Abstract
Proton magnetic resonance spectroscopy (1H-MRS) is a technique for the assay of brain neurochemistry in vivo. N-acetylaspartate (NAA), the most prominent metabolite visible within the 1H-MRS spectrum, is found primarily within neurons. The current study was designed to further elucidate NAA–cognition relationships, particularly whether such relationships are moderated by sex, or tissue type (gray or white matter). We administered standard measures of intelligence to 63 young, healthy subjects and obtained spectroscopic imaging data within a slab of tissue superior to the lateral ventricles. We found that lower NAA within right anterior gray matter predicted better performance VIQ (F=6.83, p=.011, r2=.10), while higher NAA within the right posterior gray matter region predicted better PIQ (F=8.175, p=.006, r2=.12). These findings add to the small but growing body of literature linking brain biochemistry to intelligence in normal healthy subjects using 1H-MRSI.
Keywords: Intelligence, N-acetylaspartate, Gray matter, White matter, Sex differences
1. Introduction
Modern neuroimaging techniques have been a boon for intelligence research. Numerous reports have linked brain measures to intellectual functioning using such diverse techniques as positron emission tomography (PET), functional magnetic resonance imaging (fMRI), diffusion tensor imaging (DTI), structural magnetic resonance imaging (sMRI), and magnetic resonance spectroscopy (MRS) (Jung and Haier 2007). Mechanistic underpinnings of intelligence remain elusive, however, as our neuroimaging measures only reflect indirectly what might be going on within and among populations of neurons, glia, and other brain cells: we still struggle to “see through a glass, darkly.”
Proton magnetic resonance spectroscopy (1H-MRS) allows for the assay of brain neurochemistry in vivo, thus providing information about brain metabolism. MRS can be obtained from either single voxels of tissue or across a grid of voxels (i.e. spectroscopic imaging) across a “slice” or multiple slices of tissue. Proton and phosphorous spectroscopy (1H and 31P, respectively) comprise the vast majority of clinical and normal human studies in the research literature. In 1H spectroscopy, a given metabolite of interest is reported either as a ratio to another metabolite within the same acquired spectrum, or is referenced to a water or metabolite solution scan obtained separately, allowing for what is often referred to as “absolute” quantification of a given metabolite at a millimolar level. Spectroscopic resolution has been improving steadily over time, with voxel sizes decreasing from +AH412 +AKA-cm3 to +AH41–2+AKA-cm3 over the last decade. MRS continues to make substantial contributions to the medical field, with over 10,000 PUBMED references found using the search terms “NMR spectroscopy and brain.” Interestingly, a similar search using “NMR spectroscopy and cognition” yielded only 269 references (Searches conducted July 7, 2008), suggesting that this neuroimaging technique is vastly underutilized within the cognitive neurosciences.
N-acetylaspartate (NAA) is the most prominent metabolite visible within the 1H-MRS spectrum. It has served as a marker of neuronal density and/or viability in numerous disease states (Barker, 2001), and has been postulated to serve numerous roles in neuronal and glial formation, functionality and repair (Baslow, 2003a,b; Rael et al., 2004). Produced within neuronal mitochondria, NAA is turned over within neurons at the rate of roughly 1.4+AKA-times/day through a complex exchange between neurons and oligodendrocytes (Baslow, 2003a,b), and its rate of synthesis has been demonstrated to be tightly coupled with that of glucose metabolism (Moreno et al., 2001). It is a metabolite with high sensitivity to any brain disease or disorder, as hundreds of studies have linked lower NAA to a broad spectrum of neurological and psychiatric diagnoses.
The specificity of lower NAA to particular regional pathology has yet to be firmly established (Yeo et al., 2006). Increasing NAA (following initial decrement) has been linked to resolution of neuronal dysfunction in particular diseases including traumatic brain injury (Brooks et al., 2000) and multiple sclerosis (Narayanan et al., 2001), making this metabolite a powerful marker of dynamic cellular metabolic processes. There are two emerging theories as to how NAA may modulate critical “rate limiting” biochemical processes. The first posits a role for either creatine or NAA (or both) in providing spare bioenergetic capacity in neural tissue (Jung et al., 1999; Rae et al., 2003, 2007). The second emphasizes the role of NAA in shuttling water across the hydrophobic myelin sheath during axonal firing, potentially allowing neurons to fire longer, more rapidly, and with more focused synchrony (Baslow 2003a,b).
