Epidemiology
An estimated 30 to 65 percent of pregnant women in the U.S. have genital infection with herpes simplex virus (HSV)-1 or -2.1 Neonatal HSV, defined as infection in a newborn within 28 days of birth, is an especially devastating consequence of the genital herpes epidemic. Untreated neonatal HSV has only a 40 percent survival rate and even the early initiation of high-dose intravenous acyclovir therapy results in significant disability among survivors. The frequency of neonatal HSV infection varies by patient population; between 1 in 1,700 (60 per 100,000) and 1 in 8,200 (12 per 100,000) live births in the U.S. may be complicated by neonatal HSV infection (Table 1). A retrospective study in California reported rates of 12.2 per 100,000 births from 1995 to 2003.2 Data from 30 U.S. health plans including 17 million enrollees showed a rate of 60 per 100,000 births.3 Prospective, single center studies in the U.S. have shown neonatal HSV rates as high as 31.2 per 100,000 (1 in 3,200) births.4 These incidence data for neonatal HSV are similar to those of perinatal human immunodeficiency virus (HIV) infection before the advent of routine antiretroviral use in pregnancy and are higher than those of congenital syphilis, toxoplasmosis, and congenital rubella in endemic years (Table 1).2, 5–9
Table 1.
Incidence of neonatal HSV and other congenital infections in North America
| Location | Dates | Rate (per 100,000 live births) | Source | |
|---|---|---|---|---|
| National or State Surveillance System | ||||
| Neonatal Herpes | Canada41 | 2000–2003 | 5.9 | Active surveillance of all Canadian pediatricians |
| Washington State | 2000–2004 | 2.5 | Washington State Dept. of Health | |
| Ohio | 1997–2001 | 2.6 | Ohio State Dept. of Health | |
| Hospital Discharge Data | ||||
| Washington State31 | 1987–2002 | 8.4 | Hospital discharge data | |
| New York City48 | 1994–2003 | 13.4 | Hospital discharge data | |
| California2 | 1995–2003 | 12.2 | Hospital discharge data | |
| California | 1985–1995 | 11.5 | Hospital discharge data | |
| Integrated Health Care Information Services National Managed Care Benchmark Database3 |
1997–2002 | 60 | Hospital discharge data from30 health plans covering 7 US census regions | |
| Prospective Cohort Studies | ||||
| Washington State4 | 1982–1999 | 31.2 | Prospective cohort study of pregnant women | |
| Syphilis* | Washington State | 1996–2004 | 0.8 | Jo Hofmann, DOH epidemiologist |
| US | 1995–2005 | 14.3 | CDC | |
| Toxoplasmosis† | Ohio | 1997–2003 | <1.0 | Ohio State Dept. of Health |
| Group B Strep‡ | 5–7 US metropolitan areas | 1997–2003 | 55.3 | ABC group (CDC) |
| Ohio | 1997–2001 | 43 | Ohio State Dept. of Health | |
| Rubella § | US | 1970 | 2.1 | CDC |
| US | 1995–2005 | <0.1 | CDC | |
| HIV ¶ | Washington State | 1997–2005 | 1.5 | Washington State Dept. of Health |
| Michigan49 | 1993–2000 | 5.4 | Michigan Department of Community Health | |
| US9 | 2002 | 4.5 | CDC |
Syphilis: Congenital syphilis is reportable on both a state and U.S. basis and varies greatly by regions. In Washington State, there were a total of six cases reported between 1996 and 2004, (0.8 cases per 100,000 live births). The CDC national data between 1995 and 2005 was 14.3 cases/100,000 live births.
Toxoplasmosis: Toxoplasmosis is reportable in 13 states; however, most of these states do not differentiate between congenital and non-congenital cases. Congenital Toxoplasmosis is reported in Ohio. Between 1997 and 2003 there were only 3 cases of congenital toxoplasmosis, resulting in an annual incidence <1.0 per 100,000 live births.
Group B Strep: Incidence rates of congenital group B strep come from 2 sources: the Active Bacterial Core (ABC) Surveillance group at the CDC and the Ohio State Department of Health. The ABC group monitors 5–7 U.S. metropolitan areas.
Rubella: Congenital rubella syndrome is reportable in most states. In 1970, prior to rubella vaccination, there were 77 cases of CRS, with an incidence of 2.1 cases per 100,000 live births. Since vaccination has become routine, the rate has dropped below 0.1 cases per 100,000 live births.
HIV: Pediatric HIV infection is reportable in about two-thirds of the states. Washington State, Michigan and national data from 2002 are reported. A 2002 estimate for the entire country, was formulated by extrapolating the number of cases in 33 states where perinatal herpes infection was reported to the national population.
