Abstract
A conceptual obstacle for understanding immune-to-brain signaling is the issue of the blood–brain barrier (BBB). In the last 30 years, several pathways have been investigated to address the question of how peripheral immune signals are transmitted into the brain. These pathways can be categorized into two types: BBB-dependent pathways and BBB-independent pathways. BBB-dependent pathways involve the BBB as a relay station or porous barrier, whereas BBB-independent pathways use neuronal routes that bypass the BBB. Recently, a complete BBB-dependent ascending pathway for immune-to-brain signaling has been described. Details of BBB-independent pathways are still under construction. In this review, I will summarize the current progress in unraveling immune-to-brain signaling pathways. In addition, I will provide a critical analysis of the literature to point to areas where our knowledge of the immunological afferent signaling to the central nervous system is still sorely lacking.
Keywords: Neuroimmune communication, Cytokine, Sickness behavior
Why Does CNS Need to Sense Immunological Activity?
Microbial infection in an organism is met by a powerful immunological defense. The initial reaction of the immune system uses rapid cellular and biochemical defense mechanisms collectively termed innate immunity. The invading pathogens are immediately attacked by phagocytic cells (neutrophils and macrophages) and natural killer cells as well as the fast-acting complement system. This is followed by adaptive immune responses that produce specific immunity that features exquisite targeting accuracy against microbial antigens. The adaptive immune system also retains memory from previous infections such that a second infection from the same microbial species will be met with expedited and enhanced immune reaction. Most of these intricately coordinated immunological responses appear to operate autonomously within the highly organized immune system. Therefore, one might surmise that it is entirely unnecessary for the immune system to enlist the help of the central nervous system (CNS).
From a higher perspective, however, immunological events occur not in isolation but under specific conditions that lend apparent rationales for CNS-mediated intervention. For example, inflammation often induces reduced locomotor activity [1] and decreased food and water intake [2]. This can be understood from the perspective that by sensing the immunological activity in a given environment, the CNS could orchestrate behavioral changes that help animals to avoid further acquisition of infectious pathogens. Inflammation also induces prolonged slow-wave sleep [3]. Together with reduced locomotor activity, these changes result in an increased rest during infection such that energy can be conserved for the energy-demanding immune activity. Other reasons are less obvious. Inflammation and infection induce activation of the hypothalamic–pituitary–adrenal (HPA) axis [4] and the peripheral cholinergic system [5]. These systems can exert profound anti-inflammatory effects such that potentially harmful over-inflammation can be prevented. CNS-directed physiological responses have also been found to directly alter the effectiveness of immune responses. This is highlighted by the fact that the evolutionarily conserved febrile response has been shown to contribute to enhanced bactericidal activity during infection [6]. The most profound and mysterious immune and CNS interaction was demonstrated by the experiments showing immunological modulation, either immuno-suppression [7] or immuno-enhancement [8], can be achieved by Pavlovian conditioning. Obviously, this cannot occur without precisely inputting information from the immune system to the CNS. Thus, sensing immunological activity by the CNS is an integral part of the overall host defense system. Past work has demonstrated that signals from the peripheral immune response are indeed transmitted to the CNS by several pathways. The future challenge will be to understand immune-to-brain signaling in the context of an integrated function of host defense.
BBB, a Hindrance or a Relay for Immune-to-Brain Signaling?
Initially, the blood–brain barrier (BBB) stood as a conceptual hurdle for understanding immune-to-brain signaling. Several assumptions contributed to this perceived difficulty. First, humoral inflammatory cytokines were regarded as the critical mediators of immune-to-brain communication. Ample evidence exists in the literature suggesting that interleukin (IL)-1, tumor necrosis factor (TNF) alpha, and IL-6—inflammatory cytokines that are initially released from the site of infection—are present in the circulation during infection [9]. In addition, intravenous administration of these cytokine induces CNS-controlled sickness symptoms similar to those observed during infection [10–13]. Therefore, circulating cytokines were considered the messengers for immune-to-brain signaling. The second assumption was that these inflammatory cytokines are large peptides that do not cross the BBB. This assumption draws support from the idea that inflammatory cytokines should be prevented from entering the brain because inflammation in the CNS can be particularly detrimental to this tissue that lacks expandable space and possesses a weak regenerative potential. The quandary arises when experiments showed that CNS-mediated effects in response to the peripheral immune challenge can be blocked by centrally injected cytokine antagonists [14–16], suggesting that somehow peripheral cytokines are able to transnavigate the BBB. This puzzle prompted studies that sought to establish two possible solutions. The first solution is that BBB may serve as a relay station such that its interaction with peripheral cytokines may cause the presence of central cytokines. The second solution is that BBB may be bypassed by afferent sensory nerves, arising in the periphery beyond the boundaries of the BBB, which carry information to the appropriate CNS centers that can interpret and respond to the immune stimulus.
Circumventricular Organs, an Unfinished Story
Blatteis et al. were the first to propose the circumventricular organ (CVO) theory of immune-to-brain signaling [17]. The idea was based on the fact that several specialized brain structures, the CVOs, do not appear to have intact BBB and therefore may be the gateway for cytokine entry into the brain. Among these CVOs, the organum vasculosum of the laminae terminalis (OVLT) is situated adjacent to the preoptic area of the hypothalamus. This area is known to control temperature regulation, and it is activated after peripheral immune challenge [18]. Electrolytic ablation of anterior hypothalamic regions that include OVLT prevented the peripheral lipopolysaccharide (LPS)-induced febrile response. It was concluded that the inflammatory cytokines may enter OVLT and then affect the nearby preoptic area to induce fever [17]. Other CVO lesion studies followed. The results, however, have not been consistent. For example, Stitt et al. showed that lesioning of OVLT enhanced febrile response [19], and Takahashi et al. showed that lesioning of another CVO—the subfornical organ—not the OVLT, reduced peripheral LPS induced fever [20]. Similar conflicting outcomes occurred in studies relating to another neuroimmune effect, the activation of the HPA axis. Removal of the area postrema, a CVO near the nucleus of the solitary tract (NTS), abolished peripheral IL-1-induced activation of the HPA axis in one study [21], but it did not affect peripheral IL-1-induced activation of the axis in another [22]. A more recent study by Romanovsky et al. showed that CVO lesions may produce significant untoward side effects, such as hyperthermia, which may render experimental results difficult to interpret [23]. The idea that peripheral proinflammatory cytokines simply leak into brain regions through the CVOs is probably dismissible. Elegant work from Peruzzo et al. had shown that the CVOs are shielded from the brain parenchyma by the structure of a tanycyte barrier that efficiently prevents the egress of large protein molecules in the CVOs to the nearby brain tissue [24].
What should not be dismissed, however, is that the CVOs may play critical roles in immune-to-brain signaling. Careful examination of the CVOs revealed that these specialized structures may not be the portal of entry for cytokines, but they may be important transducers for sensing peripheral systemic infection. Several lines of evidence converge to support this new hypothesis. First, peripheral immune challenge induces strong expression of proinflammatory cytokines by cells inside the CVOs [25–29]. Second, cells in the CVOs express cytokine receptors [27, 30], as well as receptors for detecting signature bacterial products—the Toll-like receptors (TLR) [31, 32] and CD14 [33]. Third, peripheral immune challenge induces the activation of specific signal transduction pathways downstream of cytokine activation in the CVOs [34, 35]. Therefore, the CVOs are strategically positioned to sense exogenous and endogenous inflammatory signal molecules. There are bidirectional neuronal projections from the CVOs to the hypothalamus [36–38], hippocampus [39], and amygdala [38]. It is possible, therefore, that the activation of neurons within the CVOs by circulating immune signal molecules may be further relayed into the brain via these nerve connections. A difficulty in ascertaining the role of the CVO–brain connection in regards to immune-to-brain signaling is that the CVOs also play critical roles in many other integrated autonomic functions including the control of cardiovascular function, body fluid regulation, feeding behavior, and reproduction [40]. Therefore, results from CVO ablation studies focused on immune-to-brain signaling alone will inevitably be confounded by the altered function in these other systems.