Despite these links between brain dysfunction and cellular metabolism established by NAA research (Ross and Sachdev 2004), relatively few studies have linked NAA to normal human cognition (Table 1). As a result, these studies do not yet provide a clear picture regarding NAA–cognition relationships due to variability in acquisition technique, different sample characteristics, and the recent emergence of possible sex differences in such relationships. Three main points can be highlighted in Table 1 that both highlight the lack of consensus in the field and inform the hypotheses for the current study. First, four of the ten studies report ratio data (usually NAA/creatine) making it difficult to determinewhether NAA or another metabolite in the equation is driving the metabolite–cognition relationship (Rae et al., 1998a,b, 1999; Valenzuela et al., 2000; Ferguson et al., 2002; Gimenez et al., 2004). Similarly, the age effects of NAA–cognition relationships are unknown, and range of the subject samples vary substantially, from an average of 12.5+AKA-years (Yeo et al., 2000), into the 90's (Charlton et al., 2007). Finally, the location of voxel placement varies widely, ranging from the frontal lobe (Pflieiderer et al., 2004) through the cerebellum (Rae et al.,1998a,b), and spanning different tissue types (gray/white). Whether NAA–cognition relationships hold true across different lobes of the brain and tissue types remains an open question. There is now some indication that sex differences might exist in the strength of NAA–cognition relationships (Pfleiderer et al., 2004; Jung et al., 2005), although support for this hypothesis will require that the methodological concerns raised above be addressed adequately.
Table 1.
| Study | Ratio/abs. | N | Age | Location | Range |
|---|---|---|---|---|---|
| Rae et al. (1998a,b)a | Cre/NAA | 20 | 22±9 | Cerebellum | .62 to .86 |
| Jung et al. (1999a) | NAA | 25 | 22±4.6 | L O-P WM | .41 to .52 |
| Jung et al. (1999b) | NAA | 45 | 23±4.9 | L O-P WM | .34 to .65 |
| Valenzuela et al. (2000) | NAA/Cre | 20 | 59–85 | Frontal WM | .54 to .59 |
| Yeo et al. (2000) | NAA | 20 | 12.5±1.5 | R frontal WM | 0.45 |
| Ferguson et al. (2002) | NAA/Cre | 85/50 | 65–70 | L parietal WM | .24 to .43 |
| Gimenez et al. (2004) | NAA/Cho | 21 | 14±2.5 | L midtemporal | .51 to .59 |
| Pfleiderer et al. (2004) | NAA | 62 | 20–75 | L/R DLPFC L ACC | .53 to .56 |
| Jung et al. (2005) | NAA | 27 | 24.8 ±5.9 | L/R FWM L O-P WM | .68 to .88 |
| Charlton et al. (2007) | NAA | 106 | 50–90 | CS WM | 0.33 to 0.53 |
Cre = Creatine; Cho = Choline; L = Left; R = Right; L/R = Left and Right; O-P = Occipito-Parietal; WM = White Matter; DLPFC = Dorsolateral Prefrontal Cortex; ACC = Anterior Cingulate Cortex; CS = Centrum Semiovale.
Rae et al. subsequently reanalyzed their data, producing semiquantitative support that their results were due to NAA as opposed to the effects of creatine. They reported these results in a correspondence (Rae et al.,1999, Neurology, 52, 898–99).
In spite of the limitations noted above, there are emerging trends, across studies in Table 1, supporting our hypotheses. First, there is a consistent, positive correlation between NAA and measures of intelligence, as obtained from Wechsler measures (e.g., Vocabulary, Similarities, Block Design). Second, in two studies, there is indication that the NAA–cognition relationship is stronger in women than for men (Pfleiderer et al., 2004; Jung et al., 2005). The current study utilizes MR Spectroscopic Imaging (SI) to further evaluate links between NAA concentration and aspects of higher cognitive function. SI allows many more voxels to be assayed, and hence provides greater detail on specific anatomic regions and patterns of metabolic variation across regions. We hypothesize that 1) higher levels of NAA will relate to broad measures of cognitive functioning as measured by intelligence tests, and 2) based on our previous findings (Jung et al., 2005), we also test the post-hoc hypothesis that higher NAA–cognition relationships will be found for women as compared to men.
2. Methods
2.1. Sample
The sample consisted of 69 experimental subjects, ranging in age from 18 to 39, from the University of New Mexico. Subjects were recruited by informational postings in various departments and classrooms around the University of New Mexico. All subjects signed a consent form approved by the institutional review board of the University of New Mexico prior to participation in the experimental protocol. Prior to entry into the study, subjects were screened by a clinical neuropsychologist (REJ) and determined to be free of neurological and psychological disorders that would impact experimental hypotheses (e.g., learning disorders, traumatic brain injury, major depressive disorder). Handedness was determined by the Edinburgh inventory (Oldfield 1971). Subjects were also screened for conditions that would prohibit undergoing an MRI scan (e.g., metal implant, orthodontic braces, severe claustrophobia). Seven subjects were removed from the sample: 1) Four experimental subjects had significant radiological abnormalities, read by a clinical neuroradiologist (cystic periventricular lesion suspicious for neurocysticercosis; low cortical volume given subject age; numerous supra and infratentorial lesions suspicious for multiple sclerosis; asymmetry of temporal horns R> L suggestive of temporal lobe epilepsy); 2) Two subjects were not administered intelligence measures, leaving a final cohort of 63 experimental subjects.