Pathophysiology
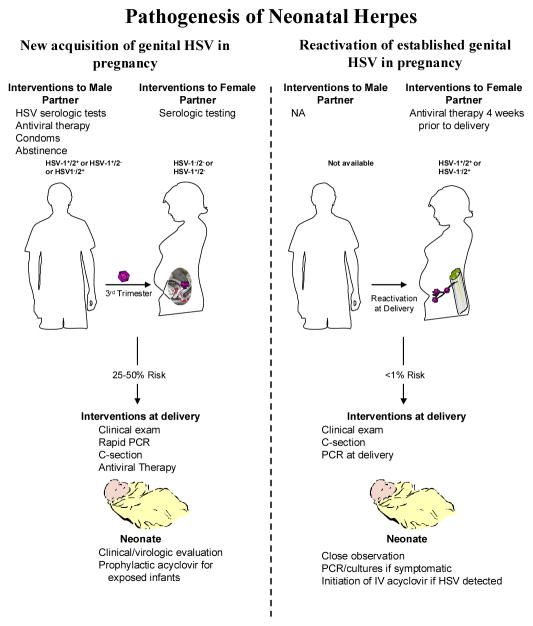
Most neonatal infection results from exposure to HSV in the genital tract during delivery, although in utero and postnatal infections can occasionally occur.10 While most clinical management guidelines for HSV infections concentrate on women with long established disease, the risk of transmission is significantly greater among women who acquire genital infection with HSV-1 or HSV-2 during pregnancy (risk of 25 to 50 percent) than among those who have longstanding HSV-2 infection and subsequently reactivate virus in the genital tract at term (risk of <1 percent) (Figure 1, Table 2). Thus, while the number of infants born to women with newly acquired HSV at the end of pregnancy is much smaller than the number of infants born to women with established HSV-2, the much greater efficiency of HSV transmission during newly acquired genital HSV accounts for the fact that 50 to 80 percent of neonatal HSV cases result from women who acquire genital HSV-1 or HSV-2 infection near term.10, 11 Most genital HSV acquisition in women occurs without signs or symptoms of disease and is associated with cervical viral shedding.
Figure 1.
Pathogenesis of Neonatal Herpes
Table 2.
Common misperceptions about neonatal herpes
| Misperception | Evidence |
|---|---|
| 1. Most infants with neonatal HSV are born to women with a past history of genital herpes. | Most maternal-fetal transmission comes from women with undiagnosed genital herpes, many of whom have acquired HSV-1 or -2 for the first time near-term. |
| 2. HSV-1 infection usually is acquired from non-maternal sources. | Neonatal HSV-1 infection accounts for 30–50% of all reported HSV-1. More than three-fourths of cases are from recently acquired genital HSV-1 in the mother, with subsequent transmission to the infant during delivery. |
| 3. Suppressive antiviral therapy at the end of pregnancy eliminates the risk of neonatal HSV infection. | Suppressive acyclovir reduces the frequency of genital lesions near-term and the frequency of cesarean delivery. There are no data to suggest it reduces the risk of neonatal herpes. |
| 4. Most infants with neonatal herpes present with vesicular lesions. | Neonatal HSV infection often presents with a sepsis-like syndrome or with new onset of seizures. Skin or mucosal lesions may not appear until late in the disease course, or not at all. |
| 5. Cutaneous HSV infection in the infant can be treated with topical or oral antivirals. | All cases of presumptive neonatal HSV should be treated with intravenous acyclovir. Confirmed cases of SEM disease should be treated with 60 mg/kg/day for 14 days and for CNS or disseminated disease for 21 days11. |
| 6. IgM antibodies are useful for the diagnosis of neonatal herpes. | IgM assays are not reliable. HSV DNA detection is the optimal method for diagnosis21. |
Approximately 2 percent of HSV-2 seropositive women by culture and 8 to 15 percent by polymerase chain reaction (PCR) have HSV-2 detected in genital secretions at term.12, 13 Almost none of this shedding is accompanied by clinically detectable genital lesions. Despite the frequent exposure to HSV during birth, <1 percent of infants delivered vaginally to women shedding HSV-2 at term develop neonatal herpes.10, 11, 14 The discrepancy between the high shedding rate among women with established HSV-2 infection and the low neonatal transmission rate suggests a role for transplacental antibody to abrogate the risk of infection. This difference in transmission risk to the neonate between the initial acquisition of HSV during pregnancy versus reactivation of prior infection contributes to the divergent patient management and public health strategies suggested to impact neonatal HSV.
Diagnosis
Genital HSV infections are often subclinical and, even if symptomatic, lack specificity in their signs and symptoms. Case series have shown that most primary genital herpes infections in pregnant women are not diagnosed accurately by clinicians.15 Women who present in pregnancy with HSV infection should have both a type-specific serological assay as well as a test of the virus to identify and type their HSV infection.12 This approach allows the clinician to objectively categorize the infant at highest risk of infection. Laboratory tests include viral isolation in culture or direct fluorescent antibody (DFA) studies to detect viral protein from genital lesions,, or PCR to test for presence of viral DNA.12 PCR assessment is the most sensitive and usually most rapid measure.16 Accurate type-specific serological assays are based on the difference in epitope specific immune responses to the HSV glycoprotein G molecule of HSV-1 v. HSV-2; occasionally tests based on whole antigen response are reported inaccurately as type-specific by diagnostic laboratories. Similarly, commercial IgM assays to HSV-1 and HSV-2 are not validated in pregnancy or in infants. Antibodies to gG1 or gG2 tend to develop reasonably late in the course of infection—2 to 12 weeks; hence, detection of virus in a seronegative woman or discordance between the type of viral isolate and antibody status, for example, HSV-2 isolate in a mother with only type specific HSV-1 antibodies, identifies women with recently acquired infection.
