Abstract
AIMS
Pulse contour analysis (PCA) obtained by finger photoplethysmography produces a digital volume pulse (DVP) including an inflection point in its down-slope. The reflection index (RI: ratio of the inflection point height over the maximal DVP) is responsive to vasodilatation. We aimed to optimize the drug dose and time interval for assessing endothelial function using PCA in healthy volunteers and patients with severe coronary artery disease.
METHODS
Time and dose to RI response relationships were constructed in 16 volunteers and nine patients to inhaled salbutamol (100–400 µg) or sublingual nitroglycerin (NTG; 25–400 µg).
RESULTS
For the volunteers, the time to maximum RI response to inhaled salbutamol and sublingual NTG was 10.73 ± 0.41 and 3.66 ± 0.21 min, respectively. A plateau in the RI response to salbutamol occurred between 5 and 15 min after inhalation and results were averaged over this period. A dose-dependent response was observed to inhaled salbutamol and sublingual NTG (P= 0.05 and P < 0.001 by repeated-measures anova, respectively) in healthy volunteers. By contrast, in patients with severe coronary artery disease inhaled salbutamol (100–400 µg) did not cause a significant change in RI.
CONCLUSIONS
In healthy volunteers the RI response to inhaled salbutamol (100–200 µg) averaged over 5–15 min after administration may be used to investigate endothelial function by PCA. The response to sublingual NTG (50 µg) should be determined at 4 min. This technique may not be suitable for the assessment of endothelial function in subjects with extensive coronary artery disease owing to the small responses observed and potential confounding effect of vasoactive medication.
Keywords: coronary artery disease, endothelial function, human, photoplethysmography, pulse contour analysis
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Pulse contour analysis is a relatively new technique used for non-invasive assessment of endothelial function.
Endothelial dysfunction is a component of a number of common conditions including hypertension, diabetes mellitus and a variety of inflammatory conditions.
A standardized method for assessing endothelial function using pulse contour analysis is required to allow comparison between studies.
WHAT THIS STUDY ADDS
This study recommends the optimal dose of vasoactive agents and timing of measurements for the assessment of endothelial function using pulse contour analysis.
Introduction
Endothelial dysfunction is a component of common conditions including hypertension, diabetes mellitus, hypercholesterolaemia and coronary artery disease. Dynamic physiological methods used to assess endothelial function include forearm plethysmography, brachial artery flow mediated dilation, applanation tonometry and pulse contour analysis (PCA) [1]. The former two techniques are unsuitable for large-scale studies. Applanation tonometry and PCA evaluate changes in the arterial waveform in response to endothelium-dependent and -independent vasodilators. These portable techniques offer the prospect of population screening to detect early endothelial dysfunction with a view to preventing cardiovascular disease.
The aim of this study was to determine the dose–response relationship to endothelium-dependent (salbutamol) and -independent [nitroglycerin (NTG)] vasodilators, and the optimum time at which to examine the drug effect using PCA.
Methods
Subjects
Following approval by the Local Ethics Committee, informed consent was obtained from all subjects. Healthy volunteers were male, aged 25–45 years. Screening excluded any history of cardiovascular disease, smoking, diabetes and concurrent medication. Patients awaiting bypass graft surgery for triple vessel coronary artery disease (8/2 male/female, aged 61–74 years) had taken their usual medications on the day of the study. All subjects abstained from caffeine for at least 6 h prior to the study. Measurements were taken with the subjects supine in a quiet, temperature-controlled (22–24°C) room following a 15-min acclimatization period. Heart rate and blood pressure were recorded, respectively, from the Pulse Trace device and from the dominant arm at 5-min intervals throughout the study using an automated oscillometric recorder.
Pulse contour analysis
PCA was assessed using a Pulse Trace (Micro Medical, Gillingham, UK) system incorporating a high-fidelity photoplethysmograph finger probe, which produces the digital volume pulse (DVP) including a characteristic inflection point in the down-slope of the waveform. The reflection index (RI) is calculated from the amplitude of the inflection point and expressed as a percentage of the maximal DVP amplitude, as previously described [1]. Vasodilation produces a marked reduction in RI [2]. Recordings were averaged over 10-s intervals.