2.2. Behavioral measures
Subjects were also administered the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler 1999) and Ravens Advanced Progressive Matrices Test (RAPMT) (Raven 1994). The WASI has high reliability (.98 for Full Scale IQ in adult samples) and validity characteristics (r=.92 with WAIS-III Full Scale IQ). The WASI consists of four subtests: Vocabulary, Similarities, Block Design, and Matrix Reasoning. Based on these four subtests, Verbal IQ, Performance IQ and Full Scale IQ scores are derived. The RAPMT was administered with a 40-minute time limit. Total testing time was 2+AKAh/subject, for which they were paid $25 for their time.
2.3. Spectroscopic acquisition
MR examinations were performed on a 1.5+AKA-T Siemens Sonata scanner using an 8-channel phased array head coil. Following Gasparovic et al. (2006) method, a sagittal scout image aligned perpendicular to the inter-hemispheric midline was acquired to prescribe T1 and T2-weighted images aligned parallel to a line drawn through the posterior and anterior commissures. T1-weighted images were obtained with a 3D transversal gradient echo sequence (TR/TE=12/4.76+AKA-ms, field of view (FOV)=256×256+AKA-mm, flip angle 200, resolution 192×192, slice thickness=1.5+AKA-mm, total scan time=6+AKA-min and 47+AKA-s).
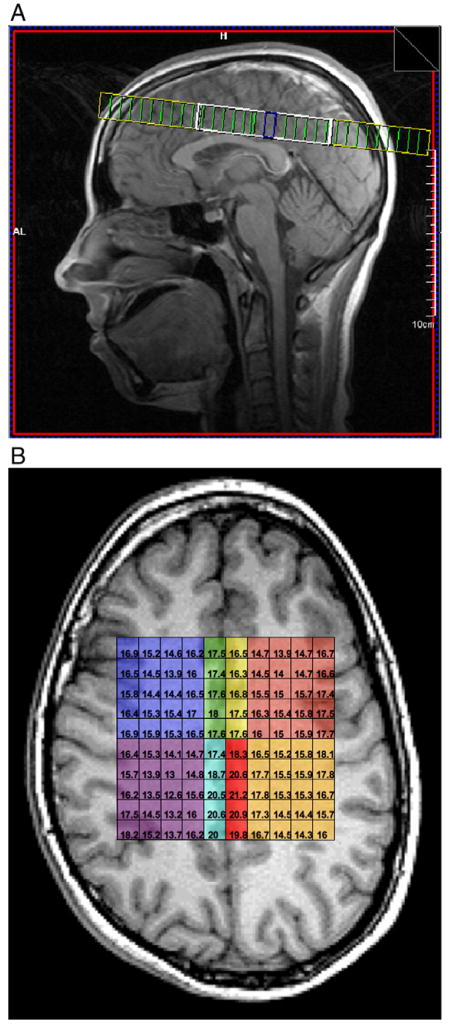
MR spectroscopy was obtained with 2D 1H-MRSI prescribed supra-ventricularly from the slices of the T1-weighted axial images (Fig. 1A). 1H-MRSI was acquired using a point-resolved spectroscopy (PRESS) sequence with phase (spatial) encoding in 2 directions and the following parameters were used: TR/TE = 1500/135+AKA-ms; field of view = 200×200+AKA-mm; slice thickness; matrix size 24×24, acquired with circular phase encoding, zero-filled to 32×32; nominal voxel size=8.3×8.3×15.0+AKA-mm; total scan time=9+AKA-min and 32+AKA-s. The 1H-MRSI volume of interest (VOI), established by the PRESS volume-selection gradients, was prescribed with a FSE image to lie immediately above the lateral ventricles and parallel to the AC-PC line. The size of the VOI was generally 75±5+AKA-mm left to right and 90±5 anterior to posterior, with nominal individual voxel size of 0.6+AKA-cm3. Both water suppressed (metabolites) and unsuppressed (water) images were acquired.
Fig. 1.
A. Sagittal image showing placement of MRSI grid (heavy white line) through supraventricular white and gray matter regions dorsal to the corpus callosum. B. Axial view of MRSI grid showing individual 0.6+AKA-cm3 voxels comprising the 8 regions of interest. Image is in radiological convention (left side of image = right hemisphere). Dark blue = right frontal white matter; purple = right posterior white matter; light red = left frontal white matter; tan = left posterior white matter; green = right anterior gray matter; yellow = left anterior gray matter; light blue = right posterior gray matter; red = left posterior gray matter.
2.4. Analysis
Absolute quantification of NAA was obtained using the unsupressed water signal as a concentration reference, and by correcting for percent tissue content within each of the spectroscopic voxels as described previously (Gasparovic et al., 2006). Data were transferred to an independent Linux workstation. After zero-filling the data to 32×32 points in k-space, applying a Hamming filer with a 50% window width, and 2D spatial Fourier transformation (FT), LC model was used for post-processing of spectra. The outermost columns and rows in the VOI were excluded from analysis.
