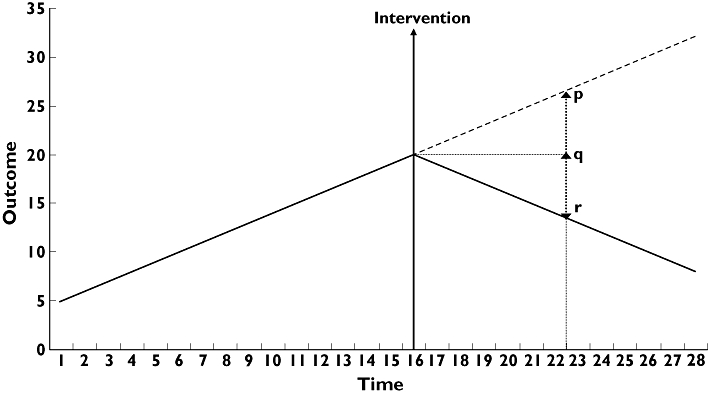
Illustration of segmented regression analysis in interrupted time series, and two methods to estimate the effect of the intervention. estimated without intervention ( ); with intervention (
); with intervention ( )
)
(b) Secondly, when there is an increasing trend in the pre-intervention period, a more conservative estimate of the absolute effect of the intervention may be calculated [22]. In our study, this applied to all the prescription data and self-poisoning with SSRIs only. Here the outcome without the intervention was taken at the earlier time (not assuming any increase in the post-intervention period). This corresponds to the distance q to r in Figure A1. Thus we have
| (5) |
and the outcome with the intervention remains unchanged
Thus, the conservative estimate of absolute effect of the intervention was
| (6) |
Standard errors for this expression also involved terms for β1 and the covariance between β1 and β2, and β1 and β3. Results are presented in Table 2.
Competing interests
None to declare.
REFERENCES
- 1.Medicines & Healthcare Products Regulatory Authority. Safety of seroxat (paroxetine) in children and adolescents under 18 years – contraindication in the treatment of depressive illness – Epinet message from Professor G Duff, Chairman of Committee on Safety of Medicines (CSM) World Wide Web. Available at http://www.mhra.gov.uk/Safetyinformation/Safetywarningsalertsandrecalls/Safetywarningsandmessagesformedicines/CON2015704 (last accessed 16 September 2008.
- 2.Medicines & Healthcare Products Regulatory Authority. Selective Serotonin Reuptake Inhibitors (SSRIs): overview of regulatory status and CSM advice relating to major depressive disorder (MDD) in children and adolescents including a summary of available safety and efficacy data. World Wide Web. Available at http://www.mhra.gov.uk/Safetyinformation/Safetywarningsalertsandrecalls/Safetywarningsandmessagesformedicines/CON019494 (last accessed 18 April 2007.
- 3.US Food and Drug Administration. Relationship between psychotropic drugs and pediatric suicidality: review and evaluation of clinical data. World Wide Web. Available at http://www.fda.gov/ohrms/dockets/ac/04/briefing/2004-4065b1-10-TAB08-Hammads-Review.pdf (last accessed 9 May 2007.