Manifestations
Congenital HSV infection is rare, shares clinical features with other congenital infections, including microcephaly, hydrocephalus, and chorioretinitis, and usually presents with clinical abnormalities at birth. Post-natal acquisition is almost always due to HSV-1 and is associated with contact with hospital personnel or family members who are shedding HSV-117. Ritual circumcision that involves suctioning of the wound with the mouth also has been associated with neonatal HSV-1.18
Most neonatal infection results from exposure to HSV during delivery. The clinical presentation has been divided into three categories, each associated with different outcomes and clinical manifestations. Neonates with infection confined to skin, eyes and mucosa (SEM), which comprise about 45% of most case series, often present with vesicular lesions on the skin, eye or mouth and, by definition, have no central nervous system (CNS) or visceral organ involvement (normal CSF indices; normal neurological and CT findings; and no evidence of pneumonitis, hepatitis, coagulation problems, etc.). Systemic therapy is required; otherwise, further progression may occur. However, with high-dose intravenous acyclovir, the long-term developmental outcome of this form of neonatal herpes is good. Children with SEM herpes often have recurrent cutaneous herpes outbreaks during early childhood. Suppressive antiviral therapy reduces the frequency of these cutaneous recurrences, but breakthrough infections may still occur. CNS-associated infection, which comprises 30% of most large case series, is associated with lethargy, poor feeding, or seizures, with or without cutaneous lesions. CSF pleocytosis is usually present, HSV DNA in cerebral spinal fluid is the most sensitive laboratory test to confirm the diagnosis, and samples obtained early in the course of the disease may be falsely negative. Morbidity of CNS HSV in infants is higher with HSV-2 than HSV-1, and may include developmental delay, epilepsy, blindness and cognitive disabilities. Prompt initiation of therapy influences outcome; unfortunately, non-specific presentation may delay diagnosis. Acyclovir therapy has substantially improved survival (Table 3); however, neonates with CNS HSV-2 infection still have high rates of developmental problems at one year and over 50 percent have moderate to severe neurological abnormalities.19, 20 Moreover, relapses of CNS infection may occur, further increasing morbidity. The highest fatality rate for neonatal HSV is associated with disseminated infection (25% of case series) involving multiple organs (such as lung, liver and brain) and appearing clinically indistinguishable from bacterial sepsis.17, 19, 20 The risk of death from disseminated neonatal HSV is still high (30 percent), even with antiviral therapy. 19, 20. Any vesicular rash in a neonate should be evaluated for HSV. As up to 50 percent of neonatal HSV infection presents without skin rash, all infants younger than 4 weeks with CNS infection or sepsis syndromes should have a laboratory evaluation, preferably with PCR, performed for HSV infection, including plasma/blood sample for HSV DNA.21, 22 CSF HSV PCR assay is considered cost-effective in febrile newborns with pleocytosis.23
Table 3.
Outcome of neonatal herpes by category of disease
| Category of disease | Mortality | Normal outcome | ||
|---|---|---|---|---|
| Without therapy50 | With IV antiviral therapy19 | Without therapy50 | With IV antiviral therapy19 | |
| Disseminated disease | 85% | 31% | rare | 83% |
| Central Nervous System disease | 50% | 6% | rare | 31% |
| Skin, eye and mucosa | 0%* | 0% | 62% | 100% |
A high proportion of infants with skin, eye and mucosa infection will progress to encephalitis or disseminated disease in the absence of antiviral therapy.
Treatment of Neonatal Herpes
Antiviral therapy with intravenous acyclovir reduces mortality to 30 percent in infants with disseminated disease and to 6 percent for those with CNS disease (Table 3). Recommended doses are acyclovir 20 mg/kg body weight intravenous every eight hours for 21 days for disseminated and CNS disease21 and for 14 days for disease limited to the skin and mucous membranes. Many experts also recommend the 14 day regimen for asymptomatic infants born to women who acquired HSV near term. Acyclovir is superior to vidarabine, the only other antiviral that has been systematically evaluated for neonatal HSV. Transient neutropenia has been noted in about 20% of infants treated with these high doses of acyclovir, but has not been reported to result in clinically significant adverse outcomes.20 Rare cases of acyclovir-resistant neonatal HSV have been reported.
Prevention
Neonatal HSV is as severe a disease as other neonatal infections for which prevention strategies have been implemented and remains one of the most serious neonatal infections (Table 3). Recent reports in the medical and popular press document an ongoing controversy about best management for the prevention of neonatal HSV infection, with diverse and sometimes opposite conclusions.24–28 As this is an area of common misunderstanding, we will review these issues.