Eight regions of interest were identified by two independent raters (RC, RB) within bilateral frontal and posterior white matter (+AH412+AKA-cm3), and bilateral anterior and posterior gray matter regions (+AH43+AKA-cm3). The gray matter regions were within the dorsal cingulate gyrus, above the lateral ventricles, with the anterior regions roughly corresponding to Brodmann area 24 and the posterior regions to Brodmann area 31. Fig. 1B shows a representative image of the eight regions of interest in a single subject.
2.5. Statistical analysis
Stepwise linear regression was used with all spectroscopic measures of NAA obtained from the eight regions of interest (left frontal white matter, left frontal gray matter, right frontal gray matter, right frontal white matter, left posterior white matter, left posterior gray matter, right posterior gray matter, right posterior white matter) serving as independent measures and the four major intelligence measures serving as the dependent measure (VIQ, PIQ, FSIQ, RAPMT) in four separate analyses. Family-wise Bonferroni correction was made for multiple comparisons with significance level set at .05/4=.0125 for the regression analyses. Post-hoc analyses were conducted comparing bivariate Pearson correlations between major indices comprising the WASI and the RAPMT separately for men and women.
3. Results
Subjects were young (23.76±4.22), generally right handed (59 right, 4 left), and well matched by sex (34 males, 29 females), and Full Scale Intelligence Quotient (Males=117.7±10.45; Females=117.5±8.91). All behavioral and spectroscopic measures, by sex, are presented in Table 2. Males had significantly higher NAA than females in two brain regions: the right anterior gray matter (Males=17.81, Females=16.66; t=2.59, p=.012), and right posterior gray matter (Males=18.99, Females=17.36; t=3.61, p=.001). Paired samples t-tests evaluated hemispheric asymmetries in NAA concentrations.
Table 2.
| Males |
Females |
t | p | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age | 23.7 | 4.1 | 23.9 | 4.4 | 0.173 | ns |
| WASI Verbal IQ | 114.3 | 12.1 | 116 | 10.6 | 0.6 | ns |
| WASI Performance IQ | 117.6 | 9.3 | 114.9 | 8.6 | 1.17 | ns |
| WASI Full Scale IQ | 117.7 | 10.5 | 117.6 | 8.9 | 0.05 | ns |
| Ravens total correct | 25.1 | 4.9 | 23.7 | 4.7 | 1.12 | ns |
| Right anterior WM NAA | 14.71 | 0.9 | 14.53 | 0.91 | 0.785 | ns |
| Right posterior WM NAA | 14.46 | 0.95 | 14.32 | 0.86 | 0.612 | ns |
| Left anterior WM NAA | 14.18 | 0.82 | 14.35 | 0.89 | 0.798 | ns |
| Left posterior WM NAA | 14.29 | 0.88 | 14.37 | 0.84 | 0.378 | ns |
| Right anterior GM NAA | 17.81 | 1.87 | 16.61 | 1.55 | 2.675 | 0.01 |
| Right posterior GM NAA | 18.99 | 2.1 | 17.32 | 1.35 | 3.625 | 0.001 |
| Left anterior GM NAA | 16.38 | 2.25 | 16.48 | 1.75 | 0.198 | ns |
| Left posterior GM NAA | 17.6 | 1.67 | 17.18 | 1.1 | 1.114 | ns |
WM = White Matter; GM = Gray Matter; NAA = N-acetylaspartate.
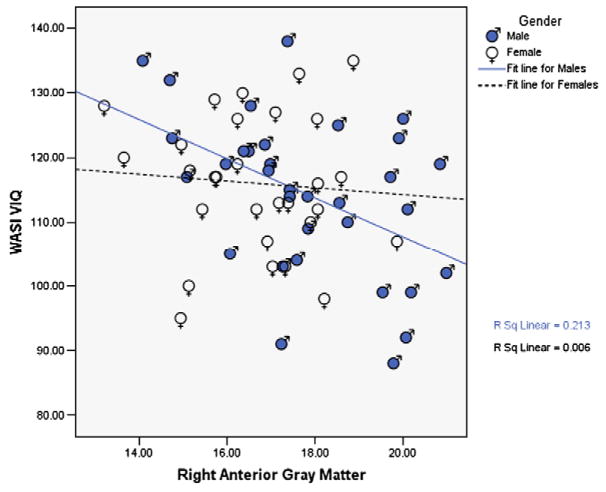
We first tested our main experimental hypothesis that NAA would predict intellectual performance. In the combined sample of men and women, we regressed NAA from the eight spectroscopic voxels of interest against the major measures of intellectual functioning (VIQ, PIQ, FSIQ, RAPMT). For VIQ, we found that a model including lower NAA within right anterior gray matter (Fig. 1B, green voxels) predicting better performance (F=6.83, p=.011, r2=.10). We also tested our post-hoc hypotheses, and found the correlation between VIQ and NAA obtained within the right anterior gray matter was stronger in males (r2 =.21) as compared to females (r2= .004). The differences between the strengths of these correlations did not reach statistical significance (p=.11). The scatterplot showing the inverse relationship between VIQ and NAA obtained from right anterior gray matter, stratified by sex, is shown in Fig. 2.
