Abstract
Cellular signaling pathways do not simply transmit data; they integrate and process signals to operate as switches, oscillators, logic gates, memory modules and many other types of control system. These complex processing capabilities enable cells to respond appropriately to the myriad of external cues that direct growth and development. The idea that crosstalk and feedback loops are used as control systems in biological signaling networks is well established. Signaling networks are also subject to exquisite spatial regulation, yet how spatial control modulates signal outputs is less well understood. Here, we explore the spatial organization of two different signal transduction circuits: receptor tyrosine kinase activation of the mitogen-activated protein kinase module; and glycosylphosphatidylinositol-anchored receptor activation of phospholipase C. With regards to these pathways, recent results have refocused attention on the crucial role of lipid rafts and plasma membrane nanodomains in signal transmission. We identify common design principals that highlight how the spatial organization of signal transduction circuits can be used as a fundamental control mechanism to modulate system outputs in vivo.
Introduction
The plasma membrane is a hydrophobic lipid bilayer that functions as a selectively permeable boundary separating cells from their external environment. For proper growth and development, external input signals must be accurately relayed across the plasma membrane into the interior of the cell to precisely regulate cell fate decisions. Cells must overcome a series of engineering challenges to accurately relay external information into the cell. Signal transmission needs to be of sufficiently high fidelity –fidelity corresponds to the similarity between input and output – so that cells can accurately replicate the external signal inside the cell to ensure that the biological response is appropriate to the external cue. The importance of this requirement is illustrated by morphogen gradients that can specify up to seven different thresholds of gene expression [1,2]. Another striking example is the determination of opposing cell fate decisions within a single cell using different amplitudes of mitogen-activated protein kinase (MAPK) activation [3,4]. Signals are particularly vulnerable to degradation when they are copied or amplified, such as occurs during transduction across the plasma membrane. To combat this problem, signal transduction circuits need to be robust. Robustness is defined as a ‘property that allows a system to maintain its function despite internal and external perturbations’, and it is fundamental to all biological systems [5]. How cells protect signal transmission from random perturbations to generate robust, high-fidelity signal transduction remains a crucial question in biology. In addition, given that the bulk output signal from the plasma membrane into the cytosol represents the integration of many thousands of individual molecular recruitment and activation events, the activation of any signaling complex needs to be precisely synchronized with the activation and inactivation of all other independent signaling units [6,7]. How cells perform this complex integration also remains unknown. Here, we explore how cells might have solved the engineering challenges of robustness, fidelity and signal integration by using the unique two-dimensional structures present within membrane environments.
Nanoclusters within the plasma membrane
It is now clear that, far from being disordered, the plasma membrane is a complex, carefully regulated dynamic structure that is compartmentalized over multiple lengths and time scales [8]. Lateral segregation within the plasma membrane is driven by a variety of lipid–lipid, lipid–protein, protein–protein and actin cytoskeleton interactions [8]. The net result of these interactions is the non-random distribution of proteins and lipids across different types of transient nanoscale domains (see Box 1). One important function ascribed to these structures is the concentration of proteins to facilitate protein–protein interactions and the assembly of signaling complexes.
Box 1. Plasma membrane nanodomains.
Ras nanoclusters
Ras proteins are arrayed in nanoclusters comprising 6–8 proteins in domains that are 12–22 nm in diameter [9,10]. Different Ras isoforms localize into spatially distinct, largely non-overlapping nanoclusters. Nanoclusters are not pre-existing stable membrane nanodomains, but transient dynamic structures that are assembled by the lipid-anchored Ras protein [8,11]. In one of the best-studied examples, the formation of H-Ras nanoclusters depends on a complex interplay between the membrane and the Ras protein. Crucial components that mediate H-Ras interaction with the plasma membrane include the H-Ras lipid anchor; charged residues within the hypervariable region (HVR) when H-ras is GDP loaded; or the helix-α4 within the G-domain when H-Ras is GTP-loaded [35-38]. Models have been proposed to explain how these molecular interactions with a membrane bilayer might drive the formation of a lipid nanodomain [39]. H-Ras–GTP nanoclusters are not perturbed by depleting cell surface cholesterol, but they are stabilized by scaffold proteins, such as galectin-1, a cytosolic prenyl-binding protein [9,40]. By contrast, H-Ras–GDP nanoclusters do not require galectin-1 but are sensitive to cholesterol depletion. Similarly, K-Ras exhibits GTP-dependent changes in lateral segregation; and K-Ras–GTP nanoclusters require a galectin-3 scaffold, whereas K-Ras–GDP nanoclusters do not. The current thinking is that Ras–GTP monomers recruit the cognate galectin scaffold from the cytosol before forming into nanoclusters [40]. The distributions of Ras proteins on the plasma membrane show an interesting general property: only a fraction (36–44%) of the Ras proteins is clustered, and the remaining proteins are randomly arrayed as monomers [10]. This ratio is constant over wide ranges of expression but, in the case of H-Ras–GTP and K-Ras–GTP, it can be modulated by varying the cellular scaffold concentration [40]. SFVT suggests that the monomeric, non-clustered Ras proteins are mobile, whereas the Ras nanoclusters are immobile. Furthermore, only Ras–GTP in nanoclusters is competent to recruit and activate the downstream signaling components Raf, MEK and ERK [12,13]. SFVT estimates that the life-time of K-Ras–GTP and H-Ras–GTP clusters is ~0.4 s, and that the life-time of H-Ras–GDP clusters is just <0.1s [8,12,41-43].
