Abstract
Background:
A few studies have observed reduced breast cancer mortality in women who used hormone therapy (HT) prior to diagnosis. Due to the high prevalence of past and current hormone use, it is important to investigate whether these preparations are related to breast cancer mortality.
Methods:
To evaluate the influence of prediagnostic use of HT on breast cancer mortality, a prospective cohort of 12,269 women aged 50 years or more diagnosed with incident invasive breast cancer and residents of Wisconsin, Massachusetts, or New Hampshire, US were enrolled in three phases beginning in 1988. They were followed for death until December 31, 2005 using the National Death Index. Cumulative mortality and multivariable adjusted hazard rate ratios (HRRs) for breast cancer and other mortality causes were calculated for women according to any HT use, and for exclusive use of estrogen or estrogen-progestin (EP).
Results:
During an average 10.3 years of follow up, 1690 deaths from breast cancer were documented. Cumulative mortality from breast cancer was lower among HT users, specifically current users at the time of diagnosis, and EP users, compared to nonusers. Adjusted survival varied by type and duration of HT prior to diagnosis. A reduced risk of death from breast cancer was associated with EP preparations (HRR 0.73; 0.59-0.91) and with ≥5 years of EP use (0.60; 0.43-0.84). No association was observed for women who were former or current users of E-alone preparations.
Conclusions:
Although use of combined EP preparations increases breast cancer risk, in this study, use of these hormones before diagnosis was associated with reduced risk of death after a breast cancer diagnosis. The better survival among users, particularly of EP, persisted after adjustment of screening, stage, and measured confounders.
Keywords: Survival, hormone replacement therapy, breast cancer, estrogen
INTRODUCTION
Compelling evidence demonstrates that hormone therapy (HT) use, particularly formulations containing progestins, increases breast cancer incidence (1, 2). However, reduced breast cancer mortality has been observed among women using HT prior to breast cancer diagnosis in several studies (3-11). It is not yet clear whether associations with survival are attributable to the hormones themselves, or to the healthier profiles, screening habits, or treatment choices of women prescribed hormones (8-10). An inverse relation between HT use and breast cancer mortality might also be explained by more favorable tumor profiles, and therefore improved prognosis, among HT users compared with non-users (11-14).
A substantial proportion of women in the U.S. have used HT in their lifetimes, including about half of postmenopausal U.S. women aged 50-69 years (15, 16). Given the large number of women with a history of HT use, an established risk factor for breast cancer incidence, it is important to establish whether the use of these preparations is also related to survival. Previous studies have been limited by modest sample sizes, restriction to high-risk groups, and inability to evaluate the characteristics of users and subtypes of tumors (3-6, 11, 17-19). We therefore examined the relation between prediagnostic HT use and mortality (from breast cancer and all causes) in a study that addressed these limitations, using data from a well-characterized cohort of 12,269 women with incident invasive breast cancer (20, 21).
MATERIALS AND METHODS
Collaborative Breast Cancer Study Cohort
The Collaborative Breast Cancer Study Cohort began in 1988 as a multi-site population-based case-control study of risk factors for breast cancer (20, 21). A total of 18,269 women with incident invasive breast cancer were enrolled during three successive phases of this study. Age eligibility varied over the course of the study which included women aged 20-74 years in phase 1 (1988-91), aged 50-79 years in phase 2 (1992-95) and aged 20-69 years in phase 3 (1997-2001). Approximately 81% of eligible case women participated in the case-control study.
Ascertainment of Exposure
All subjects completed a structured telephone interview that included detailed information on prediagnosis use of HT, including formulation, routes of administration, frequency for each episode of use, and information on other breast cancer risk factors, specifically reproductive and menstrual history, consumption of specific foods and beverages including alcohol, physical activity, height and weight history, medication use, and personal and family history of cancer. Women were asked to report exposures occurring in the year prior to diagnosis, approximately two years prior to interview. Format of the questions on HT use varied slightly depending on period of data collection; all versions after 1989 elicited a standard history of HT, including type, duration, age started and time since last use. Other questions were phrased somewhat differently, but were readily summarized across study periods. For example, questions on mammography screening always asked about regular screening the year prior to diagnosis but the completeness of history differed somewhat by study instrument.
Clinical information obtained from state cancer registries included date of diagnosis, extent of disease (local, regional and distant) and histology (22). In Wisconsin only, information was available on the first course of treatment (surgery, chemotherapy, radiation, and hormonal treatment).
Population for Analysis
The analysis was limited to women aged 50 years or more at the time of diagnosis, for consistency with all three studies (n=14,462). The following women were excluded: 1,407 were interviewed before complete HT questions were included in the interview; 662 had missing information on HT usage; 116 used hormones before age 40 or surgical menopause, and 8 women were lost during follow up. Thus, 12,265 women were included in the analysis.
Identification of Deaths
Deaths were ascertained up to December 31, 2005 using automated searches of the National Death Index (23). The underlying cause of death on the death certificate was assigned according to the International Classification of Diseases, Ninth Revision (ICD-9) (though 1998) (24) and ICD-10 (1999-2005) (25). We evaluated both death from breast cancer (ICD-9 codes 174 and ICD-10 codes C50), and all-cause mortality. Deaths from other causes, specifically cerebrovascular (ICD-10 I60-I69 and cardiovascular disease (ICD-10, I00-I09, I11, I13, I20-I51) were also examined.
Statistical Analysis
Survival time was calculated as the number of months from date of diagnosis to date of death, or December 31, 2005 for surviving women. Women were classified as ever/never having used HT; women who had ever used HT were then further classified by current use or former use of HT in the year prior to diagnosis. HT exposure by type of preparation was assessed as estrogen alone (“E-alone”) or combined estrogen and progestin (“EP”) when women had exclusively used one of these HT types; otherwise, HT was assessed as use of any preparation (“any HT”). We also examined the duration (<5, ≥ 5 years) and timing (current, former) of use. To determine the risks of dying from breast cancer according to HT (never, any HT, E-alone, EP and by recency of any HT use), we used life table techniques to calculate estimated cumulative incidence of death, a statistical method that accounts for the presence of competing risk (e.g., death from causes other than breast cancer) (26).