Fig. 2.
Right anterior gray matter.
Again testing our main hypothesis for PIQ, we found that a model that included higher NAA within the right posterior gray matter region (Fig. 1B, light blue voxels) predicted better performance (F=8.175, p=.006, r2=.12). In post-hoc hypothesis testing of sex differences mediating NAA–IQ relationships, we found the correlation between PIQ and NAA obtained within the right posterior gray matter to be similar in males (r2=.13) as compared to females (r2=.06).
No regression models inclusive of NAA from any brain regions predicted performance on either FSIQ or RAPMT for the combined sample of men and women.
4. Discussion
To our knowledge, this is the first study to report NAA–IQ correlates using multiple spectroscopic imaging voxels, sampling eight gray and white matter regions of the brain, in normal healthy subjects. Strengths of the current study include “absolute” (as opposed to ratio) measures of NAA, correction for tissue concentration and type, and simultaneous sampling throughout multiple regions of the brain. These findings add to the small but growing body of literature linking brain biochemistry to intelligence in normal healthy subjects using 1H and 31P MRS (Rae et al., 1996, 2003; Jung et al., 1999, 2000, 2005; Pfleiderer et al., 2004). We found a weak, though positive relationship between NAA and PIQ, partially replicating our earlier findings showing positive relationships between NAA and measures of intelligence (Jung et al., 1999). We also found that NAA–IQ relationships were trending (p=.11) stronger in males than females, in contrast to previous studies (Pfleiderer et al., 2004; Jung et al., 2005), although this result must be viewed with extreme caution, given the risk of Type 1 error.
The main limitation of the current study was insufficient number of subjects to achieve the desired statistical power to test our hypotheses, given the modest effect sizes. For example, given the average correlation of +AH4.50 across studies in Table 1, which yields an effect size of .33, a sample size of at least 54 subjects would be needed for a desired power of .80 with 8 predictor variables. Thus we had sufficient subjects to assess our main hypothesis (that NAA was related to IQ), but detailed inquiries (and discussion) of sex differences in NAA–IQ relationships would require far more subjects. Our current sample was above average in intelligence, a common occurrence in studies recruiting in an academic setting. This restricts range, limiting statistical power, and leaves open the question of whether results generalize across the full spectrum of individual differences. To improve resolution of NAA and cognition correlations we recommend using: 1) magnetic fields of at least 3+AKAT; 2) smaller and functionally discrete regions of interest; 3) tissue type correction to distinguish histology from biochemistry (Gasparovic et al., 2006). The lack of these in this study may have decreased the strength of the NAA/IQ correlations. We are implementing improved coregistration techniques that will allow us to correct for tissue type across individual voxels in the future. There is growing evidence that NAA levels associated with cognition in normal brain reside upon a continuum with neurometabolic changes associated with cognitive decline in disease (Rae et al., 1998a,b). The current results add to the body of literature spanning normal and clinical populations linking various brain metabolites and cognitive performance (Ross and Sachdev 2004); however, not all MRS studies have found such relationships in normal human subjects (Houkin et al., 1993; Minshew et al., 1993; Buckley et al., 1994; Deicken et al., 1995; Jarvik et al., 1996; Friedman et al., 1998; Shim et al., 2001). This is likely due to the generally weak correlation observed across studies represented in Table 1, save for samples including patients (Rae et al., 1998a,b) or reporting results exclusively for young females (Jung et al., 2005).
There is much to learn regarding how tissue volume, neurometabolism, and other neuroimaging measures (e.g., DTI) might be moderated by sex, and we ignore such differences at risk of deeper structure–function understanding. Undertaking such research, however, will require acquisition of larger sample sizes for adequate statistical power. Indeed, even at roughly thirty subjects/group, the current sample lacked the statistical power to explore sex differences a priori, much less more subtle interactions (e.g.,: hemisphere×tissue type×anterior/posterior×sex). Systematic investigation of sex differences as related to such structure–function relationships can potentially add significantly to our understanding of the way in which neural tissue gives rise to higher cognitive function under different “design” constraints (Haier et al., 2005).
There are now contradictory studies showing both positive (Pfleiderer et al., 2004) and negative (Jung et al., 2005) relationships between NAA and intelligence measures in frontal lobe regions, although these are confounded with tissue type and sex as noted above. We are not aware of any other group showing inverse relationships between NAA and cognition such that lower levels of NAA are associated with better performance on behavioral measures. Indeed, the major hypothesis revolves around the notion that NAA is a marker of neuronal mass and/or viability such that “more is better”. While this hypothesis is overwhelmingly supported, the brain is comprised of interdependent excitatory and inhibitory networks, which would tend to preclude a universal “more is better” approach to biochemical design. However, if NAA is a marker of neuronal mass, then we must begin to address the possibility that fewer neurons and/or dendritic arbor within a given voxel of interest might be associated with better performance. The field of fMRI stands as a good model in this regard in its recent recognition of both activations and deactivations within a network associated with cognition (Raichle and Snyder 2007). The “neural efficiency hypothesis”, by which the brain works to minimize the resources allocated towards higher cognitive functioning (Haier et al., 1988, 1992; Grabner et al., 2003, 2004, 2006; Neubauer et al., 2004, 2005), particularly within frontal lobe tissue, would also be an attractive alternate hypotheses to the monolithic “more is better” notion that has dominated neuroscientific research heretofore.