Turning BBB into a Relay Station
A radically different idea for immune-to-brain signaling is to turn the BBB itself into a relay station. Bank’s group is the first to show that inflammatory cytokines can be actively transported across the BBB [41]. One elegant study showed that a subcutaneous injection of recombinant human IL-1α (rhIL-1α) into mice resulted in the presence of rhIL-1α (more than murine IL-1α) in the brain and that the amounts of rhIL-1α were higher in the brain than in the blood [42]. It was concluded that IL-1 was actively transported across the BBB and that the transported IL-1 could be the major source of IL-1 in the brain during peripheral inflammation. Later, TNFα was also found to be transported across the BBB. It is interesting to note that the transport of TNFα across the BBB is abolished in TNF receptor knockout animals, suggesting that the binding to TNF receptors is a prerequisite for the transport of TNFα across the BBB [43]. In addition, Banks et al. showed that an intravenous injection of rhIL-1α induced memory impairment. This central effect of peripheral IL-1 was attenuated by an intracerebral injection of a species-specific antibody against hrIL-1α, demonstrating the functional relevance of the transported IL-1 in peripheral IL-1-induced memory impairment [44]. The number of cytokines found to be transported across the BBB expanded significantly in the last 10 years [45]. The functional consequences of many of these transported cytokines in the brain remain to be determined.
An unanticipated level of complexity in immune-to-brain signaling is that even after cytokines have entered brain parenchyma, they may not act on neuronal cells directly. Several lines of evidence suggest this possibility. First, receptors for inflammatory cytokines are typically expressed at low levels in the brain, and localization studies have found that these receptors are predominantly expressed by non-neuronal cells of the adult brain. For example, receptors for IL-1 [30, 46–48] TNFα [49], and IL-6 [50] have all been found on brain endothelial cells. Second, peripheral immune challenge induces the activation of nuclear factor (NF)-κB and STAT3 in non-neuronal brain cells. These pathways are known to be stimulated specifically by proinflammatory cytokines. Therefore, the activation of these pathways can be used as surrogate markers for direct cytokine activity in a given brain cell. After peripheral LPS injection, the activation of NF-κB[34] as well as STAT3 [51] was found in brain endothelial cells. The NF-κB pathway is sensitive to IL-1 and TNF stimulation, and STAT3 is responsive to IL-6. Although NF-κB is activated in the paraventricular nucleus(PVN), a critical hypothalamic nucleus that controls many CNS responses induced by peripheral immune challenge, double-labeling studies showed that cells in the PVN with NF-κB activation were exclusively endothelial cells [52]. These findings suggest that the direct action of inflammatory cytokines is not on neurons of the adult animals, at least not on neurons relevant for most of the commonly observed neuroimmune effects. One caveat against this reasoning is that neurons may be activated without the involvement of the canonical cytokine signal transduction pathways. For example, Bartfai’s group has shown that IL-1 may stimulate neurons via a ceremide pathway that is independent of the transcriptional activity [53]. The third line of evidence is that many CNS-controlled responses to peripheral immune challenge can be attenuated by blockers of cyclooxygenase (Cox), and the responses are absent in Cox-2 knockout animals [45]. These findings suggest that cytokine-induced Cox-2 expression is critical for immune-to-brain signaling. Peripheral immune challenge induces Cox-2 [54, 55] and mPGES-1 [56–58] expression in brain endothelial cells. Both Cox-2 and mPGES-1 are critical for the synthesis of prostaglandin-E2 (PGE2), a short-distance-acting neuromodulator. The deletion of mPGES-1 abolished peripheral LPS-induced fever [56]. Therefore, the surprising conclusion is that inflammatory cytokines inside the brain may activate neurons indirectly via the brain endothelial production of PGE2. This theory has been elegantly confirmed by a recent study that showed genetic deletion of prostaglandin receptor EP3 in the median preoptic area of the hypothalamus abrogated peripheral LPS-induced fever [59]. In addition, Ericsson et al. showed that endothelial production of PGE2 in caudal ventrolateral medulla mediates peripheral IL-1-induced activation of PVN, suggesting that the activation of the HPA axis by a peripheral immune signal is also relayed by the BBB-dependent pathways [22]. Finally, the role of brain endothelium in mediating immune-to-brain signaling was directly tested in transgenic mice using an endothelial-specific promoter to drive the expression of antisense IL-1R1. Endothelial-specific knockdown of IL-1R1 blocked CNS activation induced by both intravenously and intracerebroventricularly injected IL-1 [10]. A different approach was adopted by Gosselin and Rivest. These authors created chimeric mice with MyD88 deficiency in either hematopoietic cells or nonhematopoietic cells. IL-1-induced activation was blunted in animals that lack MyD88 in nonhematopoietic cells. MyD88 is a critical adaptor molecule in the IL-1 receptor and TLR-mediated signal transduction. The authors concluded that brain endothelial cells must be the main target of peripheral cytokines [60]. On the other hand, Steiner et al. used the same method to create animals deficient in TLR4 expression in either hematopoietic cells or nonhematopoietic cells. They showed that the early phase of fever to LPS depends on functional TLR4 on hematopoietic cells, not nonhematopoietic cells, suggesting a BBB-independent mechanism may be operative for the early phase of fever [61].
Is BBB-dependent Immune-to-Brain Signaling Sufficient for Neuroimmune Communication under Physiological Conditions?
Studies summarized in the foregoing depict a complete pathway that uses BBB as a relay station for immune-to-brain signaling. In this pathway, peripheral inflammation first causes inflammatory cytokines to enter the blood. Then, circulating cytokines act on blood side or the brain side of the BBB to stimulate the synthesis of PGE2 by brain endothelial cells, perivascular [62] or CVO cells. PGE2 then stimulates neurons bearing specific prostglandin receptors in the brainstem [22] or in the hypothalamus [59] to induce CNS-mediated effects.
An important question at this juncture is whether this BBB-dependent pathway is sufficient to transmit ascending immune-to-brain signals under physiological conditions. The following considerations are the basis for raising this question. The first consideration relates to the evolving understanding of circulating cytokines as signal molecules of an immune response. Whereas circulating proinflammatory cytokines are known to be present during endotoxic shock, a condition that often leads to multiple organ failure and mortality, proinflammatory cytokines in the circulation are either absent or not correlated with the intensity of localized, more physiological types of inflammation [63–65]. Recent studies showed that even during sepsis, cytokine responses in the body are compartmentalized such that cytokine levels in the blood compartment are not correlated with those in the inflamed tissue compartment [66]. In fact, the presence of inflammatory cytokines in the circulation has been associated with distal organ damage initiated from localized tissue inflammation [67], and significant tissue inflammation is often accompanied by preemptive increases of circulating anti-inflammatory mediators [68, 69]. These findings have led to the postulation that a systemic anti-inflammatory response may be dominant outside the affected local tissue to prevent systemic inflammation [70]. Therefore, levels of circulating inflammatory cytokines may not signal the status of tissue inflammation but rather a breakdown of physiological control of tissue inflammation, when imminent spread of inflammatory damage might ensue. It should be noted that the BBB-dependent immune-to-brain pathways were discovered largely in experiments using peripheral LPS injection. The release of free LPS during live bacterial infection, however, is known to be tightly controlled. The presence of free LPS has been associated with endotoxic shock and distal organ damage [71] but not sublethal bacterial infection. Therefore, the BBB-dependent immune-to-brain pathways may be operative under limited conditions wherein they relay alarm signals for pending spread of systemic inflammation. They are unlikely to transmit immune signals to the brain under conditions that localized tissue inflammation is successfully contained and inflammatory cytokines do not enter blood.
The second consideration relates to additional functions of cytokine receptors on brain endothelial cells. Hugh Perry’s group was the first to show intracerebral IL-1 induces leukocyte infiltration into the CNS 72]. We have confirmed this observation and determined that endothelial IL-1R1 is required for leukocyte infiltration induced by centrally injected inflammatory cytokines [10, 73]. These findings illustrate the dual roles of cytokine receptors on the endothelial cells of the BBB, viz., as part of the BBB-dependent immune-to-brain signaling pathway and as key mediators of brain inflammation. Consequently, it is possible to misactivate CNS inflammation by peripheral inflammation via the BBB-dependent immune-to-brain pathways. We and others have found that a prior peripheral inflammation activates several anti-inflammatory mechanisms such that a subsequent inflammatory stimulation in the CNS resulted in diminished CNS responses [74, 75]. These findings suggest that there may be built-in mechanisms to prevent the untoward side effects of the BBB-dependent immune-to-brain signaling. On the other hand, the risk of inauspicious activation of BBB-dependent immune-to-brain pathways may be deduced from several studies. Thus, inflammatory damage induced by either brain ischemia [76] or prion [77] can be exacerbated by peripheral LPS injection. In both cases, increased central IL-1 activity due to peripheral LPS is implicated in the worsened outcomes of brain tissue damage. The potential risk of facilitating CNS inflammation suggests that the BBB-dependent immune-to-brain pathways may be employed sparingly, perhaps only when the danger of systemic inflammation is threatened.