Cox proportional hazards regression was used to estimate the adjusted hazard rate ratio (HRR), interpreted here as a rate ratio, and corresponding 95% confidence intervals (95% CI) for death according to categories of HT use (27). All regression models were stratified on study center, year of interview, and exact age at diagnosis. Potential confounders included in multivariate models were body mass index (BMI, kg/m2) in quartiles, smoking status, history of regular mammography screening, time from date of diagnosis to interview, and menopausal status. Women were classified as postmenopausal if they reported having a natural menopause or hysterectomy with bilateral oophorectomy prior to diagnosis. Women with hysterectomy without bilateral oophorectomy were considered postmenopausal if they were ≥55 years of age at diagnosis (or ≥54 for smokers). All reported P values are two sided and statistical significance was evaluated at 0.05. All analyses were performed using SAS version 9.1 (SAS Institute, Inc., Carey, NC).
RESULTS
Women were followed, on average, for 10.3 years from diagnosis. A total of 3,953 deaths were documented, including 1,690 from breast cancer. Women who used HT were younger, of lower BMI, were more likely to have a history of mammographic screening, and more likely to be diagnosed with a local stage of disease than nonusers (Table 1). EP users were more likely than other HT users to be younger, of lower BMI, never smokers, report regular mammographic screening and to be diagnosed with local stage disease.
Table 1.
Baseline characteristics of 12,269 women with breast cancer by hormone therapy (HT) use.
| HT never users | HT ever users | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Never (N=8071) |
E-only (N=2258) |
E+P (N=1340) |
Other/Unknown (N=600) |
||||
| Age at Diagnosis | ||||||||
| 50-54 | 1342 | 16.63% | 335 | 14.84% | 379 | 28.28% | 107 | 17.83% |
| 55-59 | 1116 | 13.83% | 445 | 19.71% | 479 | 35.75% | 152 | 25.33% |
| 60-64 | 1586 | 19.65% | 497 | 22.01% | 324 | 24.18% | 154 | 25.67% |
| 65-69 | 2079 | 25.76% | 517 | 22.90% | 132 | 9.85% | 112 | 18.67% |
| 70-74 | 1332 | 16.50% | 344 | 15.23% | 22 | 1.64% | 67 | 11.17% |
| 75-79 | 616 | 7.63% | 120 | 5.31% | 4 | 0.30% | 8 | 1.33% |
| Menopausal Status | ||||||||
| Postmenopausal | 7109 | 88.08% | 2094 | 92.74% | 1154 | 86.12% | 550 | 91.67% |
| Premenopausal | 807 | 10.00% | 35 | 1.55% | 115 | 8.58% | 28 | 4.67% |
| Unknown | 155 | 1.92% | 129 | 5.71% | 71 | 5.30% | 22 | 3.67% |
| Body Mass Index | ||||||||
| Less than 22.8 | 1724 | 21.36% | 577 | 25.55% | 409 | 30.52% | 192 | 32.00% |
| 22.8-25.5 | 1878 | 23.27% | 565 | 25.02% | 348 | 25.97% | 152 | 25.33% |
| 25.6-29.1 | 2014 | 24.95% | 574 | 25.42% | 314 | 23.43% | 140 | 23.33% |
| 29.2+ | 2164 | 26.81% | 493 | 21.83% | 245 | 18.28% | 108 | 18.00% |
| Unknown | 291 | 3.61% | 49 | 2.17% | 24 | 1.79% | 8 | 1.33% |
| Smoking History | ||||||||
| Never | 3971 | 49.20% | 1114 | 49.34% | 572 | 42.69% | 262 | 43.67% |
| Former | 2509 | 31.09% | 752 | 33.30% | 524 | 39.10% | 222 | 37.00% |
| Current | 1575 | 19.51% | 389 | 17.23% | 242 | 18.06% | 115 | 19.17% |
| Missing | 16 | 0.20% | 3 | 0.13% | 2 | 0.15% | 1 | 0.17% |
| History of Mammography Screening | ||||||||
| Never | 2293 | 28.41% | 249 | 11.03% | 40 | 2.99% | 73 | 12.17% |
| Ever | 5064 | 62.74% | 1842 | 81.58% | 1263 | 94.25% | 487 | 81.17% |
| Unknown | 714 | 8.85% | 167 | 7.40% | 37 | 2.76% | 40 | 6.67% |
| Extent of Disease/Stage | ||||||||
| Local | 4836 | 59.92% | 1470 | 65.10% | 914 | 68.21% | 381 | 63.50% |
| Regional | 2191 | 27.15% | 539 | 23.87% | 322 | 24.03% | 159 | 26.50% |
| Distant | 249 | 3.09% | 36 | 1.59% | 13 | 0.97% | 10 | 1.67% |
| Unknown | 795 | 9.85% | 213 | 9.43% | 91 | 6.79% | 50 | 8.33% |
| Histologic Type | ||||||||
| Lobular | 729 | 9.03% | 219 | 9.70% | 155 | 11.57% | 56 | 9.33% |