Finally, we make note of the recent “free energy principle” of brain functioning by which the brain works to minimize the entropy involved in performing a cognitive task (Friston et al., 2006). Previous research has shown decreased tissue volume associated with increasing intelligence (Shaw et al., 2006), the resting state of brain functioning relating to intelligence (Song et al., 2008), and in the current research both positive and negative relationships between NAA and intelligence. Taken together, these findings can be interpreted to support the notion that the brain operates to minimize the free energy (or amount of work to be expended) from the “system” (in this case a biological system) during performance of a cognitive task. The human brain is an enormously expensive organ to operate, utilizing some 20% of all oxygen taken in and 25% of all glucose produced while representing only about 2% of the body's weight (Kandell et al., 2000). The hierarchical organization, the ability to make inferences with limited information, the ability to suppress repetitions of information, all play important roles in limiting the energy devoted to brain functioning and thus to ongoing biological adaptability. Future research will be necessary to better understand how the increasingly counterintuitive findings of “less is sometimes better” regarding neuroimaging correlates of intelligence fits into the “neural efficiency” and “free energy principles” of brain functioning.
Acknowledgments
This research was supported by a grant from the John Templeton Foundation entitled “The Neuroscience of Creativity.” We thank the anonymous reviewers for their very helpful suggestions, which served to improve the current manuscript substantially.
References
- Barker PB. N-acetyl aspartate—A neuronal marker? Ann Neurol. 2001;49(4):423–424. [PubMed] [Google Scholar]
- Baslow MH. Brain N-acetylaspartate as a molecular water pump and its role in the etiology of Canavan disease: A mechanistic explanation. J Mol Neurosci. 2003;21(3):185–190. doi: 10.1385/jmn:21:3:185. [DOI] [PubMed] [Google Scholar]
- Baslow MH. N-acetylaspartate in the vertebrate brain: Metabolism and function. Neurochem Res. 2003;28(6):941–953. doi: 10.1023/a:1023250721185. [DOI] [PubMed] [Google Scholar]
- Brooks WM, Stidley CA, Petropoulos H, Jung RE, Weers DC, Friedman SD, et al. Metabolic and cognitive response to human traumatic brain injury: A quantitative proton magnetic resonance study. J Neurotrauma. 2000;17(8):629–640. doi: 10.1089/089771500415382. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Moore C, Long H, Larkin C, Thompson P, Mulvany F, et al. 1H-magnetic resonance spectroscopy of the left temporal and frontal lobes in schizophrenia: Clinical, neurodevelopmental, and cognitive correlates. Biol Psychiatry. 1994;36(12):792–800. doi: 10.1016/0006-3223(94)90591-6. [DOI] [PubMed] [Google Scholar]
- Charlton RA, McIntyre DJ, Howe FA, Morris RG, Markus HS. The relationship between white matter brain metabolites and cognition in normal aging: The GENIE study. Brain Res. 2007;1164:108–116. doi: 10.1016/j.brainres.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Merrin EL, Floyd TC, Weiner MW. Correlation between left frontal phospholipids and Wisconsin Card Sort Test performance in schizophrenia. Schizophr Res. 1995;14(2):177–181. doi: 10.1016/0920-9964(94)00036-8. [DOI] [PubMed] [Google Scholar]
- Ferguson KJ, MacLullich AM, Marshall I, Deary J, Starr JM, Seckl JR, et al. Magnetic resonance spectroscopy and cognitive function in healthy elderly men. Brain. 2002;125(Pt 12):2743–2749. doi: 10.1093/brain/awf278. [DOI] [PubMed] [Google Scholar]
- Friedman SD, Brooks WM, Jung RE, Hart BL, Yeo RA. Proton MR spectroscopic findings correspond to neuropsychological function in traumatic brain injury. AJNR Am J Neuroradiol. 1998;19(10):1879–1885. [PMC free article] [PubMed] [Google Scholar]
- Friston K, Kilner J, Harrison L. A free energy principle for the brain. J Physiol Paris. 2006;100(1–3):70–87. doi: 10.1016/j.jphysparis.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, et al. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magnetic Resonance in Med. 2006;55(6):1219–1226. doi: 10.1002/mrm.20901. [DOI] [PubMed] [Google Scholar]
