Abstract
This study investigated whether delayed treatment of spinal cord injury with controlled release of neurotrophin-3 (NT-3) from fibrin scaffolds can stimulate enhanced neural fiber sprouting. Long Evans rats received a T9 dorsal hemisection spinal cord injury. Two weeks later, the injury site was re-exposed, and either a fibrin scaffold alone, a fibrin scaffold containing a heparin-based delivery system with different concentrations of NT-3 (500 and 1000 ng/mL), or a fibrin scaffold containing 1000 ng/mL of NT-3 (no delivery system) was implanted into the injury site. The injured spinal cords were evaluated for morphological differences using markers for neurons, astrocytes, and chondroitin sulfate proteoglycans 2 weeks after treatment. The addition of 500 ng/mL of NT-3 with the delivery system resulted in an increase in neural fiber density compared to fibrin alone. These results demonstrate that the controlled release of NT-3 from fibrin scaffolds can enhance neural fiber sprouting even when treatment is delayed 2 weeks following injury.
Keywords: nerve regeneration, growth factors, biomaterial, delivery system
Introduction
Spontaneous regeneration of spinal cord neurons following traumatic injury is limited by several inhibitory factors. The primary injury encompasses the focal destruction of neural tissue caused by direct mechanical trauma. Over time, the primary injury induces a progressive series of secondary injuries, which exacerbates the injury to the spinal cord (Hagg and Oudega 2006). Finally, a glial scar forms at the border of the lesion site that consists of reactive astrocytes and meningeal fibroblasts that serve as an inhibitory border to axonal regeneration (Fawcett and Asher 1999; McDonald and Sadowsky 2002). All of these factors combine to limit axonal regeneration following spinal cord injury (SCI).
To overcome some of these barriers to regeneration a family of proteins that promote the growth and survival of several different classes of neurons called neurotrophins have been investigated. Specifically, treatment with neurotrophin-3 (NT-3) has been shown to enhance sprouting of supraspinal tract axons when injected into the lesion immediately following SCI (Schnell et al. 1994). The sprouting of supraspinal axon tract axons following SCI has also been associated with increases in functional recovery, though the exact mechanism of that recovery is not known (Blits et al. 2000; Grill et al. 1997a; Lynskey et al. 2006; Novikova et al. 2002). Other research has investigated the ability of neurotrophins to stimulate regeneration of chronically injured axons following SCI. Studies have shown that the ability of certain chronically injured supraspinal axon tracts to regenerate may decrease if intervention is delayed for too long (Coumans et al. 2001; Grill et al. 1997b; Jin et al. 2002; Tobias et al. 2003; von Meyenburg et al. 1998). Recently, it was shown that the barriers to axonal regeneration that increase in chronically injured spinal cord can be overcome using a combination of diffusible growth stimulating molecules and a favorable growth stimulating extracellular matrix (ECM) (Lu et al. 2007). Lu et al. used continuous transgenic delivery of neurotrophic factors from engineered cells that also secrete ECM that is conducive to regeneration. One draw back to continuous delivery of neurotrophic factors from transplanted cells is that growth is limited to the regeneration of axons into the transplant site, which is the source of the growth stimulating neurotrophins and the axons fail to regenerate back into host tissue. Results such as these highlight the need for temporary controlled release of growth-stimulating neurotrophins that not only promote axon growth into the lesion but also foster growth into healthy spinal cord tissue.
To this end, our lab has shown that delayed treatment of SCI with fibrin scaffolds can provide a supportive regenerative environment, reducing the accumulation of reactive astrocytes surrounding the lesion and enhancing the presence of neural fibers within the lesion (Johnson et al. 2009). A heparin-based delivery system (HBDS) was developed by Sakiyama-Elbert et al. that utilizes non-covalent interactions between neurotrophins, heparin, and a covalently linked bi-domain peptide, which can be incorporated into the fibrin scaffold and allow for the controlled release of growth factors from the scaffolds (Sakiyama-Elbert and Hubbell 2000). Taylor et al. in a previous study incorporated the HBDS into a fibrin scaffold to deliver NT-3, which promoted a significant increase in the density of neural fibers sprouting in an acute SCI model (Taylor et al. 2004; Taylor et al. 2006). However, it is unclear that axons injured 2 weeks prior to treatment in a subacute SCI model are similarly responsive to treatment with NT-3, thus the purpose of this study was to examine if NT-3 was still able to promote fiber sprouting at 2 weeks after injury.
Subacute and chronic treatment studies are relevant in the development of clinical treatments for SCI, since most patients will not have access to all treatments in the first 24 hrs post-injury. While many studies have shown that delayed treatment of injured axons with neurotrophins can induce substantial regeneration, most of these studies have used neurotrophic delivery systems that deluge the injury with regeneration stimulating molecules that result in abnormal growth (Coumans et al. 2001; Grill et al. 1997b; Jin et al. 2002; Lu et al. 2007; von Meyenburg et al. 1998). The focus of the current study is to determine if the controlled release of NT-3 from fibrin scaffolds using a HBDS can enhance neural sprouting when administered at a subacute (2 weeks) time point following SCI.