BBB-independent Immune-to-Brain Signaling
The alternative to BBB-dependent immune-to-brain signaling is to transmit peripheral immune signals to the brain via sensory nerves. A historical perspective on early studies in this area can be found in a review by Romanovsky [78]. The first demonstration of functional importance for neural transmission of peripheral immune signals to the brain occurred in 1994. Bluthe et al. showed that peripheral LPS-induced change in social investigation behavior was blocked by vagotomy [79], and Watkins et al. showed that vogotomy blocked peripheral IL-1 induced hyperalgesia [80]. These authors concluded that peripheral immune signals are transmitted to the brain via vagal afferents. Numerous vagotomy studies soon followed this initial breakthrough and demonstrated that the intact vagus nerve is critical for the manifestation of a vast array of the effects induced by peripheral inflammatory challenges including hyperalgesia, fever, anorexia, taste aversions, increased levels of plasma corticosteroids, and brain norepinephrine changes [81]. Many of these vagally mediated effects, however, became controversial. For example, in initial studies, fever induced by peripheral LPS or IL-1 was blocked by vagotomy [82, 83]. Later, two studies showed that vagotomy only blocked fever induced by low doses but not high doses of these pyrogens [84, 85], and yet another study showed that fever induced by low doses of IL-1 could not be blocked by vagotomy [86]. Similarly, an early study showed that vagotomy blocked changes in food-motivated behavior induced by peripheral LPS or IL-1 [87]. This result was somewhat contradicted by later findings showing that the vagus nerve is not necessary for peripheral LPS- or IL-1-induced reduction in food intake [88, 89]. Finally, an initial study showed that peripheral LPS-induced changes in monoamine concentrations in the hypothalamus were vagally mediated [90]. This result was not replicated in two studies that showed that the vagus nerve plays a marginal role in mediating changes in brain monoamine levels induced by peripheral LPS and IL-1 [89, 91]. On the other hand, Wieczorek et al. showed that vagotomy does block low-dose peripheral IL-1-induced elevation of norepinephrine in the hypothalamus [92] in the rat. Some of these discrepant results in this field have been partially attributed to the side effects of vagotomy, such as malnutrition [78]. In addition, Ootsuka et al. showed that peripheral PGE2 could induce fever via a CNS-controlled mechanism that cannot be blocked by vagotomy, suggesting that pathways other than the vagus nerve are operative [93].
However, an under appreciated confounding factor is the existence of BBB-dependent neuroimmune pathways. Many vagotomy studies used intravenous or intraperitoneal injections of LPS or IL-1 to simulate peripheral inflammation. These injections are known to induce the presence of proinflammatory cytokines in the blood, thereby activating BBB-dependent immune-to-brain pathways. It is not surprising to note that the effects mediated by neural transmission in these experiments could be overshadowed by the effects mediated by its humoral counterpart.
A larger shadow casted by the BBB-dependent pathways over the neural pathways is perhaps a conceptual one. The initial goal of finding neural routes of immune-to-brain signaling was to identify pathways by which peripheral immune signals could be sent to the brain without the interference of the BBB. Therefore, research thus far has focused mostly on proving that the neural routes are simply additional pathways for neuroimmune communication and that they are maybe more convenient pathways to mediate the same functions as those demonstrated for the BBB-dependent pathways.
This perspective also leads to an implicit assumption that the activation of the neural transmission of peripheral immune signals will eventually lead to the same changes in the brain as those induced by the activation of BBB-dependent pathways. This is illustrated by the controversial studies regarding whether vagal afferents mediate peripheral inflammation-induced production of IL-1 in the brain. Two early studies used semiquantitative reverse transcriptase polymerase chain reaction to show that brain IL-1 messenger ribonucleic acid expression induced by peripheral LPS [94] and IL-1 [95] was blunted by vagotomy. This was not substantiated, however, by a later study that showed that vagotomy does not block peripheral LPS-induced brain IL-1 protein expression [96]. This discrepancy is not likely to be resolvable by technical issues alone because if vagal afferents were to trigger the expression of IL-1 in the brain, the induced IL-1 expression should be found in postsynaptic neurons or in glial cells at the terminal fields of the vagal pathways. Another prerequisite for this pathway to work is that brain IL-1 expression should be inducible by neurotransmitters or neuropeptides released by the vagal afferents. Thus far, the induction of IL-1 expression in the brain can only be demonstrated convincingly in non-neuronal cells [29, 97], and the induction of IL-1 by neurotransmitters and neuropeptides in any cell type has yet to be demonstrated convincingly. Further, the patterns of brain IL-1 expression induced by peripheral immune challenge is more consistent with the BBB-dependent pathways in that brain endothelial cells are found to produce IL-1 upon LPS or cytokine stimulation and there is subsequent brain microglial production of IL-1 in a widespread pattern [98] that is in accord with the stimulant of IL-1 expression being spread to the target microglial cells either via the cerebrospinal fluid or via the subvascular space. Therefore, it is unlikely that the neural routes of immune-to-brain communication will piggyback onto the cascade of the BBB-dependent pathways. Indeed, Goehler et al. showed that live bacterial infection of the gut with Campylobacter jejuni induced neuronal activation in vagal ganglia and in the primary sensory relay nucleus for the vagus, the nucleus of the solitary tract, without elevating circulating proinflammatory cytokines [99]. Similarly, Campisi et al. showed that subcutaneous injection of live Escherichia coli induced fever before increases in circulating and brain proinflammatory cytokines can be detected [100]. Finally, Ootsuka et al. showed that the vagus nerve is not involved in circulating PGE2-induced fever [93]. These results strongly suggest that the BBB-dependent and BBB-independent neuroimmune communications are mediated by separable pathways.
A clearer demonstration of the functions of neural transmission of the peripheral immune signal to the brain may emerge under conditions where BBB-dependent immune-to-brain pathways are not operative. One example for this possibility was shown by Blatteis’ group [101]. They noted that the LPS-induced early phase of fever occurs before the release of inflammatory cytokines into the blood. They demonstrated that LPS induces immediate activation of complement proteins namely, C5a, which then causes the rapid release of PGE2 from liver Kupffer cells [102]. It is hypothesized that PGE2 then activates local vagal afferents, which ultimately stimulate the preoptic area of the hypothalamus via the intermediate NTS to drive early phase of the fever [103]. This is supported by evidence that demonstrated low-dose LPS-induced fever is mediated by the vagus nerve [85] and that the hepatic branch of the vagus nerve plays a major role in the induction of this fever [104]. This series of studies showed that the neural transmission of the peripheral immune signal could engage elements of the immune-to-brain signaling that are quite different from those employed by the BBB-dependent pathways. Thus, complement factors, rather than inflammatory cytokines, could be the initiator of neuroimmune communication. Peripheral PGE2, rather than central PGE2, may be responsible for part of the fever response. The importance of peripheral PGE2 has since been definitively demonstrated by a detailed analysis by Steiner et al. [105] who showed that PGE2 is induced by peripheral LPS in the lung and liver but not in the brain at the onset of the fever. Further, peripherally but not centrally administered anti-PGE2 suppressed the first phase of fever. And finally, the presence of brain cytokines is not necessary for vagally mediated CNS activation [101]. Taking advantage of the fact that neural transmission is faster than the induction of cytokines in the blood, these studies were able to reveal features of neural routes of that are distinguishable from those of the BBB-dependent pathways during a time period when BBB-dependent pathways are not yet active.