DVP recordings during administration of NTG and salbutamol
NTG (25, 50, 100, 200 and 400 mg each in 100 ml; Faulding Pharmaceuticals plc, Royal Lemington Spa, UK) was administered sublingually. Salbutamol (100, 200, 300 and 400 mg; Baker Norton Salamol CFC-Free inhaler, Miami, FL, USA) was administered via a spacer device (Volumatic™; Allen & Hanburys, Uxbridge, UK). Each 100-mg actuation was followed by six normal breaths through the spacer device. DVP recordings were taken until measurements returned to baseline. If consecutive doses produced the same reduction in RI or the heart rate increased by > 10% from baseline for > 30 min, the study was stopped. Volunteers underwent repeatability studies at the same time on different days with NTG (n= 4) and salbutamol (n= 5).
Statistical analysis
Results are presented as mean ± standard error of the mean. Data were analysed using repeated-measures analysis of variance (anova) or paired t-tests. Repeatability data were analysed using Bland–Altman plots and expressed in terms of mean difference ± standard deviation (SD) between paired measurements. The coefficient of variation was calculated as the SD of the differences between the measurements divided by the mean of one of the measurements multiplied by 100. Values of P≤ 0.05 were considered to be significant.
Results
Time and dose–responses to NTG and salbutamol by PCA in healthy volunteers (Table 1 and Figure 1)
Table 1.
The time–response relationship and haemodynamic changes to nitroglycerin (NTG) and salbutamol in healthy male volunteers using Pulse Trace
| Time (min) | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Change in HR (bpm) | P-value | Change in MAP (mmHg)/ P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NTG 25 µg (n= 7) | 75.4 ± 5.7 | 70.8 ± 6.7 | 69.6 ± 5.3 | 65.0 ± 5.3 | 68.4 ± 4.4 | 71.0 ± 4.7 | 71.3 ± 4.4 | 70.3 ± 4.5 | 72.4 ± 5.3 | 79.3 ± 2.7 | 74.0 ± 4.8 | 78.8 ± 4.3 | 72.8 ± 4.5 | −0.3 ± 1.6 | 0.50 | 0.78 |
| NTG 50 µg (n= 7) | 74.0 ± 5.6 | 75.7 ± 6.8 | 67.2 ± 5.6 | 67.3 ± 5.9 | 67.1 ± 4.9 | 65.2 ± 5.9 | 70.3 ± 4.5 | 65.3 ± 6.1 | 71.0 ± 4.6 | 68.0 ± 5.5 | 78.0 ± 5.4 | 69.5 ± 5.6 | 71.8 ± 6.9 | 1.0 ± 1.9 | 0.91 | |
| NTG 100 µg (n= 7) | 72.8 ± 6.1 | 69.5 ± 5.5 | 64.1 ± 5.1 | 56.6 ± 4.2 | 61.7 ± 3.3 | 63.3 ± 4.1 | 68.6 ± 5.4 | 57.8 ± 2.0 | 62.3 ± 5.9 | 76.3 ± 2.9 | 61.5 ± 2.5 | 68.5 ± 4.8 | 72.7 ± 5.9 | 2.9 ± 1.5 | 0.20 | |
| NTG 200 µg (n= 7) | 80.3 ± 4.2 | 73.0 ± 3.5 | 66.0 ± 3.9 | 62.6 ± 4.2 | 56.9 ± 4.6 | 57.5 ± 5.1 | 57.8 ± 2.5 | 57.3 ± 4.2 | 64.3 ± 4.4 | 65.8 ± 4.7 | 64.6 ± 4.9 | 69.7 ± 4.0 | 70.8 ± 6.2 | 2.3 ± 1.7 | 0.58 | |