Methods
Fibrin scaffold preparation and polymerization method
All materials were purchased from Fisher Scientific (Pittsburgh, PA) unless otherwise noted. Fibrin scaffolds were made as described previously by mixing the following components: human plasminogen-free fibrinogen containing Factor XIII (10 mg/mL, Sigma, St. Louis, MO), CaCl2 (5mM), and thrombin (12.5 NIH units/mL, Sigma) in Tris-buffered saline (TBS, 137 mM NaCl, 2.7 mM KCl, 33 mM Tris, pH 7.4)(Sakiyama-Elbert and Hubbell 2000). The bi-domain peptide, denoted ATIII, was synthesized by standard solid phase Fmoc chemistry as described previously (Sakiyama-Elbert and Hubbell 2000). In scaffolds containing the delivery system (DS) ATIII peptide (0.25 mM) and heparin (6.25 μM, Sigma, sodium salt from porcine intestinal mucosa) were added to the fibrin polymerization mixture. Polymerized fibrin scaffolds (10 μL in volume) were formed by ejecting the polymerization mixture from a 20 μL pipette tip such that a sphere of the mixture formed on the tip of the pipette. The sphere was then allowed to polymerize on the pipette tip for 5 min prior to implantation into the injury site. A 20 μL in situ polymerized scaffold was then formed by ejecting the polymerization solution directly onto the sphere already in the injury site and allowing it to polymerize in the lesion site and stabilize the sphere in the injury site.
In-vivo studies – dorsal hemisection subacute SCI model
All experimental procedures on animals complied with the Guide for the Care and Use of Laboratory Animals and were performed under the supervision of the Division of Comparative Medicine at Washington University. Long-Evans female rats (250-275 g, Harlan, Indianapolis, IND) were anesthetized using 4% isoflurane gas (Vedco Inc., St Josephs, MO). The skin and muscle overlying the spinal column were incised and dissected away from the spinal column. Clamps attached to the spinous processes and a rigid frame was used to immobilize the spinal column. A dorsal laminectomy was performed using fine rongeurs at level T-9 to expose the spinal cord. A lateral slit in the dura was made, and microdissection scissors mounted to a micromanipulator were lowered into the spinal cord 1.2 mm from the dorsal spinal cord surface. Using the microdissection scissors, a lateral incision was made to form a complete dorsal hemisection of the spinal cord. Finally the microdissection scissors were run through the incision to assure the hemisection was complete. The cord was then covered with a piece of artificial dura (generous gift of Synovis Surgical Innovations, St Paul MN), the muscles were sutured with degradable sutures, and the skin was stapled close. Two weeks following initial injury the lesion was re-exposed, and scar tissue was removed from the wound site of control and experimental groups. As described above, scar tissue was removed and either no treatment (control), 10 μL fibrin sphere alone (fibrin, n = 6), fibrin sphere containing NT-3 and delivery system at two doses (500ng/mL+DS n = 6 and 1000ng/mL+DS n = 5), or fibrin sphere containing NT-3 alone (1000ng/mL no DS n = 5) were fabricated and placed in the injury site. An untreated control group (n = 6) received the same surgical removal of scar tissue but no treatment with fibrin scaffolds.
Animals were given cefazolin (15 mg/kg, twice a day) for five days following each surgical procedure as prophylactic against urinary tract infections. Bladders were expressed manually twice a day until they regained bladder control, approximately 5 to 6 days post initial injury. The animals were euthanized 2 weeks post treatment. After transcardial perfusion using 4% paraformaldehyde (Sigma), spinal cords were dissected and post fixed in 4% paraformaldehyde solution overnight. The cords were then cryoprotected in a 30% sucrose solution in phosphate buffered saline in preparation for processing. The spinal cord was embedded in Tissue-Tek OCT compound Mounting Media (Sakura Finetek, Torence, CA) and cut into 20 μm sagittal sections using a cryostat.
Immunohistochemistry
Immunohistochemistry was used to analyze morphological aspects of each experimental group. Sections were washed with phosphate-buffered saline (PBS) and permeabilized with 0.1% Triton X-100 for 5 min. After washes in PBS, the sections were blocked with 10% bovine serum albumin (BSA, Sigma) and 2% normal goat serum (NGS, Sigma). Primary antibodies against glial fibrillary acidic protein (GFAP, rabbit polyclonal, recognizing astrocytes, 1:4, ImmunoStar, Hudson, WI), neuronal class III β-tubulin (Tuj1, mouse monoclonal, recognizing neurons, 1:200, Covance Research Products, Berkeley, CA), ED-1 (mouse monoclonal, recognizing macrophages, 1:100, Serotec, Oxford, UK) and NG2 chondroitin sulfate proteoglycan (NG2, rabbit polyclonal, 1:200, Chemicon) were used to evaluate each section. Each sagittal section was double stained with Tuj1 and GFAP, ED1 and GFAP, or NG2 and Tuj1. Finally, appropriate secondary antibodies (Alexa Fluor 488, 555, and 647 conjugated, 1:300 Invitrogen, Carlsbad, CA) with 2% NGS were used and each section was stained with Hoechst nuclear stain (1:1000 Molecular Probes).