Beyond speed, a more important advantage of neural transmission of peripheral immune signals is perhaps that nerve terminals innervating the site of affected tissue may directly report the immunological status from the critical tissue locus to the brain. Studies on localized inflammation, not systemic inflammation, therefore, might be more likely to reveal the mechanisms and functions of neural routes of immune-to-brain signaling. Limited research has been done in this area. LPS has been injected into sterile subcutaneous air pouches in rats [106, 107] to induce local inflammation. Miller et al. showed that the locally applied IL-1 receptor antagonist significantly reduced fever induced by locally injected LPS, suggesting that IL-1 acting locally contributes to the induction of fever. Cartmill et al. then showed that the activity of both IL-1 and IL-6 may be required for fever induction after locally injected LPS [106]. This is consistent with results from studies that used IL-1 and IL-6 knockout mice. Febrile responses to local inflammation induced by turpentine was absent in either IL-1β or IL-6 knockouts, but fever induced by systemic injection of LPS was only attenuated in IL-1β knockouts and unchanged in IL-6 knockouts [108]. Therefore, whereas systemic inflammation uses redundant cytokine actions to activate the CNS, localized inflammation appear to require the actions of multiple cytokines to coactivate CNS. In a slightly different animal model, Roth’s group induced local inflammation by injecting LPS into artificial subcutaneous chambers in guinea pigs [109, 110]. They found the injection of ropivacaine, a local anesthetic, into the subcutaneous chamber with a low dose of LPS blocked LPS-induced fever, suggesting, under this condition, that CNS may be stimulated via primary sensory nerves. This result reinforces an earlier observation that deafferentation of C-fiber sensory neurons attenuated fever induced by intramuscularly injected turpentine (a local inflammatory agent) in rats [111]. In addition, Rummel et al. found that fever induced by locally injected LPS can be blocked by locally administered Cox inhibitor diclofenac [110]. In this experiment, LPS induced local, but not brain, Cox-2 expression. Together, these results again indicate a direct tissue-to-brain connection in which cytokines and/or PGE acting at the site of inflammation, not in the brain, are important for the activation of this pathway.
A finer picture of neuroimmune communication is being painted by the studies on neural transmission of immune-to-brain signaling. Whereas previous studies have focused on the question “do the immune system and the CNS communicate?,” these studies are beginning to shift the focus to “how do different neural routes communicate with the CNS?” Goldbach et al. showed that whereas the effects of intraperitoneal LPS were blocked by vagotomy, the effects of intramuscularly injected LPS were not [112]. Therefore, whereas peritoneal inflammation may signal the brain via vagal afferents, skeleton muscle inflammation may signal the brain via primary sensory nerves innervating the affected muscle tissue. The general assumption in the literature thus far is that these different neural routes will eventually converge to induce commonly observed CNS-mediated sickness symptoms. We have observed recently, however, that restricted localized inflammation can produce patterns of activation of the hypothalamic neurons (our unpublished results) that correspond to the peripheral locations of inflammation. These results suggest a fascinating possibility that beyond general sickness symptoms, CNS may produce responses specific for the affected tissue. The concept of an integrated immune–CNS–immune reflex loop has recently been proposed by Tracey [5]. His group found that vagal efferents play an important role in limiting the production of TNFα induced by a septic dose of LPS [113]. This theory posits that inflammatory signals may stimulate the CNS via vagal afferents, which then drive the descending cholinergic neurons to release acetylcholine. Acetylcholine then acts on macrophages to reduce the release of TNFα to limit inflammation. This theory, however, was based upon studies in which systemic LPS-induced sepsis was investigated. As discussed earlier, during LPS sepsis, widespread anti-inflammatory actions may be of paramount importance due to the threat of multiple organ failure. Whether restricted local inflammation will also activate an immune–CNS–immune reflex loop to enhance, instead of suppress, immunological activity at the site of inflammation is a tantalizing but untested possibility.
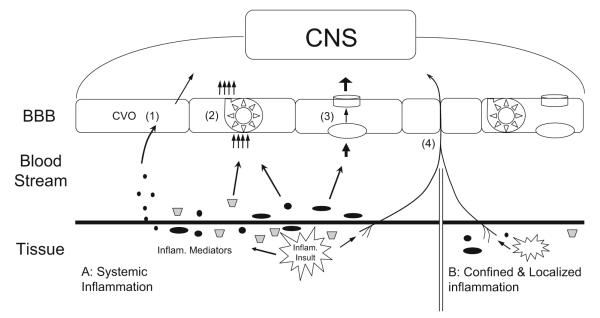
In summary, both BBB-dependent and BBB-independent pathways mediate immune-to-brain communication. A theory I would like to put forth is that BBB-dependent pathways are activated during systemic inflammation and BBB-independent pathways are activated when there is contained, nonspreading, localized tissue inflammation (Fig. 1). Whereas BBB-dependent pathways are critical for activating CNS-controlled responses for generalized neuroimmune effects, only BBB-independent pathways are capable of eliciting integrated responses from the CNS that are directed specifically toward the site of tissue infection. One should bear in mind that this thesis is an oversimplification for the benefit of erecting a temporary theoretical framework to guide future research. In reality, localized inflammation may or may not be containable. For example, in the rat model of adjuvant-induced arthritis, inflammatory swelling often occurs in both injected and noninjected paws [114], and increased production of IL-1 in distal organs has been observed [115]. Extensive burn injury is also associated with high levels of circulating proinflammatory cytokines [116]. Therefore, it is possible that in these two inflammation models, both BBB-dependent and BBB-independent signaling pathways are activated. Indeed, brain endothelial expression of Cox-2 and mPGES-1 can be demonstrated in these models [117, 118]. Another difficulty to separate BBB-dependent and BBB-independent pathways is that both pathways may employ common mediators, e.g., PGE2, albeit these common mediators may act at different sites. This may account for the fact that inhibiting the synthesis and/or activity of PGE2 is effective for blocking immune-to-brain signaling from both systemic [45] and local inflammation [119]. In addition, local inflammation induced by carrageenan results in the activation of PGE synthesis in brain endothelial cells [120, 121]. This effect was shown to be mediated by circulating IL-6. Therefore, under certain conditions, even restricted local inflammation can stimulate CNS via BBB-dependent pathways.
Fig. 1.
Schematic diagram showing BBB-dependent and BBB-independent immune-to-brain communication pathways. In scenario A, there is systemic inflammation. Inflammatory mediators either enter the circulation or act locally. Immune-to-brain signaling is mediated by (1) CVOs mediated relay, (2) BBB transport of cytokines, (3) production of inflammatory mediators by the BBB, and/or (4) peripheral afferents. In scenario B, there is contained localized inflammation. Immune-to-brain signaling is mediated by the peripheral afferents arising from the site of infection
Acknowledgment
This study is supported by R01 AI059089 and R01 AI076926. I would like to thank Dr. Miles Herkenham for his careful reading of the manuscript and many valuable suggestions. I would also like to thank Dr. Qun Chen for his help in making the illustration in this paper.