Preventing Neonatal HSV by Reducing Acquisition of HSV-1 or -2 in Late Pregnancy
Development of a vaccine that prevents acquisition of HSV-1 and HSV-2 infection would be the most effective strategy to reduce neonatal herpes. However, at present such a vaccine is not available. Protective immunity against HSV is incompletely understood, and the commonly used animal models – mice and guinea pigs – reflect only certain aspects of human HSV infections accurately. This has limited development of candidate HSV vaccines. Prior investigational vaccines have lacked efficacy against HSV-2 infection in clinical trials; a single-antigen recombinant vaccine showed partial efficacy against HSV-2 disease but demonstrated only marginal efficiency in reducing HSV-2 acquisition among seronegative women. An additional Phase 3 trial with this product is underway. An effective HSV-2 vaccine for pregnant women would need to prevent subclinical reactivation of HSV at the time of delivery to impact neonatal herpes.
Non vaccine proposed strategies to reduce acquiring HSV during pregnancy include (1) counseling all women to avoid unprotected sexual intercourse and unprotected oral-genital contact in late pregnancy, (2) HSV serological testing of women to identify those at risk of acquisition and (3) HSV serological testing of women and partners to identify those with discordant serological status. These strategies rely on sexual behavior change by pregnant women at risk. Advocates of abstinence in late pregnancy emphasize its universal applicability and low cost. However, this does not address women with prior HSV-2 infection which constitute from 30 to 60 percent of most obstetrical practices. Moreover, abstinence is an approach that alone is untested, and studies of abstinence in other situations shed doubt on its effectiveness.29, 30
Identification of women as high-risk for transmission of HSV to the neonate on the basis of demographic or clinical characteristics is a potentially cost-effective strategy; this approach is initially adopted for hepatitis B, HIV and Group B Strep testing in pregnancy. A recent population-based case control study using Washington State data on neonatal HSV infection indicated that demographic or clinical characteristics could not differentiate women at high risk of transmitting HSV to their infants, suggesting such an approach is not likely to be effective.31 Similarly, universal testing is now recommended for hepatitis B, HIV and Group B strep in pregnancy.
Type-specific HSV serological testing to identify women at risk of acquiring genital herpes near term also has been advocated as a potential prevention strategy. Knowledge of HSV status in women at special risk of acquiring infection near term may allow more effective counseling of risk reduction behavior, such as abstinence or protected coitus in the last trimester in combination with no oral-genital contact (cunnilingus). Surveys show that women are interested in testing for HSV during pregnancy, and psychosocial distress resulting from unexpectedly testing HSV-2 seropositive is small and transient.32, 33 Serologic identification of infection status is widely advocated and has been successful for HIV prevention in the U.S. and Africa.34, 35 Critical to the knowledge of serologic status is the effectiveness of strategies that target pregnant women identified as HSV-2 seronegative. Condoms appear to be 50 percent effective in reducing the risk of HSV transmission from men to women, and from women to men.36 Valacyclovir therapy of those with HSV-2 also has reduced the risk of sexual transmission to the susceptible partner by 48 percent.37 However, pregnancy may increase susceptibility to acquisition of HSV infection; it is unknown whether the use of condoms or antiviral therapy of the sexual partner with HSV-2 will have similar effects in HSV-2 seronegative pregnant women.38
Serological testing of both the pregnant woman and her partner has also been suggested. This strategy allows identification of 12 to 20 percent of situations in which the partner is HSV seropositive and the pregnant woman is seronegative (and at risk for infection).39, 40 This approach may be expensive and not applicable when there is considerable partner change during pregnancy. However, the advantage of such an approach is that it targets the high-risk couples for intensive behavioral strategies, including consistent condom use. It also is the approach most amenable to the use of antiviral therapy in the HSV-2 infected male partner. There are no clinical trials to define if identification of discordant couples by serologic testing will reduce incident maternal HSV infection, and such trials are needed.
The aforementioned approaches address reducing incident HSV-2 infection. As neonatal HSV-1 infection comprises 30 to 50 percent of neonatal herpes,41 attention to the role genital HSV-1 plays in neonatal infection seems prudent, especially as commercial assays for defining antibody status are available for both HSV-1 and HSV-2. We are unaware of any studies that have examined prevention strategies for genital HSV-1.
Reducing Neonatal Herpes in the HSV-2 Seropositive Woman
Type-specific HSV-2 serological testing during pregnancy can identify women who are HSV-2 seropositive but who have unrecognized genital herpes. Knowledge of HSV-2 may impact obstetrical management to reduce the risk of transmission of infection, such as decreasing the use of invasive monitoring devices (Figure 2); a public health advantage of such an approach is less clear. Diagnosis of newly recognized genital herpes and explanation of attendant risks during pregnancy (as well as the risk of transmission to sexual partners) require considerable effort. Approximately 20 to 25 percent of patients would require counseling about a new disease. For a practitioner who sees neonatal HSV rarely (1 in 5,000 to 10,000 deliveries), this approach may be viewed as impractical. Moreover, the optimal strategy to manage women with newly identified, established genital HSV-2 in pregnancy is unclear.