Quantification of neural fiber density, astrocyte density, and chondroitin sulfate proteoglycan density
Each spinal cord section was imaged at 40× magnification using an Olympus IX70 microscope (Olympus America, Melville, NY) and Magnafire Camera (Optronics, Goleta, CA). Imaging resulted in multiple 40× images that spanned the entire spinal cord section and that were later spliced together using Photoshop (Adobe, San Jose, CA) to yield a complete picture of the lesion and surrounding intact cord. The density of neural fibers within the lesion that stained positive for Tuj1 was quantified from 6 serial sections spaced 200μm apart from each rat. The density of Tuj1 staining was quantified inside the lesion area using the binary image processing software Ia32 (Leco, St. Joseph, MI). The lesion was defined as the space within the GFAP-positive border and a straight line connecting the dorsal surface of the injured spinal cord. An intensity threshold was set so that only Tuj1 positive neuronal fibers were quantified within the lesion. The area of positive Tuj1 staining within the lesion (the number of pixels in which the intensity was above the set intensity threshold) was divided by the total area (number of pixels within the lesion) to yield the neural fiber density.
The density of GFAP staining of astrocytes and the presence of NG2 was quantified inside the white matter that bordered the lesion for all experimental groups. Four 200× magnification images of the white matter surrounding the lesion site were taken from 6 serial sections spaced 200μm apart for each rat. Two images were taken of the intact white matter region bordering the rostral side of the lesion (as defined by GFAP staining) and two images were taken of the intact white matter region bordering the caudal side of the lesion. The images were then analyzed using the Ia32 software. An intensity threshold was set so that only areas positive for GFAP or NG2 were quantified (Figure 1B&C), then the area of GFAP or NG2 staining (the number of pixels in which the intensity was above the set threshold) was divided by the total area (number of pixels in the area) of the picture to yield GFAP or NG2 density.
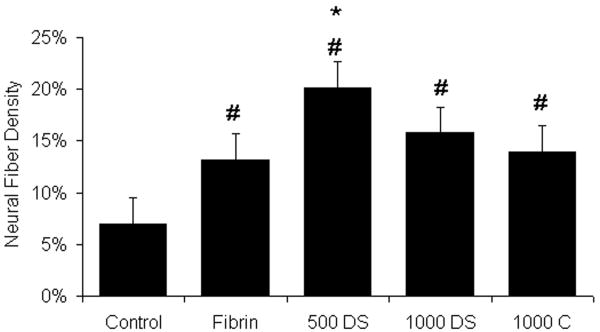
Figure 1.
Neural fiber density within the lesion site. The percent neural fiber density within the lesion site of the untreated control (Control), fibrin alone (Fibrin), fibrin containing the HBDS with 500ng/mL NT-3 (500 DS), fibrin containing the HBDS with 1000ng/mL of NT-3 (1000 DS), and fibrin containing 1000ng/mL NT-3 (no HBDS) (1000 C) groups was assessed by image analysis. Fibrin scaffolds with 500ng/mL of NT-3 containing the HBDS (500 DS) enhanced neural fiber density within the lesion site compared to fibrin alone and untreated controls. The fibrin-treated groups (Fibrin, 500 DS, 1000 DS, and 1000 C) had an increase in the density of neural fiber staining within the lesion site compared to untreated control. (Error bars denote standard deviation, * denotes p < 0.05 versus fibrin alone, # denotes p<0.05 versus untreated control)
Macrophage/Microglia immune profile analysis
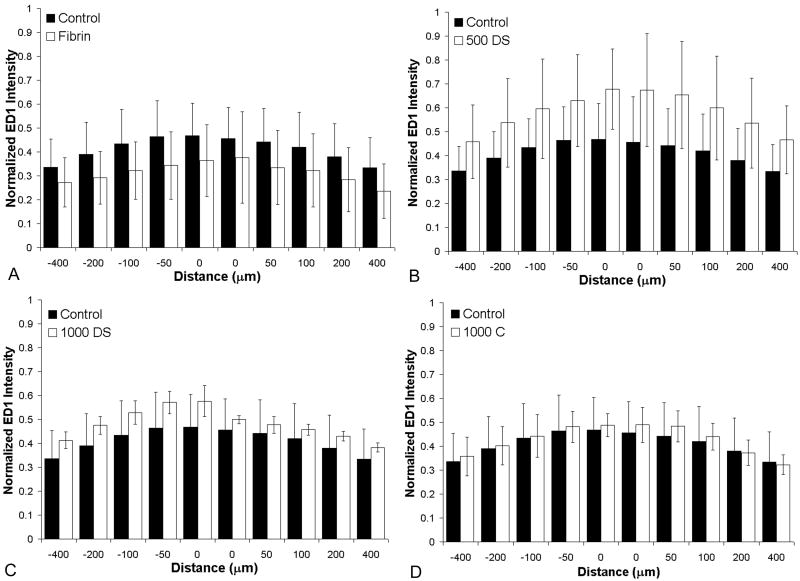