| Non-lobular | 7342 | 90.97% | 2039 | 90.30% | 1185 | 88.43% | 544 | 90.67% |
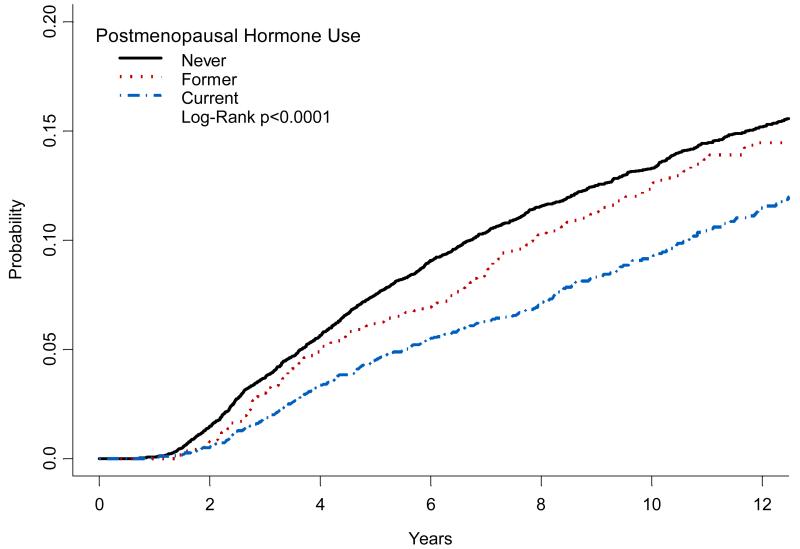
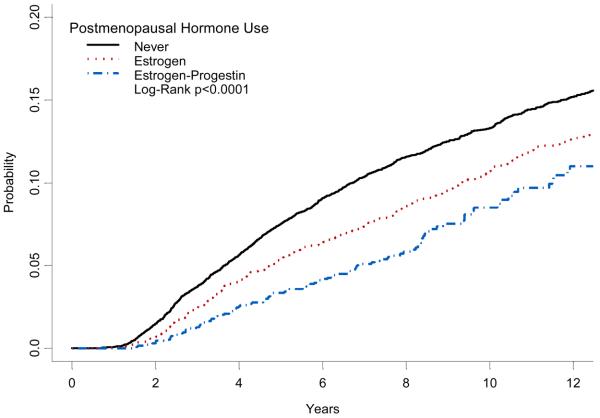
Cumulative breast cancer mortality differed depending on whether the woman had ever used HT, and was statistically significantly lower in current users in the year prior to diagnosis (Figure 1). The lowest cumulative mortality was observed among women using EP (Figure 2).
FIGURE 1.
Kaplan-meier cumulative incidence of breast cancer mortality according to history of hormone therapy use.
FIGURE 2.
Kaplan-meier cumulative incidence of breast cancer mortality by type of hormone therapy preparation.
Overall, there was a significant inverse association between ever having used any HT and breast cancer mortality (adjusted HRR 0.87, 95% CI: 0.78-0.98; Table 2). This multivariate HRR associated with ever use of HT was attenuated from the crude HRR of 0.78, suggesting appreciable confounding by body mass index, history of mammography, and other covariates in the model. Mortality was significantly reduced in current HT users (HRR 0.85, 95% CI: 0.73-0.98) but not former users. After additional adjustment for stage of disease, HRRs changed only slightly (HRR 0.87), suggesting little evidence of further confounding by extent of disease. Among users of HT, breast cancer mortality was statistically significantly reduced for EP users (HRR 0.73, 95% CI: 0.59-0.91) but not for E-alone (HRR = 0.89), however, these estimates were not statistically significantly different.
TABLE 2.
Breast cancer mortality of women by use of hormone therapy prior to breast cancer diagnosis for all women and stratified by stage of disease.
|
All Women* (n=12,269) |
Localized (n=7,601) |
Regional (n=3,270) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hormone Therapy | Number of Deaths |
Rate ratio** |
(95%CI) | Multivariate rate ratio**† |
(95%CI) | Number of Deaths |
Multivariate rate ratio**† |
(95%CI) | Number of Deaths |
Multivariate rate ratio**† |
(95%CI) |
| Never‡ | 1,235 | 1.00 | reference | 1.00 | reference | 362 | 1.00 | reference | 623 | 1.00 | reference |
| Any HT Use | 455 | 0.78 | 0.70-0.88 | 0.87 | 0.78-0.98 | 176 | 1.14 | 0.91-1.38 | 219 | 0.84 | 0.71-1.00 |
| Former HT Use | 168 | 0.86 | 0.73-1.01 | 0.92 | 0.78-1.08 | 62 | 1.05 | 0.80-1.39 | 85 | 0.97 | 0.77-1.23 |
| Current HT Use | 287 | 0.74 | 0.65-0.85 | 0.85 | 0.73-0.98 | 114 | 1.21 | 0.95-1.53 | 134 | 0.76 | 0.62-0.94 |
| Type of Exclusive Treatment | |||||||||||
| Estrogen | 270 | 0.81 | 0.71-0.93 | 0.89 | 0.78-1.02 | 108 | 1.17 | 0.94-1.47 | 131 | 0.90 | 0.74-1.09 |
| EP | 104 | 0.64 | 0.52-0.79 | 0.73 | 0.59-0.91 | 40 | 1.00 | 0.70-1.42 | 49 | 0.67 | 0.49-0.91 |
| Other/Unknown | 81 | 0.91 | 0.72-1.14 | 1.01 | 0.80-1.27 | 28 | 1.20 | 0.81-1.78 | 39 | 0.91 | 0.65-1.27 |
| Former or Current Use by Type of Exclusive Treatment | |||||||||||
| Estrogen, Former | 111 0.81 | 0.67-0.99 | 0.86 | 0.71-1.05 | 47 | 1.12 | 0.83-1.53 | 56 | 0.95 | 0.72-1.26 | |
| Estrogen, Current | 159 0.81 | 0.68-0.96 | 0.91 | 0.77-1.09 | 61 | 1.25 | 0.94-1.67 | 75 | 0.84 | 0.65-1.08 | |
| EP, Former | 20 | 0.90 | 0.58-1.40 | 0.96 | 0.62-1.50 | 5 | 0.78 | 0.32-1.90 | 11 | 0.98 | 0.53-1.80 |
| EP, Current | 84 | 0.59 | 0.47-0.75 | 0.69 | 0.55-0.88 | 35 | 1.08 | 0.74-1.58 | 38 | 0.59 | 0.41-0.84 |
| Duration by Type of Exclusive Treatment | |||||||||||
| Estrogen, < 5 years | 111 | 0.83 | 0.69-1.01 | 0.89 | 0.73-1.08 | 45 | 1.21 | 0.88-1.66 | 54 | 0.86 | 0.64-1.14 |
| Estrogen, ≥ 5 years | 159 | 0.79 | 0.67-0.94 | 0.89 | 0.75-1.06 | 63 | 1.17 | 0.89-1.54 | 77 | 0.91 | 0.71-1.16 |
| EP, < 5 years | 64 | 0.74 | 0.57-0.96 | 0.84 | 0.65-1.09 | 24 | 1.12 | 0.73-1.72 | 31 | 0.72 | 0.49-1.06 |
| EP, ≥ 5 years | 40 | 0.51 | 0.37-0.71 | 0.60 | 0.43-0.84 | 16 | 0.89 | 0.52-1.51 | 18 | 0.56 | 0.34-0.93 |
Includes all women with local, regional, and distant disease.