| NTG 400 µg (n= 4) | 62.3 ± 5.5 | 56.3 ± 5.5 | 50.8 ± 3.2 | 46.6 ± 3.5 | 48.8 ± 2.8 | 47.3 ± 3.9 | 47.3 ± 3.2 | 56.0 ± 4.4 | 53.8 ± 5.0 | 51.3 ± 5.8 | 61.0 ± 6.7 | 58.0 ± 5.2 | 68.0 ± 3.0 | 3.1 ± 1.9 | 0.27 | |
| Volunteers/patients | ||||||||||||||||
| Time (min) | 0 | 1–2 | 3–4 | 5–6 | 7–8 | 9–10 | 11–12 | 12–13 | 14–15 | 16–17 | 18–19 | |||||
| Salb 100 µg (n= 9) | 72.7 ± 4.4 | 74.6 ± 6.0 | 71.9 ± 5.1 | 73.0 ± 4.3 | 65.0 ± 5.2 | 64.4 ± 3.7 | 69.7 ± 5.2 | 63.3 ± 5.6 | 68.8 ± 5.7 | 63.0 ± 4.8 | 68.5 ± 4.3 | 2.1 ± 0.8/0.9 ± 1.1 | <0.05/ <0.05 | 0.81/0.42 | ||
| Salb 200 µg (n= 9) | 71.2 ± 4.2 | 67.7 ± 4.3 | 63.7 ± 5.2 | 61.3 ± 4.4 | 56.2 ± 3.3 | 60.8 ± 3.0 | 60.4 ± 4.6 | 62.8 ± 4.5 | 57.1 ± 4.5 | 61.0 ± 6.0 | 62.6 ± 4.1 | 4.9 ± 1.6/0.9 ± 0.9 | 0.97/0.50 | |||
| Salb 400 µg (n= 9) | 71.8 ± 4.7 | 64.7 ± 4.5 | 65.6 ± 3.8 | 60.9 ± 3.3 | 57.5 ± 3.8 | 59.5 ± 4.2 | 56.3 ± 3.5 | 60.4 ± 3.8 | 61.3 ± 5.0 | 58.8 ± 4.3 | 62.4 ± 5.4 | 6.3 ± 1.9/4.4 ± 1.4 | 0.45/0.23 | |||
Figure 1.
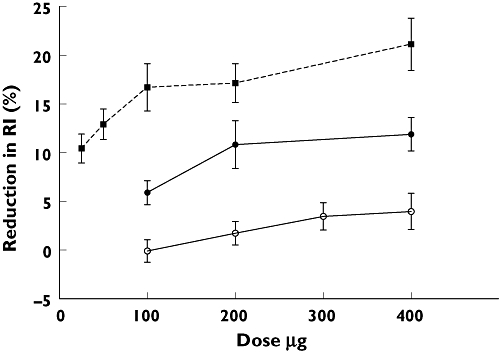
Dose–response relationship in healthy male volunteers and patients with coronary artery disease to nitroglycerin (NTG) and salbutamol. The change in reflection index (RI) in response to NTG was measured at time t= 4 min (P < 0.001, repeated-measures anova). The change in RI response to salbutamol was averaged over 5–15 min (volunteers: P= 0.05; patients: P= 0.30, repeated-measures anova). NTG Volunteers ( ); Salbutamol Volunteers (
); Salbutamol Volunteers ( ); Salbutamol Patients (
); Salbutamol Patients ( )
)
The maximum effect on RI following sublingual NTG occurred at 3.66 ± 0.21 min; there was no difference in this time between doses (P= 0.63, anova). A dose-dependent reduction in the RI response to NTG occurred at 4 min (P < 0.001, anova).
RI decreased in response to salbutamol for 5 min followed by a plateau of approximately 10 min; data are presented as the average reduction in RI over this period. No difference was observed in time to maximum response between doses of salbutamol (tmax 10.73 ± 0.41 min; P= 0.44, anova). Salbutamol induced a dose-dependent reduction in RI (P= 0.05, anova).
Administration of salbutamol but not NTG caused a dose-dependent increase in heart rate (P < 0.05 and P= 0.50, respectively, anova), but had no effect on systemic blood pressure. RI correlated with heart rate for salbutamol and NTG (P < 0.0001; r=−0.59 and r=−0.55; linear regression slope −0.57 and −0.69, respectively).