- 4.National Collaborating Centre for Mental Health. Depression in Children and Young People: Identification and Management in Primary, Community and Secondary Care. Leicester and London: The British Psychological Society and the Royal College of Psychiatrists; 2005. [PubMed] [Google Scholar]
- 5.Bridge JA, Axelson DA. The contribution of pharmacoepidemiology to the antidepressant-suicidality debate in children and adolescents. Int Rev Psychiatry. 2008;20:209–14. doi: 10.1080/09540260801889245. [DOI] [PubMed] [Google Scholar]
- 6.Volkers AC, Heerdink ER, van Dijk L. Antidepressant use and off-label prescribing in children and adolescent in Dutch general practice (2001–2005) Pharmacoepidemiol Drug Saf. 2007;16:1054–1062. doi: 10.1002/pds.1430. [DOI] [PubMed] [Google Scholar]
- 7.Olfson M, Marcus SC, Druss BG. Effects of food and drug administration warnings on antidepressant use in a national sample. Arch Gen Psychiatry. 2008;65:94–101. doi: 10.1001/archgenpsychiatry.2007.5. [DOI] [PubMed] [Google Scholar]
- 8.Dean AJ, Hendy A, McGuire T. Antidepressants in children and adolescents – changes in utilisation after safety warnings. Pharmacoepidemiol Drug Saf. 2007;16:1048–53. doi: 10.1002/pds.1396. [DOI] [PubMed] [Google Scholar]
- 9.Olfson M, Shaffer D, Marcus SC, Greenberg T. Relationship between antidepressant medication treatment and suicide in adolescents. Arch Gen Psychiatry. 2003;60:978–82. doi: 10.1001/archpsyc.60.9.978. [DOI] [PubMed] [Google Scholar]
- 10.Ludwig J, Marcotte DE. Anti-depressants, suicide and drug regulation. J Policy Anal Manage. 2005;24:249–72. doi: 10.1002/pam.20089. [DOI] [PubMed] [Google Scholar]
- 11.Gibbons RD, Brown CH, Hur K, Marcus SM, Bhaumik DK, Erkens JA, Herings RMC, Mann JJ. Early evidence on the effects of regulators' suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry. 2007;164:1356–63. doi: 10.1176/appi.ajp.2007.07030454. [DOI] [PubMed] [Google Scholar]
- 12.Wheeler BW, Gunnell D, Metcalfe C, Stephens P, Martin RM. The population impact on incidence of suicide and non-fatal self harm of regulatory action against the use of selective serotonin reuptake inhibitors in under 18s in the United Kingdom: ecological study. BMJ. 2008;336:542–5. doi: 10.1136/bmj.39462.375613.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawton K, Bergen H, Casey D, Simkin S, Palmer B, Cooper J, Kapur N, Horrocks J, House A, Lilley R, Noble R, Owens D. Self-harm in England: a tale of three cities. Multicentre study of self-harm. Soc Psychiatry Psychiatr Epidemiol. 2007;42:513–21. doi: 10.1007/s00127-007-0199-7. [DOI] [PubMed] [Google Scholar]
- 14.Bennewith O, Gunnell D, Peters TJ, Hawton K, House A. Variations in the hospital management of self-harm in adults in England: observational study. BMJ. 2004;328:1108–9. doi: 10.1136/bmj.328.7448.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Department of Health. 4-Hour checklist: reducing delays for A&E patients. World Wide Web. Available at http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4085182 (last accessed 6 November 2008.
- 16.Intercontinental Medical Statistics. IMS Health. World Wide Web. Available at http://www.imshealth.com (last accessed 27 October 2008.
- 17.Office for National Statistics. Population estimates for UK, England and Wales, Scotland and Northern Ireland – current datasets. World Wide Web. Available at http://www.statistics.gov.uk/statbase/Product.asp?vlnk=15106 (last accessed 22 August 2007.
- 18.Hawton K, Harriss L, Hall S, Simkin S, Bale E, Bond A. Deliberate self-harm in Oxford, 1990–2000: a time of change in patient characteristics. Psychol Med. 2003;33:987–96. doi: 10.1017/s0033291703007943. [DOI] [PubMed] [Google Scholar]
- 19.Stata Corporation. Stata Statistical Software. College Station, TX: Stata Corporation; 2007. Release 10. [Google Scholar]
- 20.Ramsay CR, Matowe L, Grilli R, Grimshaw JM, Thomas RE. Interrupted time series designs in health technology assessment: lessons from two systematic reviews of behavior change strategies. Int J Technol Assess Health Care. 2003;19:613–23. doi: 10.1017/s0266462303000576. [DOI] [PubMed] [Google Scholar]
- 21.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 22.Ansari F, Gray K, Nathwani D, Phillips G, Ogston S, Ramsay C, Davey P. Outcomes of an intervention to improve hospital antibiotic prescribing: interrupted time series with segmented regression analysis. J Antimicrob Chemother. 2003;52:842–8. doi: 10.1093/jac/dkg459. [DOI] [PubMed] [Google Scholar]
- 23.Buckley NA, McManus PR. Fatal toxicity of serotonergic and other antidepressant drugs: analysis of United Kingdom mortality data. BMJ. 2002;325:1332–3. doi: 10.1136/bmj.325.7376.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panorama. The secrets of seroxat. BBC News. Available at http://news.bbc.co.uk/1/hi/programmes/panorama/2310197.stm (last accessed 16 September 2008.