References
- 1.Plata-Salaman CR, Borkoski JP. Centrally administered bacterial lipopolysaccharide depresses feeding in rats. Pharmacol Biochem Behav. 1993;46(4):787–791. doi: 10.1016/0091-3057(93)90202-5. [DOI] [PubMed] [Google Scholar]
- 2.Foca A, Nicoletta P, Matera G, Mastroeni P, Caputi AP. Antidipsogenic effect of endotoxin in the rat. Circ Shock. 1983;11(4):341–350. [PubMed] [Google Scholar]
- 3.Krueger JM, Majde JA. Cytokines and sleep. Int Arch Allergy Immunol. 1995;106(2):97–100. doi: 10.1159/000236827. [DOI] [PubMed] [Google Scholar]
- 4.Eskay RL, Grino M, Chen HT. Interleukins, signal transduction, and the immune system-mediated stress response. Adv Exp Med Biol. 1990;274:331–343. doi: 10.1007/978-1-4684-5799-5_21. [DOI] [PubMed] [Google Scholar]
- 5.Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 6.Kluger MJ, Ringler DH, Anver MR. Fever and survival. Science. 1975;188(4184):166–168. [PubMed] [Google Scholar]
- 7.Bovbjerg D, Ader R, Cohen N. Behaviorally conditioned suppression of a graft-versus-host response. Proc Natl Acad Sci USA. 1982;79(2):583–585. doi: 10.1073/pnas.79.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bovbjerg D, Cohen N, Ader R. Behaviorally conditioned enhancement of delayed-type hypersensitivity in the mouse. Brain Behav Immun. 1987;1(1):64–71. doi: 10.1016/0889-1591(87)90007-9. [DOI] [PubMed] [Google Scholar]
- 9.Alisjahbana B, Netea MG, van der Meer JW. Proinflammatory cytokine response in acute infection. Adv Exp Med Biol. 2003;531:229–240. doi: 10.1007/978-1-4615-0059-9_19. [DOI] [PubMed] [Google Scholar]
- 10.Ching S, Zhang H, Belevych N, et al. Endothelial-specific knockdown of interleukin-1 (IL-1) type 1 receptor differentially alters CNS responses to IL-1 depending on its route of administration. J Neurosci. 2007;27(39):10476–10486. doi: 10.1523/JNEUROSCI.3357-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lesnikov VA, Efremov OM, Korneva EA, Van Damme J, Billiau A. Fever produced by intrahypothalamic injection of interleukin-1 and interleukin-6. Cytokine. 1991;3(3):195–198. doi: 10.1016/1043-4666(91)90016-7. [DOI] [PubMed] [Google Scholar]
- 12.Dinarello CA, Cannon JG, Mancilla J, Bishai I, Lees J, Coceani F. Interleukin-6 as an endogenous pyrogen: induction of prostaglandin E2 in brain but not in peripheral blood mononuclear cells. Brain Res. 1991;562(2):199–206. doi: 10.1016/0006-8993(91)90622-3. [DOI] [PubMed] [Google Scholar]
- 13.Sherman ML, Spriggs DR, Arthur KA, Imamura K, Frei E, 3rd, Kufe DW. Recombinant human tumor necrosis factor administered as a five-day continuous infusion in cancer patients: phase I toxicity and effects on lipid metabolism. J Clin Oncol. 1988;6(2):344–350. doi: 10.1200/JCO.1988.6.2.344. [DOI] [PubMed] [Google Scholar]
- 14.Kakucska I, Qi Y, Clark BD, Lechan RM. Endotoxin-induced corticotropin-releasing hormone gene expression in the hypothalamic paraventricular nucleus is mediated centrally by interleukin-1. Endocrinology. 1993;133(2):815–821. doi: 10.1210/endo.133.2.8344218. [DOI] [PubMed] [Google Scholar]
- 15.Imeri L, Opp MR, Krueger JM. An IL-1 receptor and an IL-1 receptor antagonist attenuate muramyl dipeptide- and IL-1-induced sleep and fever. Am J Physiol. 1993;265(4 Pt 2):R907–R913. doi: 10.1152/ajpregu.1993.265.4.R907. [DOI] [PubMed] [Google Scholar]
- 16.Lang CH, Cooney R, Vary TC. Central interleukin-1 partially mediates endotoxin-induced changes in glucose metabolism. Am J Physiol. 1996;271(2 Pt 1):E309–E316. doi: 10.1152/ajpendo.1996.271.2.E309. [DOI] [PubMed] [Google Scholar]
- 17.Blatteis CM, Bealer SL, Hunter WS, Llanos QJ, Ahokas RA, Mashburn TA., Jr Suppression of fever after lesions of the anteroventral third ventricle in guinea pigs. Brain Res Bull. 1983;11(5):519–526. doi: 10.1016/0361-9230(83)90124-7. [DOI] [PubMed] [Google Scholar]
- 18.Elmquist JK, Scammell TE, Jacobson CD, Saper CB. Distribution of Fos-like immunoreactivity in the rat brain following intravenous lipopolysaccharide administration. J Comp Neurol. 1996;371(1):85–103. doi: 10.1002/(SICI)1096-9861(19960715)371:1<85::AID-CNE5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 19.Stitt JT. Evidence for the involvement of the organum vasculosum laminae terminalis in the febrile response of rabbits and rats. J Physiol. 1985;368:501–511. doi: 10.1113/jphysiol.1985.sp015872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi Y, Smith P, Ferguson A, Pittman QJ. Circumventricular organs and fever. Am J Physiol. 1997;273(5 Pt 2):R1690–R1695. doi: 10.1152/ajpregu.1997.273.5.R1690. [DOI] [PubMed] [Google Scholar]
- 21.Lee HY, Whiteside MB, Herkenham M. Area postrema removal abolishes stimulatory effects of intravenous interleukin-1beta on hypothalamic-pituitary-adrenal axis activity and c- fos mRNA in the hypothalamic paraventricular nucleus. Brain Res Bull. 1998;46(6):495–503. doi: 10.1016/s0361-9230(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 22.Ericsson A, Arias C, Sawchenko PE. Evidence for an intramedullary prostaglandin-dependent mechanism in the activation of stress-related neuroendocrine circuitry by intravenous interleukin-1. J Neurosci. 1997;17(18):7166–7179. doi: 10.1523/JNEUROSCI.17-18-07166.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romanovsky AA, Sugimoto N, Simons CT, Hunter WS. The organum vasculosum laminae terminalis in immune-to-brain febrigenic signaling: a reappraisal of lesion experiments. Am J Physiol Regul Integr Comp Physiol. 2003;285(2):R420–R428. doi: 10.1152/ajpregu.00757.2002. [DOI] [PubMed] [Google Scholar]
- 24.Peruzzo B, Pastor FE, Blazquez JL, et al. A second look at the barriers of the medial basal hypothalamus. Exp Brain Res. 2000;132(1):10–26. doi: 10.1007/s002219900289. [DOI] [PubMed] [Google Scholar]
- 25.Breder CD, Hazuka C, Ghayur T, et al. Regional induction of tumor necrosis factor alpha expression in the mouse brain after systemic lipopolysaccharide administration. Proc Natl Acad Sci USA. 1994;91(24):11393–11397. doi: 10.1073/pnas.91.24.11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamori T, Morimoto A, Yamaguchi K, Watanabe T, Long NC, Murakami N. Organum vasculosum laminae terminalis (OVLT) is a brain site to produce interleukin-1 beta during fever. Brain Res. 1993;618(1):155–159. doi: 10.1016/0006-8993(93)90439-t. [DOI] [PubMed] [Google Scholar]
- 27.Vallieres L, Rivest S. Regulation of the genes encoding interleukin-6, its receptor, and gp130 in the rat brain in response to the immune activator lipopolysaccharide and the proinflammatory cytokine interleukin-1beta. J Neurochem. 1997;69(4):1668–1683. doi: 10.1046/j.1471-4159.1997.69041668.x. [DOI] [PubMed] [Google Scholar]
- 28.Nadeau S, Rivest S. Regulation of the gene encoding tumor necrosis factor alpha (TNF-alpha) in the rat brain and pituitary in response in different models of systemic immune challenge. J Neuropathol Exp Neurol. 1999;58(1):61–77. doi: 10.1097/00005072-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Quan N, Whiteside M, Herkenham M. Time course and localization patterns of interleukin-1b messenger RNA expression in brain and pituitary after peripheral administration of lipopolysaccharide. Neuroscience. 1998;83:281–293. doi: 10.1016/s0306-4522(97)00350-3. [DOI] [PubMed] [Google Scholar]
- 30.Ericsson A, Liu C, Hart RP, Sawchenko PE. Type 1 interleukin-1 receptor in the rat brain: distribution, regulation, and relationship to sites of IL-1-induced cellular activation. J Comp Neurol. 1995;361(4):681–698. doi: 10.1002/cne.903610410. [DOI] [PubMed] [Google Scholar]