Proportional hazards models stratified on state, year of interview, and age at diagnosis.
Proportional hazards models adjusted for body mass index, menopausal status, smoking status, mammography, and time from date of diagnosis to interview.
Reference category.
For women using EP, breast cancer mortality varied according to duration and timing of use. A significant reduction in breast cancer mortality associated with HT use was observed for current users of EP (HRR: 0.69; 95% CI: 0.55-0.88) compared to never users of HT, and the greatest benefit was observed for long-term users (≥5 years, HRR: 0.60; 95% CI: 0.43-0.84). In contrast, there was no statistically significant relation between former EP use of any duration, and breast cancer mortality. For users of E-alone preparations, there were no statistically significant associations between breast cancer mortality and current, former or duration of use. Increasing time since last use did not appear to be significantly associated with this inverse relation for either HT type (Pcontinuous >0.05, data not shown)
Results stratified by extent of disease showed statistically significantly lower HRRs among women with breast cancer diagnosed at a regional stage of disease, but not with disease diagnosed at a local stage. Among women diagnosed at a regional stage, HRR's were strongly and significantly lower for both women currently using EP (HRR 0.59; 95% CI: 0.41-0.84) and long-term users of EP (HRR 0.56; 95% CI: 0.34-0.93). The difference between HT by stage was not statistically significant for any type, recency, or duration.
Breast cancer cases diagnosed with lobular (n=1,159) and non-lobular (n=11,110) disease showed similar overall patterns in current E-alone and EP use and breast cancer mortality, with two notable exceptions (data not shown). Current users of E-alone with lobular disease experienced a halving in breast cancer mortality (HRR: 0.53; 95% CI: 0.30-0.96). In contrast, among women with lobular disease, former EP users experienced a three-fold higher breast cancer mortality (HRR 3.24; 95% CI: 1.05-9.99). Although few former EP users had lobular breast cancer as an underlying cause of death, this elevated risk contrasts with the low HRR's seen previously.
The associations with current HT were consistent according to age at diagnosis (<60 years, ≥60 years, p=0.58) and BMI (<25.7 kg/m2, ≥25.7 kg/m2, p=0.67). The results of analyses stratified by state (NH, WI, MA) were also similar to the combined results, with no significant heterogeneity observed. In a sub-analysis of Wisconsin women, where first course of treatment was available, treatment-adjusted results were similar to results unadjusted for treatment (data not shown).
Death from all causes was significantly lower both in current users of HT (adjusted HRR 0.75; 95% CI: 0.68-0.83) and former HT users (adjusted HRR 0.87; 95% CI: 0.78-0.96; Table 3). All cause mortality risks associated with type of preparation differed (p< 0.0001): current user of E-alone was less strongly associated with risk (HRR 0.80; 95% CI: 0.71-0.91) than that associated with EP (HRR 0.61; 95% CI: 0.50-0.73). Inverse relations with mortality also differed by duration and type of preparation (p< 0.0001) among long-term users of EP (HRR 0.55; 95% CI: 0.43-0.71) compared with E-alone (HRR 0.82, 95% CI: 0.73-0.91). HT use was not associated with mortality from other cancers besides breast cancer, or from cerbrovascular diseases. However both E-alone and EP users had reduced risks of mortality from cardiovascular disease (HRR 0.62; 95% CI 0.48-0.80 for E-alone and HRR 0.27; 95% CI 0.12-0.57 for EP).
TABLE 3.
All-cause mortality of women with incident breast cancer by patterns of use of hormone therapy prior to diagnosis.
| Hormone Therapy | Number of Deaths |
Rate ratio* | (95%CI) | Multivariate rate ratio*† |
(95%CI) |
|---|---|---|---|---|---|
| Never‡ | 3009 | 1.00 | reference | 1.00 | reference |
| Any HT Use | 944 | 0.74 | 0.69-0.80 | 0.81 | 0.75-0.87 |
| Former HT Use | 436 | 0.82 | 0.74-0.91 | 0.87 | 0.78-0.96 |
| Current HT Use | 507 | 0.68 | 0.61-0.75 | 0.75 | 0.68-0.83 |
| Type of Exclusive Treatment | |||||
| Estrogen Alone | 610 | 0.76 | 0.70-0.83 | 0.82 | 0.75-0.90 |
| EP | 174 | 0.63 | 0.53-0.73 | 0.68 | 0.58-0.81 |
| Other/Unknown | 160 | 0.83 | 0.71-0.98 | 0.89 | 0.76-1.04 |
| Former or Current Use by Type of Treatment | |||||
| Estrogen, Former | 312 | 0.79 | 0.70-0.89 | 0.84 | 0.74-0.94 |
| Estrogen, Current | 298 | 0.73 | 0.64-0.82 | 0.80 | 0.71-0.91 |
| EP, Former | 44 | 0.97 | 0.72-1.31 | 1.01 | 0.75-1.37 |
| EP, Current | 130 | 0.55 | 0.45-0.66 | 0.61 | 0.50-0.73 |
| Duration by Type of Treatment | |||||
| Estrogen, < 5 years | 251 | 0.78 | 0.69-0.89 | 0.83 | 0.72-0.94 |
| Estrogen, ≥ 5 years | 359 | 0.74 | 0.67-0.83 | 0.82 | 0.73-0.91 |
| EP, < 5 years | 105 | 0.74 | 0.61-0.91 | 0.80 | 0.65-0.98 |
| EP, ≥ 5 years | 69 | 0.49 | 0.39-0.63 | 0.55 | 0.43-0.71 |
Proportional hazards models stratified on state, year of interview, and age at diagnosis.