- 25.Medicines & Healthcare Products Regulatory Authority. Important safety messages: MHRA response to Panorama programme on Seroxat. World Wide Web. Available at http://www.mhra.gov.uk/Safetyinformation/Safetywarningsalertsandrecalls/Safetywarningsandmessagesformedicines/CON019522 (last accessed 16 September 2008.
- 26.Crombie IK, McLoone P. Does the availability of prescribed drugs affect rates of self poisoning. Br J Gen Pract. 2008;48:1505–6. [PMC free article] [PubMed] [Google Scholar]
- 27.Hawton K, Rodham K, Evans E, Weatherall R. Deliberate self-harm in adolescents: self report survey in schools in England. BMJ. 2002;325:1207–11. doi: 10.1136/bmj.325.7374.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang F, Wagner A, Soumerai SB, Ross-Degnan D. Estimating confidence intervals around relative changes in outcomes in segmented regression analyses of time series data. 15th Annual NESUG (NorthEast SAS Users Group Inc) Conference, Buffalo, NY, NorthEast SAS Users Group Inc. Available at http://www.nesug.info/Proceedings/nesug02/st/st005.pdf (last accessed 22 October 2008.
- 29.Ramsay CR, Matowe L, Grilli R, Grimshaw JM, Thomas RE. Interrupted Time Series Designs in Health Technology Assessment: lessons from two systematic reviews of behavior change strategies. Int J Technol Assess Health Care. 2003;19:613–23. doi: 10.1017/s0266462303000576. [DOI] [PubMed] [Google Scholar]
- 30.BBC News. Antidepressant addiction warning. World Wide Web. Available at http://newsbbc,co.uk/1/hi/health/1382551.stm (last accessed 16 September 2008.
- 31.Medicines & Healthcare Products Regulatory Authority. Safety of seroxat (paroxetine) in children and adolescents under 18 years – contraindication in the treatment of depressive illness – Epinet message from Professor G Duff, Chairman of Committee on Safety of Medicines (CSM) World Wide Web. Available at http://www.mhra.gov.uk/Safetyinformation/Safetywarningsalertsandrecalls/Safetywarningsandmessagesformedicines/CON2015704 (last accessed 16 September 2008.
- 32.Libby AM, Brent DM, Morrato EH, Orton HD, Allen S, Valuck RJ. Decline in treatment of pediatric depression after FDA advisory on risk of suicidality with SSRIs. Am J Psychiatry. 2007;164:884–91. doi: 10.1176/ajp.2007.164.6.884. [DOI] [PubMed] [Google Scholar]
- 33.Valuck RJ, Libby AM, Orton HD, Morrato EH, Allen R, Baldessarini RJ. Spillover effects on treatment of adult depression in primary care after FDA advisory on risk of pediatric suicidality with SSRIs. Am J Psychiatry. 2007;164:1198–205. doi: 10.1176/appi.ajp.2007.07010007. [DOI] [PubMed] [Google Scholar]
- 34.Hawton K, Fagg J. Trends in deliberate self poisoning and self injury in Oxford, 1976–90. BMJ. 1992;304:1409–11. doi: 10.1136/bmj.304.6839.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawton K, Fagg J, Simkin S, Bale E, Bond A. Trends in deliberate self-harm in Oxford, 1985–1995. Implications for clinical services and the prevention of suicide. Br J Psychiatry. 1997;171:556–60. doi: 10.1192/bjp.171.6.556. [DOI] [PubMed] [Google Scholar]
- 36.Hawton K, Fagg J, Marsack P, Wells P. Deliberate self-poisoning and self-injury in the Oxford area 1972–1980. Soc Psychiatry. 1982;17:175–9. [Google Scholar]
- 37.Sellar C, Goldacre MJ, Hawton K. Reliability of routine hospital data on poisoning as measures of deliberate self poisoning in adolescents. J Epidemiol Community Health. 1990;44:313–5. doi: 10.1136/jech.44.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy E, Dickson S, Donaldson I, Healey M, Kapur N, Appleby L, Cooper J. Self-harm in Manchester. Manchester: Centre for Suicide Prevention; 2007. 1.9.03-31.8.05. [Google Scholar]
- 39.Belgamwar R, Hodgson R, Waters K. Trends and characteristics of deliberate self-harm hospital presentations in an English County. Int J Psychiatr Clin Pract. 2006;10:59–63. doi: 10.1080/13651500500410679. [DOI] [PubMed] [Google Scholar]