- 31.Laflamme N, Rivest S. Toll-like receptor 4: the missing link of the cerebral innate immune response triggered by circulating Gram-negative bacterial cell wall components. FASEB J. 2001;15(1):155–163. doi: 10.1096/fj.00-0339com. [DOI] [PubMed] [Google Scholar]
- 32.Rivest S. Molecular insights on the cerebral innate immune system. Brain Behav Immun. 2003;17(1):13–19. doi: 10.1016/s0889-1591(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 33.Lacroix S, Feinstein D, Rivest S. The bacterial endotoxin lipopolysaccharide has the ability to target the brain in upregulating its membrane CD14 receptor within specific cellular populations. Brain Pathol. 1998;8(4):625–640. doi: 10.1111/j.1750-3639.1998.tb00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quan N, Whiteside M, Kim L, Herkenham M. Induction of inhibitory factor kBa mRNA in the central nervous system after peripheral lipopolysaccharide administration: an in situ hybridization histochemistry study in the rat. Proc Natl Acad Sci USA. 1997;94:10985–10990. doi: 10.1073/pnas.94.20.10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harre EM, Roth J, Gerstberger R, Hubschle T. Interleukin-6 mediates lipopolysaccharide-induced nuclear STAT3 translocation in astrocytes of rat sensory circumventricular organs. Brain Res. 2003;980(1):151–155. doi: 10.1016/s0006-8993(03)02923-8. [DOI] [PubMed] [Google Scholar]
- 36.McKinley MJ, Hards DK, Oldfield BJ. Identification of neural pathways activated in dehydrated rats by means of Fosimmunohistochemistry and neural tracing. Brain Res. 1994;653(1–2):305–314. doi: 10.1016/0006-8993(94)90405-7. [DOI] [PubMed] [Google Scholar]
- 37.Larsen PJ, Mikkelsen JD. Functional identification of central afferent projections conveying information of acute “stress” to the hypothalamic paraventricular nucleus. J Neurosci. 1995;15(4):2609–2627. doi: 10.1523/JNEUROSCI.15-04-02609.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverman AJ, Hoffman DL, Zimmerman EA. The descending afferent connections of the paraventricular nucleus of the hypothalamus (PVN) Brain Res Bull. 1981;6(1):47–61. doi: 10.1016/s0361-9230(81)80068-8. [DOI] [PubMed] [Google Scholar]
- 39.Ciriello J, Gutman MB. Functional identification of central pressor pathways originating in the subfornical organ. Can J Physiol Pharmacol. 1991;69(7):1035–1045. doi: 10.1139/y91-154. [DOI] [PubMed] [Google Scholar]
- 40.Cottrell GT, Ferguson AV. Sensory circumventricular organs: central roles in integrated autonomic regulation. Regul Pept. 2004;117(1):11–23. doi: 10.1016/j.regpep.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood–brain barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- 42.Banks WA, Kastin AJ. Relative contributions of peripheral and central sources to levels of IL-1 alpha in the cerebral cortex of mice: assessment with species- specific enzyme immunoassays. J Neuroimmunol. 1997;79(1):22–28. doi: 10.1016/s0165-5728(97)00103-3. [DOI] [PubMed] [Google Scholar]
- 43.Pan W, Kastin AJ. TNFa transport across the blood–brain barrier is abolished in receptor knockout mice. Exp Neurol. 2002;174:193–200. doi: 10.1006/exnr.2002.7871. [DOI] [PubMed] [Google Scholar]
- 44.Banks WA, Farr SA, La Scola ME, Morley JE. Intravenous human interleukin-1alpha impairs memory processing in mice: dependence on blood–brain barrier transport into posterior division of the septum. J Pharmacol Exp Ther. 2001;299(2):536–541. [PubMed] [Google Scholar]
- 45.Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21(6):727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Cunningham ETJ, Wada E, Carter DB, Tracey DE, Battey JF, De Souza EB. In situ histochemical localization of type I interleukin-1 receptor messenger RNA in the central nervous system, pituitary, and adrenal gland of the mouse. J Neurosci. 1992;12(3):1101–1114. doi: 10.1523/JNEUROSCI.12-03-01101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Dam AM, De Vries HE, Kuiper J, et al. Interleukin-1 receptors on rat brain endothelial cells: a role in neuroimmune interaction? FASEB J. 1996;10(2):351–356. doi: 10.1096/fasebj.10.2.8641570. [DOI] [PubMed] [Google Scholar]
- 48.Konsman JP, Vigues S, Mackerlova L, Bristow A, Blomqvist A. Rat brain vascular distribution of interleukin-1 type-1 receptor immunoreactivity: relationship to patterns of inducible cyclooxygenase expression by peripheral inflammatory stimuli. J Comp Neurol. 2004;472(1):113–129. doi: 10.1002/cne.20052. [DOI] [PubMed] [Google Scholar]
- 49.Bebo BF, Jr, Linthicum DS. Expression of mRNA for 55-kDa and 75-kDa tumor necrosis factor (TNF) receptors in mouse cerebrovascular endothelium: effects of interleukin-1 beta, interferon-gamma and TNF-alpha on cultured cells. J Neuroimmunol. 1995;62(2):161–167. doi: 10.1016/0165-5728(95)00113-5. [DOI] [PubMed] [Google Scholar]
- 50.Vallieres L, Rivest S. Interleukin-6 is a needed proinflammatory cytokine in the prolonged neural activity and transcriptional activation of corticotropin-releasing factor during endotoxemia. Endocrinology. 1999;140(9):3890–3903. doi: 10.1210/endo.140.9.6983. [DOI] [PubMed] [Google Scholar]
- 51.Rummel C, Voss T, Matsumura K, et al. Nuclear STAT3 translocation in guinea pig and rat brain endothelium during systemic challenge with lipopolysaccharide and interleukin-6. J Comp Neurol. 2005;491(1):1–14. doi: 10.1002/cne.20653. [DOI] [PubMed] [Google Scholar]
- 52.Quan N, He L, Lai W. Endothelial activation is an intermediate step for peripheral lipopolysaccharide induced activation of paraventricular nucleus. Brain Res Bull. 2003;59(6):447–452. doi: 10.1016/s0361-9230(02)00951-6. [DOI] [PubMed] [Google Scholar]
- 53.Sanchez-Alavez M, Tabarean IV, Behrens MM, Bartfai T. Ceramide mediates the rapid phase of febrile response to IL-1beta. Proc Natl Acad Sci USA. 2006;103(8):2904–2908. doi: 10.1073/pnas.0510960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quan N, Whiteside M, Herkenham M. Cyclooxygenase 2 mRNA expression in rat brain after peripheral injection of lipopolysaccharide. Brain Res. 1998;802(1–2):189–197. doi: 10.1016/s0006-8993(98)00402-8. [DOI] [PubMed] [Google Scholar]
- 55.Cao C, Matsumura K, Yamagata K, Watanabe Y. Involvement of cyclooxygenase-2 in LPS-induced fever and regulation of its mRNA by LPS in the rat brain. Am J Physiol. 1997;272(6 Pt 2):R1712–R1725. doi: 10.1152/ajpregu.1997.272.6.R1712. [DOI] [PubMed] [Google Scholar]
- 56.Engblom D, Saha S, Engstrom L, et al. Microsomal prostaglandin E synthase-1 is the central switch during immune-induced pyresis. Nat Neurosci. 2003;6(11):1137–1138. doi: 10.1038/nn1137. [DOI] [PubMed] [Google Scholar]
- 57.Ek M, Engblom D, Saha S, Blomqvist A, Jakobsson PJ, Ericsson-Dahlstrand A. Inflammatory response: pathway across the blood–brain barrier. Nature. 2001;410(6827):430–431. doi: 10.1038/35068632. [DOI] [PubMed] [Google Scholar]
- 58.Yamagata K, Matsumura K, Inoue W, et al. Coexpression of microsomal-type prostaglandin E synthase with cyclooxygenase-2 in brain endothelial cells of rats during endotoxin-induced fever. J Neurosci. 2001;21(8):2669–2677. doi: 10.1523/JNEUROSCI.21-08-02669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lazarus M, Yoshida K, Coppari R, et al. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci. 2007;10(9):1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- 60.Gosselin D, Rivest S. MyD88 signaling in brain endothelial cells is essential for the neuronal activity and glucocorticoid release during systemic inflammation. Mol Psychiatry. 2008;13:480–497. doi: 10.1038/sj.mp.4002122. [DOI] [PubMed] [Google Scholar]