To analyze the immune response, the lesion area was imaged at 40× magnification and individual 40× images of the lesion area were spliced together to yield a complete picture of the lesion area and surrounding intact cord. ED-1 staining of macrophages and microglia was analyzed surrounding the lesion site in 6 serial sections spaced 200μm apart from each rat to evaluate the immune response to the fibrin scaffolds. Immune response was analyzed by using a program written in the Matlab Image Processing Toolbox (Mathworks, Natick, MA). The program allowed the user to define the lesion border based on the GFAP stained lesion border as it was defined for the neural fiber density analysis. Based on this border, the program generates line profiles of a specific distance spaced 5μm apart originating at the lesion border and preceding either rostrally or caudally from the border. The program then analyzed the pixel intensity along this line and extracted relative ED-1 fluorescence intensity as a function of distance from the glial scar border (Jain et al. 2006). The analysis yielded an immune profile for each sample. To concisely convey this data, the normalized intensity was displayed at distances of 0, 50, 100, 200, and 400μm rostral (-) and caudal (+) to the lesion border.
Statistical analysis
All statistics were performed with Analysis of Variance (ANOVA, planned comparison post-hoc test) using Statistica (StatSoft, Tulsa, OK). Significance was determined to be p<0.05.
Results
Neural fiber density
Administration of NT-3 has been shown to promote regeneration of central nervous system neurons following SCI. To evaluate the effect of controlled release of NT-3 from the HBDS on neural fiber sprouting, the density of neural fiber staining within the lesion site was quantified following subacute SCI. The addition of fibrin scaffolds with or without NT-3 enhanced the density of neural fibers staining in the lesion site when compared to the untreated control (Figure 1). Additionally, the group treated with fibrin scaffolds containing the delivery system and 500 ng/mL of NT-3 (500 DS) showed an increase in neural fiber density within the lesion site compared to fibrin alone (p<0.05). At 2 weeks, 500 DS group exhibited an average neural fiber density of ∼20 ± 6% in the lesion site, where as the fibrin alone group and untreated control group exhibited only ∼13± 6% and ∼7± 3% neural fiber density, respectively. In the remaining experimental groups, the addition of 1000ng/mL of NT-3 with and without the delivery system did not elicit an increase in neural fiber density compared to fibrin alone. As seen in previous studies, sections from the group treated with fibrin scaffolds consistently exhibited Tuj1 positive neural fibers that extend past regions of GFAP staining and were found in the lesion (Figure 2). The addition of NT-3 appears to increase this effect. These findings demonstrate that the controlled release of NT-3 at the appropriate dose from a fibrin scaffold enhances neural fiber density within the lesion site following subacute SCI.
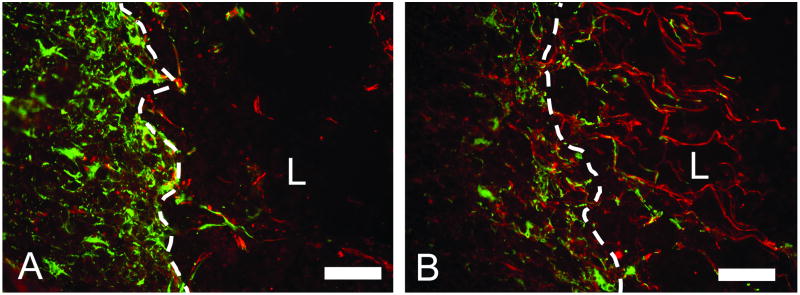
Figure 2.