GPI nanoclusters
GPI-anchored proteins are arrayed in cholesterol-dependent nanoclusters on the plasma membrane [24,44]. As discussed in several recent papers and reviews, these nanoclusters should be considered as the modern-day equivalent of lipid rafts. The original raft hypothesis envisioned rafts as relatively stable, liquid-ordered (Lo) domains enriched with cholesterol [20]. As exemplified by GPI-anchored receptor (GPI-AR) nanoclusters, lipid rafts are viewed by many investigators as small, highly dynamic structures with an Lo structure that is stabilized by the engagement of specific lipid-anchored proteins through lipid–lipid and protein–protein interactions [8,42]. An alternative terminology is therefore cholesterol-dependent nanocluster, which acknowledges the role of the interactions among lipid-anchors, the lipid-bilayer and cholesterol, and also takes into account the potential role for protein–lipid and protein–protein interactions to help drive formation of the domain [8,24]. The functional similarity with Ras nanoclusters then becomes apparent. These small dynamic Lo domains might be stabilized and assembled into larger structures by cross-linking the constituent protein components [25,26,42]. The proposed transient nature of the small lipid rafts – their life-times can be <0.1 s – has prompted questions regarding the ability of such domains to concentrate and promote protein–protein interactions [31]. However, computational studies suggest that these concerns are not warranted [45,46] and, as discussed in this review, the rapid turnover of nanodomains, which operate as signaling platforms, carries important advantages in circuit design.
A specific example is provided by the non-random organization of Ras proteins on the inner leaflet of the plasma membrane. Ras proteins are small guanine nucleotide-binding proteins that operate in many signaling circuits that control cell growth and differentiation. Ras proteins operate in discrete nanodomains called nanoclusters, which can be visualized by electron microscopy (EM) and single fluorophore video tracking (SFVT) [9-11]. Ras nanoclusters contain on average seven Ras proteins, have radii of 6–12 nm, and short life-times (~0.4 s) [10,12]. Importantly, the three different Ras isoforms, H-Ras, N-Ras and K-Ras, occupy spatially distinct nanoclusters that have different structural requirements for actin, cholesterol and various scaffold proteins, such as galectin-1, galectin-3 and Sur-8 (Box 1). In addition, only a fixed fraction (~40%) of any given Ras isoform is localized to nanoclusters at any time point, with the remainder being randomly dispersed over the cell surface as monomers [9]. Consistent with the idea that nanoclusters have a role as signaling platforms, it is Ras nanoclusters, not randomly arrayed Ras monomers, that recruit downstream effectors [13]. One of the core, signaling pathways activated by Ras is the MAPK cascade that comprises Raf, MEK and ERK (Box 2) [14]. SFVT of Ras and Raf interactions in live cells agrees with EM and fluorescence resonance energy transfer (FRET) imaging studies showing that Raf is recruited to immobile Ras nanoclusters [15]. Recent extensions of this work also show that, at least in fibroblasts, K-Ras nanoclusters are the primary site for the recruitment and activation of Raf [13,16]. Blocking the formation of Ras nanoclusters by ablating crucial scaffolds or by perturbing the actin cytoskeleton disrupts Ras-dependent MAPK signaling [9-11]. These observations confirm that Ras nanoclusters are essential for MAPK activation, but how and why these highly dynamic signaling platforms are used to generate a MAPK response has only recently been elucidated.
Box 2. Biochemistry and general role of membrane interactions in regulating the mammalian MAPK module.