Proportional hazards models adjusted for body mass index, mammography, smoking status and time from date of diagnosis to interview.
Reference category.
DISCUSSION
The extent to which specific HT use influences the risk of mortality among breast cancer cases had been largely unknown, and no prior research has investigated whether or not this risk varies by either patient or tumor characteristics. In this large population-based cohort of women with breast cancer, current use of HT was associated with a moderately lower breast cancer specific mortality when compared to never use of these preparations. Mortality was lowest among current and long-term users of combined EP therapy. The present results provide the strongest evidence to date that HT use is associated with the subsequent development of less aggressive breast cancers through mechanisms that are not yet fully clear.
Evidence is limited on the relationship between HT use before breast cancer diagnosis and mortality from this disease. This and other studies evaluated self-reported HT use before the diagnosis of invasive breast cancer (3-5). Only one showed a statistically significant lower risk of the association of pre-diagnostic HT use with case fatality in a cohort (n= 2,614 women) with breast cancers assembled in a large breast cancer screening program (5). After adjustment for age, race, BMI, tumor size, and number of positive lymph nodes, women using HT at the time of diagnosis experienced approximately half the risk of dying of breast cancer in both node-negative and node-positive disease, although this effect waned with increasing time since diagnosis. These authors reported that the inverse association was no longer apparent after 4 years for node-positive disease and 12 years for node-negative disease, and thus this association may reflect residual confounding due to screening for node-positive disease, but this is less likely for node-negative disease, given the prolonged protection conferred. Limitations of the study are that the results were not stratified by type of HT, and other relevant personal and tumor characteristics.
In an earlier study, Bergkvist et al compared a group of 261 cases of breast cancer that had taken E-alone prior to diagnosis with 6627 breast cancer cases identified through a population cancer registry whose estrogen exposure status was unknown (3). After consideration of mortality attributable to competing risks of death, the relative survival rate among previous users of HT was suggestively higher when compared with the general cancer registry cases with a greater reduction in breast cancer mortality in users of EP. Other investigators have reported decreased all-cause mortality among women with breast cancer who had used HT, though these studies made no adjustment for competing risks of death, potentially leading to bias (4, 6, 11, 28).
Studies have also generally shown lower breast cancer mortality with HT use in women initially without cancer, although in one study the mortality effects observed with HT use appear to wane over time, with increased breast cancer mortality observed among women using HT for 10 years of more (29). Because studies have consistently indicated a modestly increased risk of developing breast cancer in HT users (30-33), these results suggest that breast cancers that develop in HT users may be associated with a less aggressive course than breast cancers that develop in nonusers (9, 29, 34-42). A further reason for lower case-fatality may be that the cancers developing in women using HT are selected to be more hormonally responsive. Thus, with termination of the promoting factor at diagnosis (HT use) and the use of anti-estrogen treatment, now standard of care, these tumors would be expected to be associated with improved prognosis.
It has been suggested that the reduction in breast cancer mortality associated with HT use is attributable to an earlier stage at diagnosis (3, 19), which may be due to a higher likelihood of screening among HT users (surveillance bias) (5) or the tendency for women who develop a serious illness to stop taking HT (healthy estrogen-user effect) (43), rather than a modifying effect of hormone use on tumor biology. We observed that the inverse association between HT use and breast cancer mortality was limited to women originally diagnosed with regional, but not localized, disease. It has been well-documented that HT users are likely to be screened more aggressively than non-users (44) and have cancers that are diagnosed at an earlier stage (45), despite evidence that use of postmenopausal hormones reduces both sensitivity and specificity of screening mammograms (46). However, even in analyses that adjust for screening, cancers that develop in HT users tend to be smaller (11, 19), of lower grade (47), have fewer positive axillary lymph nodes (11, 19, 48), lower tumor cell proliferation rate (49, 50), and have other clinically more favorable features (14, 48). Yet, in the Women's Health Initiative (WHI) randomized trial of the combined EP regimen, the rate of incident metastatic breast cancer was similar regardless of HT assignment (2).
It may also be relevant to consider an effect of HT's on tumor growth after diagnosis. Although rare, HT use initiated after diagnosis of breast cancer has been shown to have a beneficial (5, 17, 51) or neutral (18) association with survival, and there has been no observed improvement in survival associated with duration of use or route of administration (oral or vaginal cream) (51).
In our study, we found better breast cancer survival among women who used combined EP therapy before diagnosis. Widespread use of combined EP preparations began in the 1980's (52) and most earlier mortality studies evaluated the use of E-alone formulations. Two previous studies have reported more favorable prognostic profiles associated with combined estrogen-progestin therapy relative to other types of HT. Magnusson et al. found that women receiving a combined EP regimen were less likely to have tumors >20mm in diameter, but to have axillary lymph node dissemination, and poorly differentiated, or aneuploid tumors at diagnosis (19). Daling et al. observed that the tumors of users of continuous EP therapy (relative to E-alone therapy or sequential EP therapy) were more likely to be estrogen receptor and progesterone receptor positive (53), features that are associated with better prognosis. (14) Thus, our observation of reduced mortality among users of combined HT might be expected, based upon the generally favorable profiles of the tumors occurring among women using HT compared to the tumors developing in non-users, or users of other regimens.