The presence of neural processes that cross the glial scar border at the lesion site. Images of the lesion border stained with Tuj1 (red) and GFAP (green). A) The lesion border from the untreated control group showing neural fibers growing to the glial scar border and failing to cross the border into the lesion site. B) Image of the lesion border from the group treated with 500ng/mL NT-3 containing the HBDS showing neural fibers that cross glial scar border at the lesion site. The neural fibers are accompanied by elongated glial processes of GFAP positive astrocytes that accompany the neural fibers across the border. (White dotted line marks glial scar border, L indicates lesion site, scale bar = 100 μm)
Astrocyte density
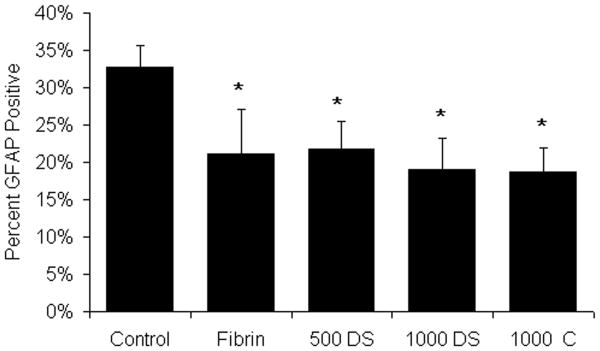
Glial scarring following CNS injury has been associated with decreased axonal regeneration and functional recovery. Astrocytes are the major component of the glial scar that forms following SCI, and may play a role in preventing the regeneration following injury. The density of astrocyte staining surrounding the lesion site was analyzed at 2 weeks following treatment. Those groups treated with fibrin scaffolds had a significant decrease in the density of GFAP staining surrounding the lesion site when compared to untreated controls at 2 weeks (∼32% for untreated control versus less than 20% for fibrin-treated groups) (Figure 3). The results shown here are consistent with previous studies showing that fibrin scaffolds effectively delay the formation of a mature glial scar and that the controlled release of NT-3 from an HBDS does not affect this delayed formation (Taylor et al. 2006).
Figure 3.
Effect of fibrin scaffolds on astrocyte density. The percent GFAP positively stained area surrounding the lesion site of the untreated control (Control), fibrin alone (Fibrin), fibrin containing the HBDS with 500ng/mL NT-3 (500 DS), fibrin containing the HBDS with 1000ng/mL of NT-3 (1000 DS), and fibrin containing 1000ng/mL NT-3 (no HBDS) (1000 C) groups was assessed by image analysis. Fibrin-treated groups (Fibrin, 500 DS, 1000 DS, and 1000 C) decreased the density of astrocyte staining surrounding the lesion site following implantation compared to untreated controls. (Error bars denote standard deviation, * denotes p < 0.05 versus untreated control)
NG2 density
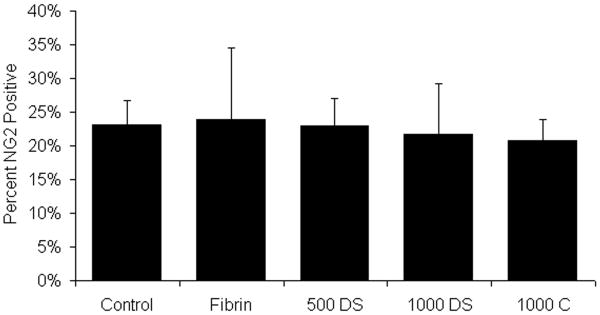
Chondroitin sulfate proteoglycans (CSPGs) are a major inhibitory component preventing the regeneration of neural tissue following SCI. Here the density of NG2 CSPGs surrounding the injury site was quantified following subacute SCI. The density of NG2 surrounding the lesion site in all experimental groups was not significantly different from untreated control groups (Figure 4). Treatment with fibrin scaffolds did not increase NG2 deposition, which has been associated with an increase in the inhibition of axonal regeneration.
Figure 4.
Effect of fibrin scaffolds on chondroitin sulfate proteoglycan (NG2) density. The percent NG2 positively stained area surrounding the lesion site of the control (Control), fibrin alone (Fibrin), fibrin containing the HBDS with 500ng/mL NT-3 (500 DS), fibrin containing the HBDS with 1000ng/mL of NT-3 (1000 DS), and fibrin containing 1000ng/mL NT-3 (no HBDS) (1000 C) groups was assessed by image analysis. No significant difference was observed in NG2 density surrounding the lesion site between untreated control and fibrin treated groups (Fibrin, 500 DS, 1000 DS, and 1000 C). (Error bars denote standard deviation)
Macrophage/Microglia immune profile
A profile of macrophage/microglia present adjacent to the lesion site was used to assess inflammation in response to treatment with fibrin scaffolds. As described in the methods, a program was developed analyze the immune profile of each experimental group (Figure 5). In all experimental groups, ED-1 positive cells accumulated at the lesion border. There was no statistical difference between untreated controls and all other experimental groups, indicating that the treatment does not induce an increase in immune response following injury.
Figure 5.
Effect of fibrin scaffold on macrophage/microglia staining. Immune response following SCI was analyzed by measuring the intensity of ED1 staining as a function of distance from the lesion border for each experimental group (untreated control (Control), fibrin treated group (Fibrin), fibrin containing the HBDS with 500ng/mL NT-3 (500 DS), fibrin containing the HBDS with 1000ng/mL of NT-3 (1000 DS), and fibrin containing 1000ng/mL NT-3 (no HBDS) (1000 C)). The analysis yielded an immune profile for each experimental group that was normalized by a positive control. The normalized intensity is displayed at distances of 0, 50, 100, 200, and 400μm rostral (-) and caudal (+) to the lesion border. A) ED1 profile for the Control (black bars) and fibrin treated groups (white bars) 2 weeks following implantation. B) ED1 profile of the untreated Control (black bars) and 500 DS (white bars). C) ED1 profile of the untreated Control (black bars) and 1000 DS (white bars). D) ED1 profile of the untreated control (black bars) and 1000 C (white bars). The experimental groups were not statistically different from the control group at any distance (p>0.05). (Error bars represent standard deviation).