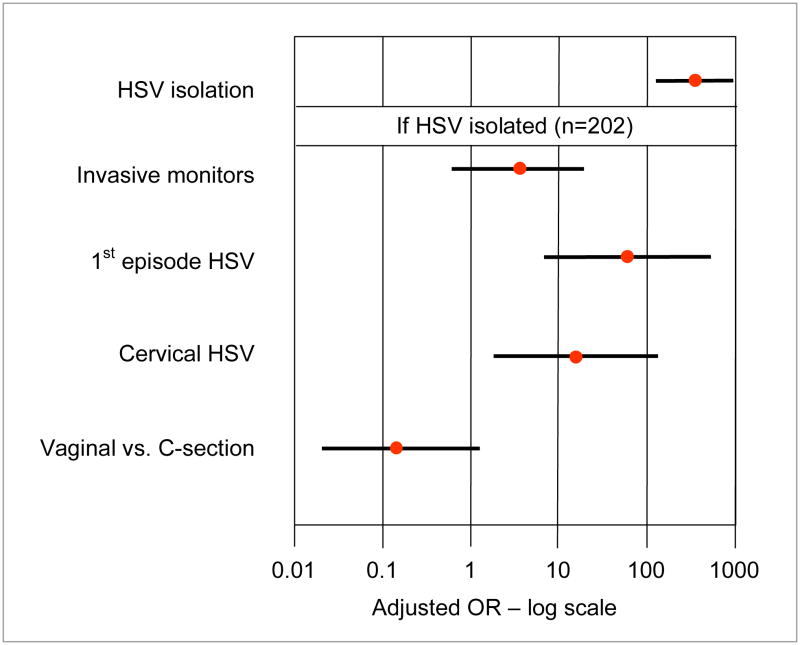
Figure 2. Risk of Neonatal HSV Among 40,023 Women with Genital Cultures for HSV at Delivery.
The data are adapted from Brown et al.4
Current guidelines recommend cesarean deliveries for women with clinical recurrent genital herpes at term (Figure 2).12 Several small studies have shown that daily antivirals at the end of pregnancy can reduce genital HSV recurrences and shedding at term, as well as the need for subsequent cesarean deliveries,42 but have not provided insight on the potential to reduce neonatal herpes. Transmission to the neonate from women who are HSV-2 seropositive is rare; as such, one would expose a large number of mothers and neonates to antiviral therapy to prevent each case of neonatal HSV. The levels of acyclovir reached in amniotic fluid can be similar to those seen in infants treated with systemic acyclovir, of which up to 20 percent develop neutropenia.20 While a pharmacologic approach may offer potential benefit for reducing morbidity from HSV-related cesarean deliveries, widespread exposure of neonates to acyclovir has not been tested and could result in unnecessary toxicity. As such, routine use of antivirals in late pregnancy in HSV-2 seropositive women, especially the majority of those without a history of genital herpes, should require evidence of efficacy in reducing neonatal herpes and minimal toxicity to the infant.
Because of the morbidity to the mother, many authorities recommend that recently acquired genital HSV infections in pregnant women should be treated with antiviral medications.12 Acyclovir is not teratogenic and may be administered either orally to pregnant women with first episode genital herpes or intravenously to pregnant women with severe HSV infection. A common regimen for pregnant women with first episode genital herpes is acyclovir 400 mg orally three times a day or valacyclovir 500 mg BID for seven to 10 days. No data are available whether such therapy reduces the infection rate in the infant.
Identifying the Infant at Risk
The isolation of HSV from the maternal genital tract at delivery is associated with >300-fold risk of neonatal herpes.4 Other risk factors associated with acquisition include fetal scalp monitors and cervical HSV infection (Figure 2). Identifying HSV-exposed infants allows resources to be focused on those at highest risk and defines a strategy that could be applied to infants born to mothers with first or recurrent HSV episodes. Once exposure is identified, one could initiate antiviral prophylaxis (or early expectant therapy). This approach requires (1) a rapid, accurate assay to define HSV shedding at delivery, (2) initiation of early antiviral therapy for those at risk, and (3) determination of the effectiveness of the intervention to reduce acquisition of HSV or to improve the outcome of infected infants (Table 3).
Rapid PCR assays have been developed for many diseases, implemented in field hospitals and used for testing samples from women in labor.43, 44 In addition, point-of care tests to identify HSV-2 specific antibodies have been developed and are commercially available, allowing clinicians to define the risk of neonatal infections as high (seronegative mother) or low (seropositive mother)—a factor that many authorities use in determining if systemic acyclovir prophylaxis should be administered.45 At present, little data exist to guide optimal intervention for managing infants exposed to HSV at birth. Use of antiviral prophylaxis has been quite effective in preventing HSV-1 or HSV-2 infection in neonatal animal models.46 One recommendation for infants born to mothers shedding HSV-2 at term is to follow them with sequential sampling for virus in urine and on the mucosa in conjunction with close clinical follow-up for sign of illness and to initiate systemic therapy if HSV infection is present.21 This approach could result in early initiation of therapy for those with neonatal HSV, which is a major determinant of favorable outcome.19, 20, 47 Thus, the identification and observation of exposed infants would at least provide “best practices” monitoring. Alternatively, antiviral therapy could be initiated at birth in infants whose mothers lacked HSV antibody, as their risk of invasive disease is high. Infants born to women with antibodies to the viral type detected could be closely followed. These approaches, while potentially attractive, need to be empirically evaluated.