Our confidence in these study results is enhanced by the large sample size, mature follow-up, and availability of comprehensive information on tumor stage and other covariates associated with breast cancer mortality. Arising from a population-based study with high response rates, the cohort reflected the spectrum of breast cancer as it occurs in the population. However, some limitations should be considered when interpreting our results. This evaluation was based upon HT use before diagnosis, approximately two years prior to interview. Participants were not followed-up for changes in HT practices after breast cancer diagnosis, except on a subset of the population that participated in a study of post-diagnosis diet and other factors, including HT, in relation to breast cancer survival. In this actively followed sub-group, few women (4.5%) reported use of HT—which has generally not been recommended after breast cancer diagnosis (54). Thus, the uncommon use of post diagnostic HT is unlikely to have biased our results. However, other exposures sustained or initiated after diagnosis may affect survival. Unmeasured post-diagnosis characteristics of HT users, such as changes in weight and physical activity, could influence the observed differences in survival according to HT use. To reduce this possibility we excluded from the analysis women whose breast cancer was diagnosed at a late stage and the results were unchanged.
Screening is a particularly important covariate affecting breast cancer survival. In our population, HT was associated with mammography; only 10% of HT users had never been screened compared with 30% of never HT users. Surprisingly, stratification by mammography screening suggested stronger inverse relations with HT, particularly with respect to EP use, among women who were not screened compared to women who reported regular screening. Limited sample sizes made it impossible to rule out chance in these associations, but the results are generally reassuring in that characteristics as measured by screening use are unlikely to have introduced bias. The examination of cause specific mortality may suggest some artifact of unmeasured confounders, since HT users did have statistically significantly reduced cardiovascular disease. Follow-up of cases randomized to receive HT prior to diagnosis, such as in the WHI or HERS studies, will help address this limitation of observational studies.
We were unable to consider the ER/PR status of tumors in our analysis. As a common phenotype of breast cancer tumors, the inability to control for receptor status is unlikely to overestimate our estimates of survival by HT use; rather, the combination of all tumor types increases the heterogeneity of our sample and may attenuate our results if HT use is related to survival only among those with tumors expressing ER/PR. However, since ER/PR positivity increases with increasing age (55), and our sample was postmenopausal, most women's tumors would have been hormone receptor positive.
In summary, we found that use of HT prior to diagnosis in a large population-based cohort of women with breast cancer was associated with improved breast cancer survival. Survival was best among current and long-term users among women using combination regimens of EP, and appeared limited to women with regional disease. The better breast cancer survival in users of HT prior to diagnosis persisted after adjustment for screening, stage, and measured risk factors.
Acknowledgments
Financial Support: Grants CA47147, CA67264, and CA47305 from the National Institutes of Health.
REFERENCES
- 1.Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350(9084):1047–59. [PubMed] [Google Scholar]
- 2.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 3.Bergkvist L, Adami HO, Persson I, Bergstrom R, Krusemo UB. Prognosis after breast cancer diagnosis in women exposed to estrogen and estrogen-progestogen replacement therapy. Am J Epidemiol. 1989;130(2):221–8. doi: 10.1093/oxfordjournals.aje.a115328. [DOI] [PubMed] [Google Scholar]
- 4.Ewertz M, Gillanders S, Meyer L, Zedeler K. Survival of breast cancer patients in relation to factors which affect the risk of developing breast cancer. Int J Cancer. 1991;49(4):526–30. doi: 10.1002/ijc.2910490409. [DOI] [PubMed] [Google Scholar]
- 5.Schairer C, Gail M, Byrne C, Rosenberg PS, Sturgeon SR, Brinton LA, et al. Estrogen replacement therapy and breast cancer survival in a large screening study. J Natl Cancer Inst. 1999;91(3):264–70. doi: 10.1093/jnci/91.3.264. [DOI] [PubMed] [Google Scholar]
- 6.Strickland DM, Gambrell RD, Jr., Butzin CA, Strickland K. The relationship between breast cancer survival and prior postmenopausal estrogen use. Obstet Gynecol. 1992;80(3 Pt 1):400–4. [PubMed] [Google Scholar]
- 7.Colditz GA, Hankinson SE, Hunter DJ, Willett WC, Manson JE, Stampfer MJ, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332(24):1589–93. doi: 10.1056/NEJM199506153322401. [DOI] [PubMed] [Google Scholar]
- 8.Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prior to use of estrogen replacement therapy, are users healthier than nonusers? Am J Epidemiol. 1996;143(10):971–8. doi: 10.1093/oxfordjournals.aje.a008678. [DOI] [PubMed] [Google Scholar]
- 9.Sturgeon SR, Schairer C, Brinton LA, Pearson T, Hoover RN. Evidence of a healthy estrogen user survivor effect. Epidemiology. 1995;6(3):227–31. doi: 10.1097/00001648-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Cheek J, Lacy J, Toth-Fejel S, Morris K, Calhoun K, Pommier RF. The impact of hormone replacement therapy on the detection and stage of breast cancer. Arch Surg. 2002;137(9):1015–9. doi: 10.1001/archsurg.137.9.1015. [DOI] [PubMed] [Google Scholar]