Repeatability studies
The within-observer difference in RI for healthy subjects given NTG and salbutamol at the time of maximum effect was, respectively, 2.48 ± 5.04, n= 4, coefficient of variation 8.8%, and 0.9 ± 17.2, n= 5, coefficient of variation 27.5% or 2.77 ± 7.42, coefficient of variation 11.8% when the RI values were averaged over 5–15 min. No difference was observed in baseline heart rate and blood pressure between the two studies.
Time and dose–response to salbutamol by PCA in patients with coronary artery disease
Three patients failed to complete the study following inhalation of salbutamol (300 µg) owing to the onset of angina (n= 1) and persistent tachycardia (n= 2). There was no significant dose–response relationship to salbutamol (P= 0.30, anova; Figure 1). The patients' RI responses to all doses were less than those of the healthy volunteers. Heart rate increased with increasing doses of salbutamol (P= 0.049, anova, n= 9; linear regression slope −0.20). Blood pressure did not change (Table 1).
Discussion
Our data suggest that the RI responses to sublingual NTG (50 mg) and inhaled salbutamol (100–200 mg) should be analysed at 4 and between 5 and 15 min after administration, as indices of endothelium-independent and -dependent vasodilation, respectively. Using the lowest effective drug dose will avoid confounding variables and minimize adverse effects like tachycardia. For example, salbutamol-associated tachycardia affects the measurement of augmentation index, a similar technique using pulse wave analysis to assess endothelial function [3]. Increasing the heart rate allows less time for wave reflection and hence reductions in the inflection point.
Our results are comparable to previous repeatability studies of PCA using the Sphygmocor (ATCOR, Sydney, Australia) [4, 5] and the Pulse Trace systems [5, 6]. Repeatability studies using flow-mediated vasodilation, arguably the gold standard method for assessing endothelial function, have derived coefficients of variation of 7.1% [5] and 2.3% [7].
In patients with advanced coronary artery disease there was no effect of salbutamol on PCA, but 80% and 10% of patients had recently taken β antagonists and nitrates, respectively, making the data difficult to interpret. Hence, protocols for assessment by PCA derived from healthy volunteers should be re-evaluated in patients with conditions that affect endothelial function. Another limitation with regard to the generalizability of these studies is that female subjects were excluded from the volunteer studies owing to the monthly variation in endothelial function in pre-menopausal women [8].
Competing interests
None declared.
Work carried out in this study was supported by a Young Investigator Award from the Intensive Care Society. We are grateful for statistical advice from Winston Banya.
REFERENCES
- 1.Chowienczyk PJ, Kelly RP, MacCallum H, Millasseau SC, Andersson TL, Gosling RG, Ritter JM, Anggard EE. Photoplethysmographic assessment of pulse wave reflection: blunted response to endothelium-dependent beta2-adrenergic vasodilation in type II diabetes mellitus. J Am Coll Cardiol. 1999;34:2007–14. doi: 10.1016/s0735-1097(99)00441-6. [DOI] [PubMed] [Google Scholar]
- 2.Morikawa Y. Characteristic pulse wave caused by organic nitrates. Nature. 1967;213:841–2. doi: 10.1038/213841a0. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525(1):263–70. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16:2079–84. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]
- 5.Donald AE, Charakida M, Cole TJ, Friberg P, Chowienczyk PJ, Millasseau SC, Deanfield JE, Halcox JP. Non-invasive assessment of endothelial function: which technique? J Am Coll Cardiol. 2006;48:1846–50. doi: 10.1016/j.jacc.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 6.Rambaran C, Jiang B, Ritter JM, Shah A, Kalra L, Chowienczyk PJ. Assessment of endothelial function: comparison of the pulse wave response to beta-adrenoceptor stimulation with flow mediated dilatation. Br J Clin Pharmacol. 2007;65:238–43. doi: 10.1111/j.1365-2125.2007.03006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto M, Akishita M, Eto M, Ishikawa M, Kozaki K, Toba K, Sagara Y, Taketani Y, Orimo H, Ouchi Y. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation. 1995;92:3431–5. doi: 10.1161/01.cir.92.12.3431. [DOI] [PubMed] [Google Scholar]