Every eukaryote possesses mitogen-activated protein (MAP) kinase pathways that are used in multiple ways to control growth and development [47]. All MAPK pathways follow the same fundamental design: a MAP kinase kinase kinase (MKKK) phosphorylates and activates a MAP kinase kinase (MKK); this, in turn, phosphorylates and activates a MAP kinase (MAPK) [47]. The evolutionary conservation of this three-tiered modular design indicates that there are significant advantages inherent in this configuration. MAPK modules display a variety of signal outputs in vivo: high-fidelity analog circuits that accurately relay information [13]; switch-like circuits that convert graded inputs into digital all-or-nothing responses [48]; and more complex outputs that include oscillations [49] and long-range signaling by phospho-protein waves [50], in addition to functioning as logic gates in which multiple inputs are integrated to generate single outputs [51]. In mammalian cells, the three major Ras proteins, K-Ras, H-Ras and N-Ras, transmit signals from activated receptors on the plasma membrane through a canonical MAPK cascade comprising Raf (a MAPKKK), MEK (a MAPKK) and ERK (a MAPK) [14]. Ras is a membrane-bound molecular switch, cycling between active GTP-bound and inactive GDP-bound conformations [52]. Raf is recruited to the plasma membrane by binding to Ras–GTP, and this is the crucial first step in a complex Raf activation process that also involves interaction with phospholipids, de-phosphorylation of inhibitory residues, phosphorylation of activating residues, and changes in protein–protein interactions [53]. Inactive MEK forms a complex with ERK in the cytosol [54,55]. Raf activates MEK by phosphorylating two serine residues within the MEK activation loop. MEK, in turn, phosphorylates ERK at threonine and tyrosine residues in a ‘TEY’ motif within the ERK activation loop, generating active ERK (ERKpp). Activated ERK dissociates from MEK and phosphorylates >150 substrates throughout the cell [56]. Although neither MEK nor ERK contain membrane-targeting sequences, MEK binds to both Raf and ERK, providing a direct method for recruiting the entire MAPK cascade to membrane environments [57-59]. Scaffold proteins act as docking platforms that bind two or more components of the MAPK module, and it is now clear that a crucial role of scaffold proteins is the localization of MAPK modules to distinct sub-cellular membrane compartments (Table I). Importantly, genetic studies in yeast, nematodes, flies and mammals have confirmed that scaffolds are essential for MAPK signal transduction. In combination, these findings demonstrate that spatial regulation is an integral property of the MAPK pathway that is absolutely essential for proper signal transmission in vivo.
In contrast to Ras proteins, glycosylphosphatidylinositol (GPI)-anchored receptors (GPI-ARs) are attached to the outer leaflet of the plasma membrane [17]. Cross-linking of GPI-ARs on the outer leaflet of the plasma membrane drives the formation of molecular complexes containing Src-family kinases (SFKs) on the inner leaflet of the plasma membrane, resulting in SFK activation [18]. How the extracellular GPI-ARs are able to communicate with the intracellular SFKs through the lipid barrier has remained unclear. Observations that GPI-anchored proteins and many SFKs have an affinity for cholesterol-rich membrane fractions suggests that lipid-raft domains are involved in the communication between the inner and outer leaflets of the plasma membrane [19-21]. Consistent with this hypothesis, cross-linking GPI-ARs induces cholesterol-dependent clustering and co-localization of lipid-anchored fluorophores attached to the inner leaflet of the plasma membrane [22,23]. Additional sophisticated analysis combining hetero- and homo-FRET with computational modeling has revealed that a small fraction of GPI-ARs are present in dynamic, cholesterol-dependent, nanoscale clusters that are highly reminiscent of Ras nanoclusters (Figure 1a) [24]. The precise role of GPI-AR spatial organization during in trans membrane signaling has also recently been clarified [23,25,26]. We briefly discuss the Ras and GPI-AR systems individually before identifying some important common design principles.
Figure 1.