- 11.Bonnier P, Romain S, Giacalone PL, Laffargue F, Martin PM, Piana L. Clinical and biologic prognostic factors in breast cancer diagnosed during postmenopausal hormone replacement therapy. Obstet Gynecol. 1995;85(1):11–7. doi: 10.1016/0029-7844(94)00324-7. [DOI] [PubMed] [Google Scholar]
- 12.Stahlberg C, Pedersen AT, Andersen ZJ, Keiding N, Hundrup YA, Obel EB, et al. Breast cancer with different prognostic characteristics developing in Danish women using hormone replacement therapy. Br J Cancer. 2004;91(4):644–50. doi: 10.1038/sj.bjc.6601996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerlikowske K, Miglioretti DL, Ballard-Barbash R, Weaver DL, Buist DS, Barlow WE, et al. Prognostic characteristics of breast cancer among postmenopausal hormone users in a screened population. J Clin Oncol. 2003;21(23):4314–21. doi: 10.1200/JCO.2003.05.151. [DOI] [PubMed] [Google Scholar]
- 14.Schnitt SJ. Traditional and newer pathologic factors. J Natl Cancer Inst Monogr. 2001;(30):22–6. doi: 10.1093/oxfordjournals.jncimonographs.a003456. [DOI] [PubMed] [Google Scholar]
- 15.Brett KM, Madans JH. Use of postmenopausal hormone replacement therapy: estimates from a nationally representative cohort study. Am J Epidemiol. 1997;145(6):536–45. doi: 10.1093/oxfordjournals.aje.a009142. [DOI] [PubMed] [Google Scholar]
- 16.Kelly JP, Kaufman DW, Rosenberg L, Kelley K, Cooper SG, Mitchell AA. Use of postmenopausal hormone therapy since the Women's Health Initiative findings. Pharmacoepidemiol Drug Saf. 2005;14(12):837–42. doi: 10.1002/pds.1103. [DOI] [PubMed] [Google Scholar]
- 17.diSaia PJ, Brewster WR, Ziogas A, Anton-Culver H. Breast cancer survival and hormone replacement therapy: a cohort analysis. Am J Clin Oncol. 2000;23(6):541–5. doi: 10.1097/00000421-200012000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Durna EM, Heller GZ, Leader LR, Sjoblom P, Eden JA, Wren BG. Breast cancer in premenopausal women: recurrence and survival rates and relationship to hormone replacement therapy. Climacteric. 2004;7(3):284–91. doi: 10.1080/13697130400001380. [DOI] [PubMed] [Google Scholar]
- 19.Magnusson C, Holmberg L, Norden T, Lindgren A, Persson I. Prognostic characteristics in breast cancers after hormone replacement therapy. Breast Cancer Res Treat. 1996;38:325–34. doi: 10.1007/BF01806152. [DOI] [PubMed] [Google Scholar]
- 20.Newcomb PA, Egan KM, Titus-Ernstoff L, Trentham-Dietz A, Greenberg ER, Baron JA, et al. Lactation in relation to postmenopausal breast cancer. Am J Epidemiol. 1999;150(2):174–182. doi: 10.1093/oxfordjournals.aje.a009977. [DOI] [PubMed] [Google Scholar]
- 21.Newcomb PA, Titus-Ernstoff L, Egan KM, Trentham-Dietz A, Baron JA, Storer BE, et al. Postmenopausal estrogen and progestin use in relation to breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11(7):593–600. [PubMed] [Google Scholar]
- 22.Percy C, Van Holten V, Muir C, editors. International Classification of Diseases for Oncology. 2nd Ed. World Health Organization; Geneva: 2000. [Google Scholar]
- 23.Calle EE, Terrell DD. Utility of the National Death Index for ascertainment of mortality among cancer prevention study II participants. Am J Epidemiol. 1993;137(2):235–41. doi: 10.1093/oxfordjournals.aje.a116664. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization . International Classification of Diseases (ICD-9) WHO; Geneva: 1977. [Google Scholar]
- 25.World Health Organization . International Classification of Diseases (ICD-10) WHO; Geneva: 1994. [Google Scholar]
- 26.Pepe MS, Mori M. Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Statistics in Medicine. 1993;12(8):737–51. doi: 10.1002/sim.4780120803. [DOI] [PubMed] [Google Scholar]
- 27.Breslow NE, Day NE. Volume II-The design and analysis of cohort studies. IARC Scientific Publications; Lyon: 1987. Statistical methods in cancer research. [PubMed] [Google Scholar]
- 28.Jernstrom H, Bendahl PO, Lidfeldt J, Nerbrand C, Agardh CD, Samsioe G. A prospective study of different types of hormone replacement therapy use and the risk of subsequent breast cancer: the women's health in the Lund area (WHILA) study (Sweden) Cancer Causes Control. 2003;14(7):673–80. doi: 10.1023/a:1025635720208. [DOI] [PubMed] [Google Scholar]
- 29.Grodstein F, Stampfer MJ, Colditz GA, Willett WC, Manson JE, Joffe M, et al. Postmenopausal hormone therapy and mortality. N Engl J Med. 1997;336(25):1769–75. doi: 10.1056/NEJM199706193362501. [DOI] [PubMed] [Google Scholar]
- 30.Persson I, Weiderpass E, Bergkvist L, Bergstrom R, Schairer C. Risks of breast and endometrial cancer after estrogen and estrogen- progestin replacement. Cancer Causes Control. 1999;10(4):253–60. doi: 10.1023/a:1008909128110. [DOI] [PubMed] [Google Scholar]
- 31.Ross RK, Paganini-Hill A, Wan PC, Pike MC. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst. 2000;92(4):328–32. doi: 10.1093/jnci/92.4.328. [DOI] [PubMed] [Google Scholar]
- 32.Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000;283(4):485–91. doi: 10.1001/jama.283.4.485. [DOI] [PubMed] [Google Scholar]
- 33.Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. JAMA. 2003;289(24):3243–53. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 34.Henderson BE, Paganini-Hill A, Ross RK. Decreased mortality in users of estrogen replacement therapy. Arch Intern Med. 1991;151(1):75–8. [PubMed] [Google Scholar]