- 61.Steiner AA, Chakravarty S, Rudaya AY, Herkenham M, Romanovsky AA. Bacterial lipopolysaccharide fever is initiated via Toll-like receptor 4 on hematopoietic cells. Blood. 2006;107(10):4000–4002. doi: 10.1182/blood-2005-11-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schiltz JC, Sawchenko PE. Distinct brain vascular cell types manifest inducible cyclooxygenase expression as a function of the strength and nature of immune insults. J Neurosci. 2002;22(13):5606–5618. doi: 10.1523/JNEUROSCI.22-13-05606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wiik H, Karttunen R, Haukipuro K, Syrjala H. Maximal local and minimal systemic cytokine response to colorectal surgery: the influence of perioperative filgrastim. Cytokine. 2001;14(3):188–192. doi: 10.1006/cyto.2001.0870. [DOI] [PubMed] [Google Scholar]
- 64.Yamamoto T, Umegae S, Kitagawa T, Matsumoto K. Systemic and local cytokine production in quiescent ulcerative colitis and its relationship to future relapse: a prospective pilot study. Inflamm Bowel Dis. 2005;11(6):589–596. doi: 10.1097/01.mib.0000161917.97136.e2. [DOI] [PubMed] [Google Scholar]
- 65.Hedges SR, Barrientes F, Desmond RA, Schwebke JR. Local and systemic cytokine levels in relation to changes in vaginal flora. J Infect Dis. 2006;193(4):556–562. doi: 10.1086/499824. [DOI] [PubMed] [Google Scholar]
- 66.Cavaillon JM, Annane D. Compartmentalization of the inflammatory response in sepsis and SIRS. J Endotoxin Res. 2006;12(3):151–170. doi: 10.1179/096805106X102246. [DOI] [PubMed] [Google Scholar]
- 67.Makhija R, Kingsnorth AN. Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9(4):401–410. doi: 10.1007/s005340200049. [DOI] [PubMed] [Google Scholar]
- 68.O’Nuallain EM, Puri P, Mealy K, Reen DJ. Induction of interleukin-1 receptor antagonist (IL-1ra) following surgery is associated with major trauma. Clin Immunol Immunopathol. 1995;76(1 Pt 1):96–101. doi: 10.1006/clin.1995.1093. [DOI] [PubMed] [Google Scholar]
- 69.Cavaillon JM, Adib-Conquy M, Fitting C, Adrie C, Payen D. Cytokine cascade in sepsis. Scand J Infect Dis. 2003;35(9):535–544. doi: 10.1080/00365540310015935. [DOI] [PubMed] [Google Scholar]
- 70.Munford RS, Pugin J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med. 2001;163(2):316–321. doi: 10.1164/ajrccm.163.2.2007102. [DOI] [PubMed] [Google Scholar]
- 71.Creasey AA, Stevens P, Kenney J, et al. Endotoxin and cytokine profile in plasma of baboons challenged with lethal and sublethal Escherichia coli. Circ Shock. 1991;33(2):84–91. [PubMed] [Google Scholar]
- 72.Anthony DC, Bolton SJ, Fearn S, Perry VH. Age-related effects of interleukin-1 beta on polymorphonuclear neutrophil-dependent increases in blood–brain barrier permeability in rats. Brain. 1997;120(Pt 3):435–444. doi: 10.1093/brain/120.3.435. [DOI] [PubMed] [Google Scholar]
- 73.Ching S, He L, Lai W, Quan N. IL-1 type I receptor plays a key role in mediating the recruitment of leukocytes into the central nervous system. Brain Behav Immun. 2005;19(2):127–137. doi: 10.1016/j.bbi.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 74.Nadeau S, Rivest S. Endotoxemia prevents the cerebral inflammatory wave induced by intraparenchymal lipopolysaccharide injection: role of glucocorticoids and CD14. J Immunol. 2002;169(6):3370–3381. doi: 10.4049/jimmunol.169.6.3370. [DOI] [PubMed] [Google Scholar]
- 75.Ching S, Zhang H, Lai W, Quan N. Peripheral injection of lipopolysaccharide prevents brain recruitment of leukocytes induced by central injection of interleukin-1. Neuroscience. 2006;137(2):717–726. doi: 10.1016/j.neuroscience.2005.08.087. [DOI] [PubMed] [Google Scholar]
- 76.McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27(16):4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25(40):9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Romanovsky AA. Signaling the brain in the early sickness syndrome: are sensory nerves involved? Front Biosci. 2004;9:494–504. doi: 10.2741/1247. [DOI] [PubMed] [Google Scholar]
- 79.Bluthe RM, Walter V, Parnet P, et al. Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C R Acad Sci Ser III. 1994;317(6):499–503. [PubMed] [Google Scholar]
- 80.Watkins LR, Wiertelak EP, Goehler LE, Smith KP, Martin D, Maier SF. Characterization of cytokine-induced hyper-algesia. Brain Res. 1994;654(1):15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- 81.Maier SF, Goehler LE, Fleshner M, Watkins LR. The role of the vagus nerve in cytokine-to-brain communication. Ann N Y Acad Sci. 1998;840:289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- 82.Sehic E, Blatteis CM. Blockade of lipopolysaccharide-induced fever by subdiaphragmatic vagotomy in guinea pigs. Brain Res. 1996;726(1–2):160–166. [PubMed] [Google Scholar]
- 83.Watkins LR, Goehler LE, Relton JK, et al. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Lett. 1995;183(1–2):27–31. doi: 10.1016/0304-3940(94)11105-r. [DOI] [PubMed] [Google Scholar]
- 84.Hansen MK, O’Connor KA, Goehler LE, Watkins LR, Maier SF. The contribution of the vagus nerve in interleukin-1beta-induced fever is dependent on dose. Am J Physiol Regul Integr Comp Physiol. 2001;280(4):R929–R934. doi: 10.1152/ajpregu.2001.280.4.R929. [DOI] [PubMed] [Google Scholar]
- 85.Romanovsky AA, Simons CT, Szekely M, Kulchitsky VA. The vagus nerve in the thermoregulatory response to systemic inflammation. Am J Physiol. 1997;273(1 Pt 2):R407–R413. doi: 10.1152/ajpregu.1997.273.1.R407. [DOI] [PubMed] [Google Scholar]
- 86.Luheshi GN, Bluthe RM, Rushforth D, et al. Vagotomy attenuates the behavioural but not the pyrogenic effects of interleukin-1 in rats. Auton Neurosci. 2000;85(1–3):127–132. doi: 10.1016/S1566-0702(00)00231-9. [DOI] [PubMed] [Google Scholar]
- 87.Bret-Dibat JL, Bluthe RM, Kent S, Kelley KW, Dantzer R. Lipopolysaccharide and interleukin-1 depress food-motivated behavior in mice by a vagal-mediated mechanism. Brain Behav Immun. 1995;9(3):242–246. doi: 10.1006/brbi.1995.1023. [DOI] [PubMed] [Google Scholar]
- 88.Porter MH, Hrupka BJ, Langhans W, Schwartz GJ. Vagal and splanchnic afferents are not necessary for the anorexia produced by peripheral IL-1beta, LPS, and MDP. Am J Physiol. 1998;275(2 Pt 2):R384–R389. doi: 10.1152/ajpregu.1998.275.2.R384. [DOI] [PubMed] [Google Scholar]
- 89.Wieczorek M, Swiergiel AH, Pournajafi-Nazarloo H, Dunn AJ. Physiological and behavioral responses to interleukin-1beta and LPS in vagotomized mice. Physiol Behav. 2005;85(4):500–511. doi: 10.1016/j.physbeh.2005.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fleshner M, Goehler LE, Hermann J, Relton JK, Maier SF, Watkins LR. Interleukin-1 beta induced corticosterone elevation and hypothalamic NE depletion is vagally mediated. Brain Res Bull. 1995;37(6):605–610. doi: 10.1016/0361-9230(95)00051-f. [DOI] [PubMed] [Google Scholar]
- 91.MohanKumar SM, MohanKumar PS, Quadri SK. Effects of bacterial lipopolysaccharide on central monoamines and fever in the rat: involvement of the vagus. Neurosci Lett. 2000;284(3):159–162. doi: 10.1016/s0304-3940(00)01025-9. [DOI] [PubMed] [Google Scholar]
- 92.Wieczorek M, Dunn AJ. Effect of subdiaphragmatic vagotomy on the noradrenergic and HPA axis activation induced by intraperitoneal interleukin-1 administration in rats. Brain Res. 2006;1101(1):73–84. doi: 10.1016/j.brainres.2006.04.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ootsuka Y, Blessing WW, Steiner AA, Romanovsky AA. Fever response to intravenous prostaglandin E2 is mediated by the brain but does not require afferent vagal signaling. Am J Physiol Regul Integr Comp Physiol. 2008;294(4):R1294–R1303. doi: 10.1152/ajpregu.00709.2007. [DOI] [PubMed] [Google Scholar]