In summary, whether caused by HSV-1 or HSV-2, neonatal HSV infection is severe and persistent in the U.S., exceeding in incidence other infectious diseases for which nationwide prevention strategies exist. The tools to devise better prevention strategies have been developed, and several strategies to conceptually reduce infection have been outlined. Current guidelines by the American College of Obstetrics and Gynecology provide useful patient management tools but are not directed at neonatal HSV prevention and appear not to have altered the epidemiology of neonatal HSV in the U.S. in the last decade.12 A concentrated effort to conduct studies that may provide guidance to effectively reduce neonatal herpes is needed and will require an alliance between practitioners and academicians.
Acknowledgments
Grant Acknowledgement and Contributions
Supported by grants PO1 AI-30731, R37 AI-42528, and CA-15704. LC and AW discussed the content of the Tables, Figures and text. LC wrote the first draft of the paper, which was then critically revised by both authors.
Footnotes
Conflict of Interest
Dr. Corey is director of the University of Washington Virology Division, which has received grant support from GlaxoSmithKline, a company that make antiviral drugs for the treatment of HSV-2. Dr. Corey receives no salary support from these studies. He is a consultant for Aicuris, which is developing a drug for treating HSV infection. Dr. Corey is a co-inventor on several patents describing antigens/epitopes to which T cell responses to HSV-2 are directed. These proteins have the potential to be utilized in candidate HSV vaccines.
Dr. Wald has received grant support from the National Institutes of Health, GlaxoSmithKline, Antigenics and Astellas. She has been a consultant for Aicuris and Medigene, as well as a speaker for Merck Vaccines. No other potential conflict of interest relevant to this article is reported.
References
- 1.Genital Herpes: CDC Fact Sheet. Centers for Disease Control and Prevention; 2007. [Accessed July 8, 2008]. http://www.cdc.gov/std/herpes/STDFact-herpes.htm. [Google Scholar]
- 2.Morris SR, Bauer HM, Samuel MC, Gallagher D, Bolan G. Neonatal herpes morbidity and mortality in California, 1995–2003. Sex Transm Dis. 2008;35:14–8. [PubMed] [Google Scholar]
- 3.Whitley R, Davis EA, Suppapanya N. Incidence of neonatal herpes simplex virus infections in a managed-care population. Sex Transm Dis. 2007;34:704–8. doi: 10.1097/01.olq.0000258432.33412.e2. [DOI] [PubMed] [Google Scholar]
- 4.Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203–9. doi: 10.1001/jama.289.2.203. [DOI] [PubMed] [Google Scholar]
- 5.Meissner HC, Reef SE, Cochi S. Elimination of rubella from the United States: a milestone on the road to global elimination. Pediatrics. 2006;117:933–5. doi: 10.1542/peds.2005-1760. [DOI] [PubMed] [Google Scholar]
- 6.Reef SE, Cochi SL. The evidence for the elimination of rubella and congenital rubella syndrome in the United States: a public health achievement. Clin Infect Dis. 2006;43 (Suppl 3):S123–5. doi: 10.1086/505943. [DOI] [PubMed] [Google Scholar]
- 7.Reef SE, Redd SB, Abernathy E, Zimmerman L, Icenogle JP. The epidemiological profile of rubella and congenital rubella syndrome in the United States, 1998–2004: the evidence for absence of endemic transmission. Clin Infect Dis. 2006;43 (Suppl 3):S126–32. doi: 10.1086/505944. [DOI] [PubMed] [Google Scholar]
- 8.Averhoff F, Zucker J, Vellozzi C, et al. Adequacy of surveillance to detect endemic rubella transmission in the United States. Clin Infect Dis. 2006;43 (Suppl 3):S151–7. doi: 10.1086/505948. [DOI] [PubMed] [Google Scholar]
- 9.Achievements in public health. Reduction in perinatal transmission of HIV infection--United States, 1985–2005. MMWR Morb Mortal Wkly Rep. 2006;55:592–7. [PubMed] [Google Scholar]
- 10.Brown ZA, Selke SA, Zeh J, et al. Acquisition of herpes simplex virus during pregnancy. N Engl J Med. 1997;337:509–15. doi: 10.1056/NEJM199708213370801. [DOI] [PubMed] [Google Scholar]
- 11.Sullender WM, Yasukawa LL, Schwartz M, et al. Type-specific antibodies to herpes simplex virus type 2 (HSV-2) glycoprotein G in pregnant women, infants exposed to maternal HSV-2 infection at delivery, and infants with neonatal herpes. J Infect Dis. 1988;157:164–71. doi: 10.1093/infdis/157.1.164. [DOI] [PubMed] [Google Scholar]