- Gimenez M, Junque C, Narberhaus A, Caldu X, Segarra D, Vendrell P, et al. Medial temporal MR spectroscopy is related to memory performance in normal adolescent subjects. Neuroreport. 2004;15(4):703–707. doi: 10.1097/00001756-200403220-00026. [DOI] [PubMed] [Google Scholar]
- Grabner RH, Fink A, Stipacek A, Neuper C, Neubauer AC. Intelligence and working memory systems: Evidence of neural efficiency in alpha band ERD. Brain Research Cognitive Brain Research. 2004;20(2):212–225. doi: 10.1016/j.cogbrainres.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Grabner RH, Neubauer AC, Stern E. Superior performance and neural efficiency: The impact of intelligence and expertise. Brain Research Bulletin. 2006;69(4):422–439. doi: 10.1016/j.brainresbull.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Grabner RH, Stern E, Neubauer AC. When intelligence loses its impact: Neural efficiency during reasoning in a familiar area. International Journal of Psychophysiology. 2003;49(2):89–98. doi: 10.1016/s0167-8760(03)00095-3. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Jung RE, Yeo A, Head K, Alkire MT. The neuroanatomy of general intelligence: Sex matters. Neuroimage. 2005;25(1):320–327. doi: 10.1016/j.neuroimage.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Siegel BV, Jr, MacLachlan A, Soderling E, Lottenberg S, Buchsbaum MS. Regional glucose metabolic changes after learning a complex visuospatial/motor task: A positron emission tomographic study. Brain Research. 1992;570(1–2):134–143. doi: 10.1016/0006-8993(92)90573-r. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Siegel BV, Nuechterlein KH, Hazlett E, Wu JC, Paek J, et al. Cortical glucose metabolic rate correlates of abstract reasoning and attention studied with positron emission tomography. Intelligence. 1988;12(2):199–217. [Google Scholar]
- Houkin K, Kamada K, Kamiyama H, Iwasaki Y, Abe H, Kashiwaba T. Longitudinal changes in proton magnetic resonance spectroscopy in cerebral infarction. Stroke. 1993;24(9):1316–1321. doi: 10.1161/01.str.24.9.1316. [DOI] [PubMed] [Google Scholar]
- Jarvik JG, Lenkinski RE, Saykin AJ, Jaans A, Frank I. Proton spectroscopy in asymptomatic HIV-infected adults: Initial results in a prospective cohort study. Journal of Acquired Immune Deficiency Syndroms and Human Retrovirology. 1996;13(3):247–253. doi: 10.1097/00042560-199611010-00006. [DOI] [PubMed] [Google Scholar]
- Jung RE, Brooks WM, Yeo RA, Chiulli SJ, Weers DC, Sibbitt WL., Jr Biochemical markers of intelligence: A proton MR spectroscopy study of normal human brain. Proceedings of the Royal Society of London B Biological Sciences. 1999;266(1426):1375–1379. doi: 10.1098/rspb.1999.0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: Converging neuroimaging evidence. Behavioral and Brain Sciences. 2007:135–154. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Jung RE, Haier RJ, Yeo RA, Rowland LM, Petropoulos H, Levine AS, et al. Sex differences in N-acetylaspartate correlates of general intelligence: An 1H-MRS study of normal human brain. Neuroimage. 2005;26(3):965–972. doi: 10.1016/j.neuroimage.2005.02.039. [DOI] [PubMed] [Google Scholar]
- Jung RE, Yeo RA, Chiulli SJ, Sibbitt WL, Jr, Brooks WM. Myths of neuropsychology: Intelligence, neurometabolism, and cognitive ability. The Clinical Neuropsychologist. 2000;14(4):535–545. doi: 10.1076/clin.14.4.535.7198. [DOI] [PubMed] [Google Scholar]
- Kandell ER, Schwartz JH, Jessell TM. Principles of Neural Science. New York: McGraw-Hill; 2000. [Google Scholar]
- Minshew NJ, Goldstein G, Dombrowski SM, Panchalingam K, Pettegrew JW. A preliminary 31P MRS study of autism: Evidence for undersynthesis and increased degradation of brain membranes. Biological Psychiatry. 1993;33(11–12):762–773. doi: 10.1016/0006-3223(93)90017-8. [DOI] [PubMed] [Google Scholar]
- Moreno A, Ross BD, Bluml S. Direct determination of the N-acetyl-L-aspartate synthesis rate in the human brain by (13)C MRS and [1-(13)C]glucose infusion. Journal of Neurochemistry. 2001;77(1):347–350. doi: 10.1046/j.1471-4159.2001.t01-1-00282.x. [DOI] [PubMed] [Google Scholar]
- Narayanan S, De Stefano N, Francis GS, Arnaoutelis R, Caramanos Z, Collins DL, et al. Axonal metabolic recovery in multiple sclerosis patients treated with interferon beta-1b. Journal of Neurology. 2001;248(11):979–986. doi: 10.1007/s004150170052. [DOI] [PubMed] [Google Scholar]