Discussion
Following SCI, the environment within the spinal cord is intensely inhibitory to axonal regeneration. By providing an environment containing regeneration-promoting cellular and molecular cues, multiple studies have shown that it is possible to overcome some aspects of the inhibitory environment and increase regeneration (Lu et al. 2007; Piantino et al. 2006; Taylor et al. 2004). These studies however, analyzed the efficacy of treatments immediately after injury. Other studies that analyzed the effects of delayed treatment of SCI have been published and show that a time delay between injury and treatment can affect outcome (Lynskey et al. 2006; Shumsky et al. 2003; Tobias et al. 2003). The major findings of this study were that delayed treatment with the controlled release of NT-3 increased the neural fiber density within the lesion site and that treatment with fibrin scaffolds decrease the accumulation of reactive astrocytes following subacute SCI.
Several studies have reported the stimulation of axonal regeneration following SCI through the exogenous administration of NT-3 (Blits et al. 2000; Grill et al. 1997a; Lynskey et al. 2006; Novikova et al. 2002; Schnell et al. 1994; Taylor et al. 2006). In the current study, it was found that a dose of 500 ng/mL of NT-3 delivered from our HBDS was sufficient to stimulate an increase in neural fiber density within the lesion site. The incorporation of the HBDS has been shown in in vitro studies to promote the retention of NT-3 within fibrin scaffolds (Taylor et al. 2004) and to increase neural fiber density at 9 days following acute SCI in rats (Taylor et al. 2006). The total mass of NT-3 used in this study was 15 ng and is significantly less (∼70× less) than the total mass of NT-3 used in other studies (Lynskey et al. 2006; Novikova et al. 2002; Piantino et al. 2006; Schnell et al. 1994). Given that the total mass of NT-3 that elicited an increase in neural fiber density was considerably less than other studies and the ability of the HBDS to retain NT-3 in vitro within fibrin scaffolds, it is likely the HBDS effectively increased the local concentration of NT-3 and thus enhanced its therapeutic effect in the lesion site.
Increased neural fiber density compared to the fibrin alone group was seen only in the 500 ng/mL dose of NT-3 with the HBDS (500 DS). The observation that increasing the concentration of NT-3 with the HBDS (1000 DS) does not result in an increase in neural fiber density compared to fibrin alone implies that there may be saturation of the NT-3 receptor (Trk C). Although there is some literature evidence suggesting a down regulation of the receptor level in response to high concentrations of NT-3 (Xu et al. 2002), from the results of our study it cannot be determined whether the biphasic response was caused by saturation. Additionally, because heparin has been shown to interact with multiple growth factors it is possible that in an in vivo environment the sequestering of growth factors would not be limited to exogenous NT-3 loaded into the scaffold prior to implantation, rather other endogenous growth factors may also retained within the lesion site. However, thorough characterization of our delivery system in a similar SCI model showed no difference between groups treated with fibrin plus delivery system alone and those treated with fibrin alone (in the absence of NT-3) (Taylor et al. 2004; Taylor et al. 2006; Taylor and Sakiyama-Elbert 2006).
Neural fiber density analysis was performed in this study using the LECO IA32 binary image process software that measures the density of neural fiber staining in the lesion site. Using this analysis, a quantitative statement regarding the number of regenerating neural fibers seen in each experimental group cannot be made. As such, the differences in neural fiber density observed in this study could be a result of different anatomical results. The increase in neural fiber density could have resulted from an increased number of regenerating neural fibers in the lesion site of those animals treated in the 500+DS group compared to the fibrin alone and control groups. Alternatively, it is possible that each experimental group had similar numbers of regenerating fibers but the presence of NT-3 with the HBDS (500+DS) stimulated differences in the length of those fibers that resulted in differences in neural fiber density. In either scenario, fibrin scaffolds containing 500 ng/mL of NT-3 and the HBDS increased the overall neural fiber staining density of injured CNS neural fibers after delayed treatment.
Treatment with fibrin scaffolds significantly decreased the density of astrocyte staining surrounding the lesion site. This observation is in agreement with previous studies using fibrin scaffolds to treat SCI in our lab (Taylor et al. 2006). Dense astroglial scars that often form after SCI contain inhibitory molecules that hinder the regeneration of axons (Hsu and Xu 2005; Patist et al. 2004; Prang et al. 2006). It is possible that the presence of the fibrin scaffold facilitated the migration of astrocytes into the lesion. This migration could have resulted in a more diffuse accumulation of astrocytes at the lesion border. In the current study, the combination of reducing the density of accumulated astrocytes and the addition of NT-3 may have further enhanced the neural fiber density compared to fibrin scaffolds alone. Previously it has been shown that glial cells can readily migrate into fibrin scaffolds (Knoops et al. 1991). In accordance with this explanation, others have shown that enhanced migration of astrocytes into fibrin scaffolds resulted in elongated glial processes crossing the lesion border similar to the structures seen in this study (Figure 2), which provided an environment more permissive to axonal sprouting (Taylor et al. 2006).