- 12.ACOG practice bulletin. Management of herpes in pregnancy (No. 82, June 2007). Clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2007;109:1489–98. doi: 10.1097/01.aog.0000263902.31953.3e. [DOI] [PubMed] [Google Scholar]
- 13.Watts DH, Brown ZA, Money D, et al. A double-blind, randomized, placebo-controlled trial of acyclovir in late pregnancy for the reduction of herpes simplex virus shedding and cesarean delivery. Am J Obstet Gynecol. 2003;188:836–43. doi: 10.1067/mob.2003.185. [DOI] [PubMed] [Google Scholar]
- 14.Andrews WW, Kimberlin DF, Whitley R, Cliver S, Ramsey PS, Deeter R. Valacyclovir therapy to reduce recurrent genital herpes in pregnant women. Am J Obstet Gynecol. 2006;194:774–81. doi: 10.1016/j.ajog.2005.11.051. [DOI] [PubMed] [Google Scholar]
- 15.Hensleigh PA, Andrews WW, Brown Z, Greenspoon J, Yasukawa L, Prober CG. Genital herpes during pregnancy: inability to distinguish primary and recurrent infections clinically. Obstet Gynecol. 1997;89:891–5. doi: 10.1016/s0029-7844(97)00121-x. [DOI] [PubMed] [Google Scholar]
- 16.Wald A, Huang ML, Carrell D, Selke S, Corey L. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis. 2003;188:1345–51. doi: 10.1086/379043. [DOI] [PubMed] [Google Scholar]
- 17.Kimberlin DW. Herpes simplex virus infections in neonates and early childhood. Semin Pediatr Infect Dis. 2005;16:271–81. doi: 10.1053/j.spid.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Gesundheit B, Grisaru-Soen G, Greenberg D, et al. Neonatal genital herpes simplex virus type 1 infection after Jewish ritual circumcision: modern medicine and religious tradition. Pediatrics. 2004;114:e259–63. doi: 10.1542/peds.114.2.e259. [DOI] [PubMed] [Google Scholar]
- 19.Kimberlin DW, Lin CY, Jacobs RF, et al. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics. 2001;108:223–9. doi: 10.1542/peds.108.2.223. [DOI] [PubMed] [Google Scholar]
- 20.Kimberlin DW, Lin CY, Jacobs RF, et al. Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics. 2001;108:230–8. doi: 10.1542/peds.108.2.230. [DOI] [PubMed] [Google Scholar]
- 21.AAP. Herpes simplex. In: Pickering L, editor. Red book 2006 report of the committee on infectious disease. Elk Grove Village, IL: American Academy of Pediatrics; 2006. pp. 361–71. [Google Scholar]
- 22.Diamond C, Mohan K, Hobson A, Frenkel L, Corey L. Viremia in neonatal herpes simplex virus infections. Pediatr Infect Dis J. 1999;18:487–9. doi: 10.1097/00006454-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Caviness AC, Demmler GJ, Swint JM, Cantor SB. Cost-effectiveness analysis of herpes simplex virus testing and treatment strategies in febrile neonates. Arch Pediatr Adolesc Med. 2008;162:665–74. doi: 10.1001/archpedi.162.7.665. [DOI] [PubMed] [Google Scholar]
- 24.Urato AC, Caughey AB. Universal prenatal herpes screening is a bad idea in pregnancy. Lancet. 2006;368:898–9. doi: 10.1016/S0140-6736(06)69348-3. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong D. Baby talk: drug firm’s cash sways debate over test for pregnant women. Wall Street Journal. 2006 Dec 15; [Google Scholar]
- 26.Thung SF, Grobman WA. The cost-effectiveness of routine antenatal screening for maternal herpes simplex virus-1 and -2 antibodies. Am J Obstet Gynecol. 2005;192:483–8. doi: 10.1016/j.ajog.2004.09.134. [DOI] [PubMed] [Google Scholar]
- 27.Cleary KL, Pare E, Stamilio D, Macones GA. Type-specific screening for asymptomatic herpes infection in pregnancy: a decision analysis. BJOG. 2005;112:731–6. doi: 10.1111/j.1471-0528.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 28.Qutub M, Klapper P, Vallely P, Cleator G. Genital herpes in pregnancy: is screening cost-effective? Int J STD AIDS. 2001;12:14–6. [PubMed] [Google Scholar]
- 29.Underhill K, Montgomery P, Operario D. Sexual abstinence only programmes to prevent HIV infection in high income countries: systematic review. BMJ. 2007;335:248. doi: 10.1136/bmj.39245.446586.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiCenso A, Guyatt G, Willan A, Griffith L. Interventions to reduce unintended pregnancies among adolescents: systematic review of randomised controlled trials. BMJ. 2002;324:1426. doi: 10.1136/bmj.324.7351.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mark KE, Kim HN, Wald A, Gardella C, Reed SD. Targeted prenatal herpes simplex virus testing: can we identify women at risk of transmission to the neonate? Am J Obstet Gynecol. 2006;194:408–14. doi: 10.1016/j.ajog.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 32.Miyai T, Turner KR, Kent CK, Klausner J. The psychosocial impact of testing individuals with no history of genital herpes for herpes simplex virus type 2. Sex Transm Dis. 2004;31:517–21. doi: 10.1097/01.olq.0000137901.71284.6b. [DOI] [PubMed] [Google Scholar]