- 35.Hunt K, Vessey M, McPherson K. Mortality in a cohort of long term users of hormone replacement therapy: an update analysis. Br J Obstet Gynaecol. 1990;97:1080–1086. doi: 10.1111/j.1471-0528.1990.tb02494.x. al. e. [DOI] [PubMed] [Google Scholar]
- 36.Sellers TA, Mink PJ, Cerhan JR, Zheng W, Anderson KE, Kushi LH, et al. The role of hormone replacement therapy in the risk for breast cancer and total mortality in women with a family history of breast cancer. Ann Intern Med. 1997;127(11):973–80. doi: 10.7326/0003-4819-127-11-199712010-00004. [DOI] [PubMed] [Google Scholar]
- 37.Willis DB, Calle EE, Miracle-McMahill HL, Heath CW., Jr. Estrogen replacement therapy and risk of fatal breast cancer in a prospective cohort of postmenopausal women in the United States. Cancer Causes Control. 1996;7(4):449–57. doi: 10.1007/BF00052671. [DOI] [PubMed] [Google Scholar]
- 38.Sourander L, Rajala T, Raiha I, Makinen J, Erkkola R, Helenius H. Cardiovascular and cancer morbidity and mortality and sudden cardiac death in postmenopausal women on oestrogen replacement therapy (ERT) Lancet. 1998;352(9145):1965–9. doi: 10.1016/S0140-6736(98)05066-1. [DOI] [PubMed] [Google Scholar]
- 39.Paganini-Hill A. Morbidity and mortality changes with estrogen replacement therapy. In: Lobo RA, editor. Treatment of the postmenopausal woman: basic and clinical aspects. Raven Press; New York: 1994. pp. 399–404. [Google Scholar]
- 40.Ettinger B, Friedman GD, Bush T, Quesenberry CP., Jr. Reduced mortality associated with long-term postmenopausal estrogen therapy. Obstet Gynecol. 1996;87(1):6–12. doi: 10.1016/0029-7844(95)00358-4. [DOI] [PubMed] [Google Scholar]
- 41.Persson I, Yuen J, Bergkvist L, Schairer C. Cancer incidence and mortality in women receiving estrogen and estrogen-progestin replacement therapy--long-term follow-up of a Swedish cohort. Int J Cancer. 1996;67(3):327–32. doi: 10.1002/(SICI)1097-0215(19960729)67:3<327::AID-IJC4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 42.Vakil DV, Morgan RW, Halliday M. Exogenous estrogens and development of breast and endometrial cancer. Cancer Detect Prev. 1983;6(45):415–24. [PubMed] [Google Scholar]
- 43.Yuen J, Persson I, Bergkvist L, Hoover R, Schairer C, Adami HO. Hormone replacement therapy and breast cancer mortality in Swedish women: results after adjustment for ‘healthy drug-user’ effect. Cancer Causes Control. 1993;4(4):369–74. doi: 10.1007/BF00051340. [DOI] [PubMed] [Google Scholar]
- 44.Carney PA, Kasales CJ, Tosteson AN, Weiss JE, Goodrich ME, Poplack SP, et al. Likelihood of additional work-up among women undergoing routine screening mammography: the impact of age, breast density, and hormone therapy use. Prev Med. 2004;39(1):48–55. doi: 10.1016/j.ypmed.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 45.Antoine C, Liebens F, Carly B, Pastijn A, Rozenberg S. Influence of HRT on prognostic factors for breast cancer: a systematic review after the Women's Health Initiative trial. Hum Reprod. 2004;19(3):741–56. doi: 10.1093/humrep/deh112. [DOI] [PubMed] [Google Scholar]
- 46.Laya MB, Larson EB, Taplin SH, White E. Effect of estrogen replacement therapy on the specificity and sensitivity of screening mammography. J Natl Cancer Inst. 1996;88(10):643–9. doi: 10.1093/jnci/88.10.643. [DOI] [PubMed] [Google Scholar]
- 47.Harding C, Knox WF, Faragher EB, Baildam A, Bundred NJ. Hormone replacement therapy and tumour grade in breast cancer: prospective study in screening unit. BMJ. 1996;312(7047):1646–7. doi: 10.1136/bmj.312.7047.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Squitieri R, Tartter PI, Ahmed S, Brower ST, Theise ND. Carcinoma of the breast in postmenopausal hormone user and nonuser control groups. J Am Coll Surg. 1994;178(2):167–70. [PubMed] [Google Scholar]
- 49.Oestreicher N, White E, Malone KE, Porter PL. Hormonal factors and breast tumor proliferation: do factors that affect cancer risk also affect tumor growth? Breast Cancer Res Treat. 2004;85(2):133–42. doi: 10.1023/B:BREA.0000025402.70958.3e. [DOI] [PubMed] [Google Scholar]
- 50.Holli K, Isola J, Cuzick J. Low biologic aggressiveness in breast cancer in women using hormone replacement therapy. J Clin Oncol. 1998;16(9):3115–20. doi: 10.1200/JCO.1998.16.9.3115. [DOI] [PubMed] [Google Scholar]
- 51.O'Meara ES, Rossing MA, Daling JR, Elmore JG, Barlow WE, Weiss NS. Hormone replacement therapy after a diagnosis of breast cancer in relation to recurrence and mortality. J Natl Cancer Inst. 2001;93(10):754–61. doi: 10.1093/jnci/93.10.754. [DOI] [PubMed] [Google Scholar]
- 52.International Agency for Research on Cancer . IARC monographs on the evaluation of carcinogenic risks to humans: postmenopausal hormone therapy and hormonal contraception. IARC Press; Lyon: 1999. [Google Scholar]
- 53.Daling JR, Malone KE, Doody DR, Voight LF, Bernstein L, Coates RJ, et al. Relation of regimens of combined hormone replacement therapy to lobular, ductal, and other histologic types of breast carcinoma. Cancer. 2002;95(12):2455–64. doi: 10.1002/cncr.10984. [DOI] [PubMed] [Google Scholar]
- 54.Zielinski SL. Hormone replacement therapy for breast cancer survivors: an answered question? J Natl Cancer Inst. 2005;97(13):955. doi: 10.1093/jnci/dji198. [DOI] [PubMed] [Google Scholar]
- 55.Kardinal CG. Hormonal and Endocrine Therapy of Breast Cancer. In: Donegan WL, Spratt JS, editors. Cancer of the Breast. 5th ed. Saunders; St. Louis, MO: 2002. pp. 693–737. [Google Scholar]