In the current study, the inhibitory molecules produced by astrocytes that are associated with inhibition of axonal regeneration were not directly targeted. However, decreasing the accumulation of astrocytes at the lesion border may also decrease the presentation of these inhibitory molecules and result an increase in regeneration potential of axons across the lesion border. Finally, combining this potential with the controlled release of NT-3 may have caused the increase in fiber density that was seen.
None of the treatment examined in this study affected the density of NG2 staining surrounding the lesion site. The consistent presence of this inhibitory molecule in groups with increased neural fiber density seems counterintuitive, however a recent study has shown that axonal regeneration can occur in the presence of inhibitory cues. Lu et al. demonstrated that substantial delivery of NT-3 via cells genetically altered to constitutively produce the growth factor can stimulate growth of axons through unaltered glial scars that are otherwise inhibitory to any axonal sprouting (Lu et al. 2007).
Overall, this study demonstrated that controlled release of NT-3 promotes increased neural fiber density within the lesion and decreased reactive astrocyte staining around the lesion of subacute SCI. As discussed above, many studies have shown that delayed treatment of injured axons with neurotrophins can induce substantial regeneration, however most of these studies have used genetically engineered cells, osmotic pumps, or other delivery methods that deliver large continuous doses of growth factor to the spinal cord (Coumans et al. 2001; Grill et al. 1997b; Jin et al. 2002; Lu et al. 2007; von Meyenburg et al. 1998). The current study determined that the controlled release of NT-3 from fibrin scaffolds using a HBDS can enhance neural fiber sprouting when administered at a subacute (2 weeks) time point following SCI. The finding is promising because local temporary delivery of neurotrophic factors from an implantable degradable drug delivery device to treat SCI would have a lower risk of systemic side effects than cellular or osmotic minipump delivery of trophic factors.
Acknowledgments
The authors acknowledge Dan Hunter for assistance with tissue preparation and image analysis, and Angela Guzmán for assistance with animal care. This study was supported by NIH R01 NS051454.
Footnotes
No benefit of any kind will be received either directly or indirectly by the authors.
References
- Blits B, Dijkhuizen PA, Boer GJ, Verhaagen J. Intercostal nerve implants transduced with an adenoviral vector encoding neurotrophin-3 promote regrowth of injured rat corticospinal tract fibers and improve hindlimb function. Exp Neurol. 2000;164(1):25–37. doi: 10.1006/exnr.2000.7413. [DOI] [PubMed] [Google Scholar]
- Coumans JV, Lin TT, Dai HN, MacArthur L, McAtee M, Nash C, Bregman BS. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J Neurosci. 2001;21(23):9334–44. doi: 10.1523/JNEUROSCI.21-23-09334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49(6):377–91. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Grill R, Murai K, Blesch A, Gage FH, Tuszynski MH. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J Neurosci. 1997a;17(14):5560–72. doi: 10.1523/JNEUROSCI.17-14-05560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill RJ, Blesch A, Tuszynski MH. Robust growth of chronically injured spinal cord axons induced by grafts of genetically modified NGF-secreting cells. Exp Neurol. 1997b;148(2):444–52. doi: 10.1006/exnr.1997.6704. [DOI] [PubMed] [Google Scholar]
- Hagg T, Oudega M. Degenerative and spontaneous regenerative processes after spinal cord injury. J Neurotrauma. 2006;23(3-4):264–80. doi: 10.1089/neu.2006.23.263. [DOI] [PubMed] [Google Scholar]
- Hsu JY, Xu XM. Early profiles of axonal growth and astroglial response after spinal cord hemisection and implantation of Schwann cell-seeded guidance channels in adult rats. J Neurosci Res. 2005;82(4):472–83. doi: 10.1002/jnr.20662. [DOI] [PubMed] [Google Scholar]
- Jain A, Kim YT, McKeon RJ, Bellamkonda RV. In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials. 2006;27(3):497–504. doi: 10.1016/j.biomaterials.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Jin Y, Fischer I, Tessler A, Houle JD. Transplants of fibroblasts genetically modified to express BDNF promote axonal regeneration from supraspinal neurons following chronic spinal cord injury. Exp Neurol. 2002;177(1):265–75. doi: 10.1006/exnr.2002.7980. [DOI] [PubMed] [Google Scholar]