- Neubauer AC, Grabner RH, Freudenthaler HH, Beckmann JF, Guthke J. Intelligence and individual differences in becoming neurally efficient. Acta Psychologica (Amsterdam) 2004;116(1):55–74. doi: 10.1016/j.actpsy.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Neubauer AC, Grabner RH, Fink A, Neuper C. Intelligence and neural efficiency: Further evidence of the influence of task content and sex on the brain–IQ relationship. Brain Research Cognitive Brain Research. 2005;25(1):217–225. doi: 10.1016/j.cogbrainres.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pfleiderer B, Ohrmann P, Suslow T, Wolgast M, Gerlach AL, Heindel W, et al. N-acetylaspartate levels of left frontal cortex are associated with verbal intelligence in women but not in men: A proton magnetic resonance spectroscopy study. Neuroscience. 2004;123(4):1053–1058. doi: 10.1016/j.neuroscience.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Rae C. Piece of mind; a full systems approach is required. Behavioral and Brain Sciences. 2007;30(2):167–168. [Google Scholar]
- Rae C, Digney AL, McEwan SR, Bates TC. Oral creatine monohydrate supplementation improves brain performance: A double-blind, placebo-controlled, cross-over trial. Proceedings of the Royal Society London B. 2003;270:2147–2150. doi: 10.1098/rspb.2003.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae C, Karmiloff-Smith A, Lee MA, Dixon RM, Grant J, Blamire AM, et al. Brain biochemistry in Williams syndrome: Evidence for a role of the cerebellum in cognition? Neurology. 1998;51(1):33–40. doi: 10.1212/wnl.51.1.33. [DOI] [PubMed] [Google Scholar]
- Rae C, Lee MA, et al. Metabolic abnormalities in developmental dyslexia detected by 1H magnetic resonance spectroscopy. Lancet. 1998;351:1849–1852. doi: 10.1016/S0140-6736(97)99001-2. [DOI] [PubMed] [Google Scholar]
- Rae C, Scott RB, Thompson CH, Kemp GJ, Dumughn I, Styles P, et al. Is pH a biochemical marker of IQ. Proceedings of the Royal Society of London B Biological Sciences. 1996;263:1061–1064. doi: 10.1098/rspb.1996.0156. [DOI] [PubMed] [Google Scholar]
- Rae C, Scott RB, Lee M, Simpson JM, Hines N, Paul C, et al. Brain bioenergetics and cognitive ability. Developmental Neuroscience. 2003;25(5):324–331. doi: 10.1159/000073509. [DOI] [PubMed] [Google Scholar]
- Rae C, Thompson C, Karmiloff-Smith A, Grant J, Lee M, Dixon RM, et al. Reply from the authors. Neurology. 1999;52(4):898–899. [Google Scholar]
- Rael LT, Thomas GW, Bar-Or R, Craun ML, Bar-Or D. An anti-inflammatory role for N-acetyl aspartate in stimulated human astroglial cells. Biochemical and Biophysical Research Communications. 2004;319(3):847–853. doi: 10.1016/j.bbrc.2004.04.200. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37(4):1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Raven JC. Manual Sections 1 & 4 (Sets I, II) Oxford: Oxford Psychologists Press; 1994. Advanced Progressive Matrices. [Google Scholar]
- Ross AJ, Sachdev PS. Magnetic resonance spectroscopy in cognitive research. Brain Research Brain Research Reviews. 2004;44(2–3):83 –102. doi: 10.1016/j.brainresrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shim TS, Lee JH, Kim SY, Lim TH, Kim SJ, Kim DS, et al. Cerebral metabolic abnormalities in COPD patients detected by localized proton magnetic resonance spectroscopy. Chest. 2001;120(5):1506–1513. doi: 10.1378/chest.120.5.1506. [DOI] [PubMed] [Google Scholar]
- Song M, Zhou Y, Li J, Liu Y, Tian L, Yu C, et al. Brain spontaneous functional connectivity and intelligence. Neuroimage. 2008;41(3):1168–1176. doi: 10.1016/j.neuroimage.2008.02.036. [DOI] [PubMed] [Google Scholar]
- Valenzuela MJ, Sachdev PS, Wen W, Shnier R, Brodaty H, Gillies D. Dual voxel proton magnetic resonance spectroscopy in the healthy elderly: Subcortical–frontal axonal N-acetylaspartate levels are correlated with fluid cognitive abilities independent of structural brain changes. Neuroimage. 2000;12(6):747–756. doi: 10.1006/nimg.2000.0629. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Yeo RA, Brooks WM, Jung RE. NAA and higher cognitive function in humans. Advances in Experimental Medicine and Biology. 2006;576:215–226. doi: 10.1007/0-387-30172-0_15. (discussion 361–3) [DOI] [PubMed] [Google Scholar]
- Yeo RA, Hill D, Campbell R, Vigil J, Brooks WM. Developmental instability and working memory ability in children: A magnetic resonance spectroscopy investigation. Developmental Neuropsychology. 2000;17(2):143–159. doi: 10.1207/S15326942DN1702_01. [DOI] [PubMed] [Google Scholar]