- 33.Rosenthal SL, Zimet GD, Leichliter JS, et al. The psychosocial impact of serological diagnosis of asymptomatic herpes simplex virus type 2 infection. Sex Transm Infect. 2006;82:154–7. doi: 10.1136/sti.2005.016311. discussion 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States--an analysis of cost-effectiveness. N Engl J Med. 2005;352:586–95. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 35.Marum E, Taegtmeyer M, Chebet K. Scale-up of voluntary HIV counseling and testing in Kenya. JAMA. 2006;296:859–62. doi: 10.1001/jama.296.7.859. [DOI] [PubMed] [Google Scholar]
- 36.Wald A, Langenberg AG, Krantz E, et al. The relationship between condom use and herpes simplex virus acquisition. Ann Intern Med. 2005;143:707–13. doi: 10.7326/0003-4819-143-10-200511150-00007. [DOI] [PubMed] [Google Scholar]
- 37.Corey L, Wald A, Patel R, et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med. 2004;350:11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- 38.Kaushic C, Ashkar AA, Reid LA, Rosenthal KL. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J Virol. 2003;77:4558–65. doi: 10.1128/JVI.77.8.4558-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kulhanjian JA, Soroush V, Au DS, et al. Identification of women at unsuspected risk of primary infection with herpes simplex virus type 2 during pregnancy. N Engl J Med. 1992;326:916–20. doi: 10.1056/NEJM199204023261403. [DOI] [PubMed] [Google Scholar]
- 40.Gardella C, Brown Z, Wald A, et al. Risk factors for herpes simplex virus transmission to pregnant women: a couples study. Am J Obstet Gynecol. 2005;193:1891–9. doi: 10.1016/j.ajog.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 41.Kropp RY, Wong T, Cormier L, et al. Neonatal herpes simplex virus infections in Canada: results of a 3-year national prospective study. Pediatrics. 2006;117:1955–62. doi: 10.1542/peds.2005-1778. [DOI] [PubMed] [Google Scholar]
- 42.Sheffield JS, Hollier LM, Hill JB, Stuart GS, Wendel GD. Acyclovir prophylaxis to prevent herpes simplex virus recurrence at delivery: a systematic review. Obstet Gynecol. 2003;102:1396–403. doi: 10.1016/j.obstetgynecol.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 43.Bergeron MG, Ke D, Menard C, et al. Rapid detection of group B streptococci in pregnant women at delivery. N Engl J Med. 2000;343:175–9. doi: 10.1056/NEJM200007203430303. [DOI] [PubMed] [Google Scholar]
- 44.Money D, Dobson S, Cole L, et al. An evaluation of a rapid real time polymerase chain reaction assay for detection of group B streptococcus as part of a neonatal group B streptococcus prevention strategy. J Obstet Gynaecol Can. 2008;30:770–5. doi: 10.1016/S1701-2163(16)32940-1. [DOI] [PubMed] [Google Scholar]
- 45.Morrow RA, Friedrich D, Meier A, Corey L. Use of “biokit HSV-2 Rapid Assay” to improve the positive predictive value of Focus HerpeSelect HSV-2 ELISA. BMC Infect Dis. 2005;5:84. doi: 10.1186/1471-2334-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bravo FJ, Bourne N, Harrison CJ, et al. Effect of antibody alone and combined with acyclovir on neonatal herpes simplex virus infection in guinea pigs. J Infect Dis. 1996;173:1–6. doi: 10.1093/infdis/173.1.1. [DOI] [PubMed] [Google Scholar]
- 47.Whitley RJ, Corey L, Arvin A, et al. Changing presentation of herpes simplex virus infection in neonates. J Infect Dis. 1988;158:109–16. doi: 10.1093/infdis/158.1.109. [DOI] [PubMed] [Google Scholar]
- 48.Schillinger J, Klingler E, Pathela P, et al. Estimating the incidence of neonatal herpes infection in New York City, 1994–2003: implications for formulating a national case definition. No. 229. CDC National STD Prevention Conference; Jacksonville, FL. 2006. [Google Scholar]
- 49.Progress toward elimination of perinatal HIV infection--Michigan. MMWR Morb Mortal Wkly Rep 2002. 1993–2000;51:93–7. [PubMed] [Google Scholar]
- 50.Whitley RJ, Nahmias AJ, Soong SJ, Galasso GG, Fleming CL, Alford CA. Vidarabine therapy of neonatal herpes simplex virus infection. Pediatrics. 1980;66:495–501. [PubMed] [Google Scholar]