- Johnson PJ, Parker SR, Sakiyama-Elbert SE. Fibrin-based tissue engineering scaffolds enhance neural fiber sprouting and delay the accumulation of reactive astrocytes at the lesion in a subacute model of spinal cord injury. J Biomed Mater Res A. 2009 doi: 10.1002/jbm.a.32343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoops B, Hubert I, Hauw JJ, van den Bosch de Aguilar P. Axonal growth and glial migration from co-cultured hippocampal and septal slices into fibrin-fibronectin-containing matrix of peripheral regeneration chambers: a light and electron microscope study. Brain Res. 1991;540(1-2):183–94. doi: 10.1016/0006-8993(91)90506-q. [DOI] [PubMed] [Google Scholar]
- Lu P, Jones LL, Tuszynski MH. Axon regeneration through scars and into sites of chronic spinal cord injury. Exp Neurol. 2007;203(1):8–21. doi: 10.1016/j.expneurol.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Lynskey JV, Sandhu FA, Dai HN, McAtee M, Slotkin JR, MacArthur L, Bregman BS. Delayed intervention with transplants and neurotrophic factors supports recovery of forelimb function after cervical spinal cord injury in adult rats. J Neurotrauma. 2006;23(5):617–34. doi: 10.1089/neu.2006.23.617. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359(9304):417–25. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- Novikova LN, Novikov LN, Kellerth JO. Differential effects of neurotrophins on neuronal survival and axonal regeneration after spinal cord injury in adult rats. J Comp Neurol. 2002;452(3):255–63. doi: 10.1002/cne.10381. [DOI] [PubMed] [Google Scholar]
- Patist CM, Mulder MB, Gautier SE, Maquet V, Jerome R, Oudega M. Freeze-dried poly(D,L-lactic acid) macroporous guidance scaffolds impregnated with brain-derived neurotrophic factor in the transected adult rat thoracic spinal cord. Biomaterials. 2004;25(9):1569–82. doi: 10.1016/s0142-9612(03)00503-9. [DOI] [PubMed] [Google Scholar]
- Piantino J, Burdick JA, Goldberg D, Langer R, Benowitz LI. An injectable, biodegradable hydrogel for trophic factor delivery enhances axonal rewiring and improves performance after spinal cord injury. Exp Neurol. 2006;201(2):359–67. doi: 10.1016/j.expneurol.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Prang P, Muller R, Eljaouhari A, Heckmann K, Kunz W, Weber T, Faber C, Vroemen M, Bogdahn U, Weidner N. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials. 2006;27(19):3560–9. doi: 10.1016/j.biomaterials.2006.01.053. [DOI] [PubMed] [Google Scholar]
- Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65(3):389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- Schnell L, Schneider R, Kolbeck R, Barde YA, Schwab ME. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994;367(6459):170–3. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- Shumsky JS, Tobias CA, Tumolo M, Long WD, Giszter SF, Murray M. Delayed transplantation of fibroblasts genetically modified to secrete BDNF and NT-3 into a spinal cord injury site is associated with limited recovery of function. Exp Neurol. 2003;184(1):114–30. doi: 10.1016/s0014-4886(03)00398-4. [DOI] [PubMed] [Google Scholar]
- Taylor SJ, McDonald JW, 3rd, Sakiyama-Elbert SE. Controlled release of neurotrophin-3 from fibrin gels for spinal cord injury. J Control Release. 2004;98(2):281–94. doi: 10.1016/j.jconrel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Taylor SJ, Rosenzweig ES, McDonald JW, 3rd, Sakiyama-Elbert SE. Delivery of neurotrophin-3 from fibrin enhances neuronal fiber sprouting after spinal cord injury. J Control Release. 2006;113(3):226–35. doi: 10.1016/j.jconrel.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SJ, Sakiyama-Elbert SE. Effect of controlled delivery of neurotrophin-3 from fibrin on spinal cord injury in a long term model. J Control Release. 2006;116(2):204–10. doi: 10.1016/j.jconrel.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias CA, Shumsky JS, Shibata M, Tuszynski MH, Fischer I, Tessler A, Murray M. Delayed grafting of BDNF and NT-3 producing fibroblasts into the injured spinal cord stimulates sprouting, partially rescues axotomized red nucleus neurons from loss and atrophy, and provides limited regeneration. Exp Neurol. 2003;184(1):97–113. doi: 10.1016/s0014-4886(03)00394-7. [DOI] [PubMed] [Google Scholar]
- von Meyenburg J, Brosamle C, Metz GA, Schwab ME. Regeneration and sprouting of chronically injured corticospinal tract fibers in adult rats promoted by NT-3 and the mAb IN-1, which neutralizes myelin-associated neurite growth inhibitors. Exp Neurol. 1998;154(2):583–94. doi: 10.1006/exnr.1998.6912. [DOI] [PubMed] [Google Scholar]
- Xu B, Michalski B, Racine RJ, Fahnestock M. Continuous infusion of neurotrophin-3 triggers sprouting, decreases the levels of TrkA and TrkC, and inhibits epileptogenesis and activity-dependent axonal growth in adult rats. Neuroscience. 2002;115(4):1295–308. doi: 10.1016/s0306-4522(02)00384-6. [DOI] [PubMed] [Google Scholar]