- 94.Laye S, Bluthe RM, Kent S, et al. Subdiaphragmatic vagotomy blocks induction of IL-1 beta mRNA in mice brain in response to peripheral LPS. Am J Physiol. 1995;268(5 Pt 2):R1327–R1331. doi: 10.1152/ajpregu.1995.268.5.R1327. [DOI] [PubMed] [Google Scholar]
- 95.Hansen MK, Taishi P, Chen Z, Krueger JM. Vagotomy blocks the induction of interleukin-1beta (IL-1beta) mRNA in the brain of rats in response to systemic IL-1beta. J Neurosci. 1998;18(6):2247–2253. doi: 10.1523/JNEUROSCI.18-06-02247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hansen MK, Nguyen KT, Goehler LE, et al. Effects of vagotomy on lipopolysaccharide-induced brain interleukin-1beta protein in rats. Auton Neurosci. 2000;85(1–3):119–126. doi: 10.1016/s1566-0702(00)00230-7. [DOI] [PubMed] [Google Scholar]
- 97.van Dam A-M, Brouns M, Louisse S, Berkenbosch F. Appearance of interleukin-1 in macrophages and in ramified microglia in the brain of endotoxin-treated rats: a pathway for the induction of non-specific symptoms of sickness? Brain Res. 1992;588:291–296. doi: 10.1016/0006-8993(92)91588-6. [DOI] [PubMed] [Google Scholar]
- 98.Proescholdt MG, Chakravarty S, Foster JA, Foti SB, Briley EM, Herkenham M. Intracerebroventricular but not intravenous interleukin-1beta induces widespread vascular-mediated leukocyte infiltration and immune signal mRNA expression followed by brain-wide glial activation. Neuroscience. 2002;112(3):731–749. doi: 10.1016/s0306-4522(02)00048-9. [DOI] [PubMed] [Google Scholar]
- 99.Goehler LE, Gaykema RP, Opitz N, Reddaway R, Badr N, Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun. 2005;19(4):334–344. doi: 10.1016/j.bbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 100.Campisi J, Hansen MK, O’Connor KA, et al. Circulating cytokines and endotoxin are not necessary for the activation of the sickness or corticosterone response produced by peripheral E. coli challenge. J Appl Physiol. 2003;95(5):1873–1882. doi: 10.1152/japplphysiol.00371.2003. [DOI] [PubMed] [Google Scholar]
- 101.Blatteis CM. The onset of fever: new insights into its mechanism. Prog Brain Res. 2007;162:3–14. doi: 10.1016/S0079-6123(06)62001-3. [DOI] [PubMed] [Google Scholar]
- 102.Blatteis CM, Li S, Li Z, Perlik V, Feleder C. Signaling the brain in systemic inflammation: the role of complement. Front Biosci. 2004;9:915–931. doi: 10.2741/1297. [DOI] [PubMed] [Google Scholar]
- 103.Blatteis CM. Endotoxic fever: new concepts of its regulation suggest new approaches to its management. Pharmacol Ther. 2006;111(1):194–223. doi: 10.1016/j.pharmthera.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 104.Simons CT, Kulchitsky VA, Sugimoto N, Homer LD, Szekely M, Romanovsky AA. Signaling the brain in systemic inflammation: which vagal branch is involved in fever genesis? Am J Physiol. 1998;275(1 Pt 2):R63–R68. doi: 10.1152/ajpregu.1998.275.1.R63. [DOI] [PubMed] [Google Scholar]
- 105.Steiner AA, Ivanov AI, Serrats J, et al. Cellular and molecular bases of the initiation of fever. PLoS Biol. 2006;4(9):e284. doi: 10.1371/journal.pbio.0040284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cartmell T, Poole S, Turnbull AV, Rothwell NJ, Luheshi GN. Circulating interleukin-6 mediates the febrile response to localised inflammation in rats. J Physiol. 2000;526(Pt 3):653–661. doi: 10.1111/j.1469-7793.2000.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miller AJ, Hopkins SJ, Luheshi GN. Sites of action of IL-1 in the development of fever and cytokine responses to tissue inflammation in the rat. Br J Pharmacol. 1997;120(7):1274–1279. doi: 10.1038/sj.bjp.0701049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kozak W, Kluger MJ, Soszynski D, et al. IL-6 and IL-1 beta in fever. Studies using cytokine-deficient (knockout) mice. Ann NY Acad Sci. 1998;856:33–47. doi: 10.1111/j.1749-6632.1998.tb08310.x. [DOI] [PubMed] [Google Scholar]
- 109.Roth J, De Souza GE. Fever induction pathways: evidence from responses to systemic or local cytokine formation. Braz J Med Biol Res. 2001;34(3):301–314. doi: 10.1590/s0100-879x2001000300003. [DOI] [PubMed] [Google Scholar]
- 110.Rummel C, Barth SW, Voss T, et al. Localized vs. systemic inflammation in guinea pigs: a role for prostaglandins at distinct points of the fever induction pathways? Am J Physiol Regul Integr Comp Physiol. 2005;289(2):R340–R347. doi: 10.1152/ajpregu.00104.2005. [DOI] [PubMed] [Google Scholar]
- 111.Cooper AL, Rothwell NJ. Mechanisms of early and late hypermetabolism and fever after localized tissue injury in rats. Am J Physiol. 1991;261(6 Pt 1):E698–E705. doi: 10.1152/ajpendo.1991.261.6.E698. [DOI] [PubMed] [Google Scholar]
- 112.Goldbach JM, Roth J, Zeisberger E. Fever suppression by subdiaphragmatic vagotomy in guinea pigs depends on the route of pyrogen administration. Am J Physiol. 1997;272(2 Pt 2):R675–R681. doi: 10.1152/ajpregu.1997.272.2.R675. [DOI] [PubMed] [Google Scholar]
- 113.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 114.Lussier A, de Medicis R, Tetreault L. Adjuvant arthritis: influence of the adjuvant volume and composition on the non-specific inflammation. Int J Tissue React. 1981;3(1):11–15. [PubMed] [Google Scholar]
- 115.Connolly KM, Stecher VJ, Danis E, Pruden DJ, LaBrie T. Alteration of interleukin-1 production and the acute phase response following medication of adjuvant arthritic rats with cyclosporin-A or methotrexate. Int J Immunopharmacol. 1988;10(6):717–728. doi: 10.1016/0192-0561(88)90025-2. [DOI] [PubMed] [Google Scholar]
- 116.Eski M, Sahin I, Sengezer M, Serdar M, Ifran A. Thalidomide decreases the plasma levels of IL-1 and TNF following burn injury: is it a new drug for modulation of systemic inflammatory response. Burns. 2008;34(1):104–108. doi: 10.1016/j.burns.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 117.Engblom D, Ek M, Andersson IM, et al. Induction of microsomal prostaglandin E synthase in the rat brain endothelium and parenchyma in adjuvant-induced arthritis. J Comp Neurol. 2002;452(3):205–214. doi: 10.1002/cne.10380. [DOI] [PubMed] [Google Scholar]
- 118.Ozaki-Okayama Y, Matsumura K, Ibuki T, et al. Burn injury enhances brain prostaglandin E2 production through induction of cyclooxygenase-2 and microsomal prostaglandin E synthase in cerebral vascular endothelial cells in rats. Crit Care Med. 2004;32(3):795–800. doi: 10.1097/01.ccm.0000114576.60077.fc. [DOI] [PubMed] [Google Scholar]
- 119.Saha S, Engstrom L, Mackerlova L, Jakobsson PJ, Blomqvist A. Impaired febrile responses to immune challenge in mice deficient in microsomal prostaglandin E synthase-1. Am J Physiol Regul Integr Comp Physiol. 2005;288(5):R1100–R1107. doi: 10.1152/ajpregu.00872.2004. [DOI] [PubMed] [Google Scholar]
- 120.Oka Y, Ibuki T, Matsumura K, et al. Interleukin-6 is a candidate molecule that transmits inflammatory information to the CNS. Neuroscience. 2007;145(2):530–538. doi: 10.1016/j.neuroscience.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 121.Guay J, Bateman K, Gordon R, Mancini J, Riendeau D. Carrageenan-induced paw edema in rat elicits a predominant prostaglandin E2 (PGE2) response in the central nervous system associated with the induction of microsomal PGE2 synthase-1. J Biol Chem. 2004;279(23):24866–24872. doi: 10.1074/jbc.M403106200. [DOI] [PubMed] [Google Scholar]