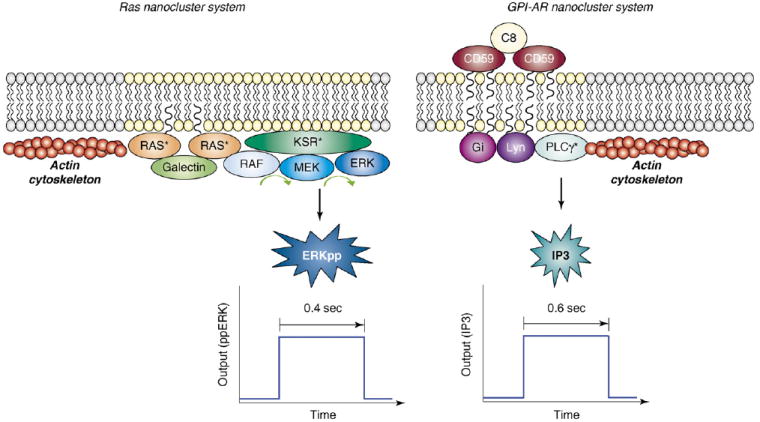
Ras and GPI-AR nanoclusters share many key biophysical properties. (a) K-Ras nanoclusters. Binding of EGF to the EGF receptor activates K-Ras, triggering the formation of Ras nanoclusters on the inner leaflet of the plasma membrane. These nanoclusters consist of active Ras (Ras*), the MAPK module (Raf, MEK and ERK), and scaffold proteins (galectin and KSR1). Ras nanoclusters have approximately seven Ras proteins, an average radius of 6–12 nm and short life-times (~0.4 s). SFVT and EM studies show that Raf is recruited to immobile Ras nanoclusters for activation. Formation of K-Ras nanoclusters requires an intact actin cytoskeleton but is lipid-raft-independent. During their brief life-times, each nanocluster generates a digital burst of active ERK (ERKpp). (b) GPI-AR nanoclusters. Cross-linking of GPI-ARs by ligands (e.g. the cross-linking of GPI-AR CD59 by C8) generates GPI-AR nanoclusters of approximately six GPI-AR molecules on the outer leaflet of the plasma membrane. CD59 GPI-AR nanoclusters recruit single molecules of Gαi2 and Lyn to the inner leaflet of the plasma membrane underneath the CD59 nanocluster. This is achieved through both protein–protein and lipid-raft interaction, as shown by the fact that GPI-AR clustering is cholesterol-dependent. Gαi2 activates Lyn, thereby inducing the binding of CD59 nanocluster to F-actin, and promoting brief (~0.6 s) immobilization of the GPI-AR nanocluster. PLCγ2 molecules are transiently recruited to immobilized CD59 clusters to generate a digital burst of inositol-(1,4,5) triphosphate (IP3). The similarity between the Ras and GPI-AR nanocluster systems is striking. Both Ras and GPI-AR systems contain few molecules (approximately seven molecules of Ras, and approximately six molecules of CD59), both systems have short lifespans (Ras nanoclusters ~0.4s, immobilized GPI-AR nanocluster ~0.6s) and both systems are active only when immobilized on the membrane. Most importantly, both systems generate digital bursts of output.
K-Ras nanoclusters use digital signaling to build a transmembrane analog circuit
Insights into how Ras nanoclusters are used to build biological circuits in vivo have come from a combination of computational modeling and cell-biological experiments [13,27]. These studies have revealed that MAPK activation is under tight spatial regulation. Activation of the MAPK module does not occur from the cytosol, but is confined to the plasma membrane [13,27]. A key player here is the Raf kinase inhibitor protein (RKIP), which blocks activation of the MAPK module by preventing any interaction between Raf and MEK [28,29]. RKIP is localized to the cytosol and therefore functions as a cytosol-specific inhibitor of the MAPK pathway [27]. On the plasma membrane, MAPK activation is further restricted to Ras nanoclusters, which are the sites to which Raf, MEK and ERK scaffolded on the kinase suppressor of Ras 1 (KSR1) protein are selectively recruited and activated (Figure 1) [13]. An important feature of individual K-Ras nanoclusters is that they respond maximally to very low ‘inputs’ of Raf kinase activity [13]. K-Ras nanoclusters therefore function as high-gain signal amplifiers, converting any strength of Raf kinase input into a maximal double-phosphorylated (ERKpp) output (Figure 2). Functionally, therefore, each nanocluster operates as a low-threshold digital switch. To capture the spatiotemporal scale and type of signal output from an individual K-Ras nanocluster, they have been termed ‘nanoswitches’. The dominant parameter within this Ras system is the very short life-time of a nanocluster; each nanoswitch therefore only remains ‘on’ for ~0.4s before disassembling [13]. The other crucial system behavior is the observed strict linear relationship between the number of Ras nanoswitches generated on the plasma membrane and the concentration of stimulating epidermal growth factor (EGF) (Figure 2) [13]. As a consequence of this linear relationship, the plasma membrane digitizes the analog continuous EGF input signal with an appropriate, matched number of nanoswitches that, in turn, generate discrete ERKpp output quanta [13]. The plasma membrane as a system therefore functions as a biological analog-to-digital converter (ADC) [27]. In the cytosol, the ERKpp digital pulses from all the individual nanoswitches are summed to recreate a final analog output that matches the original analog EGF signal. In essence, the cytosol now functions as a simple digital-to-analog converter (DAC) [27], and so the whole Ras system operates as an analog–digital–analog relay (Figure 2).
Figure 2.
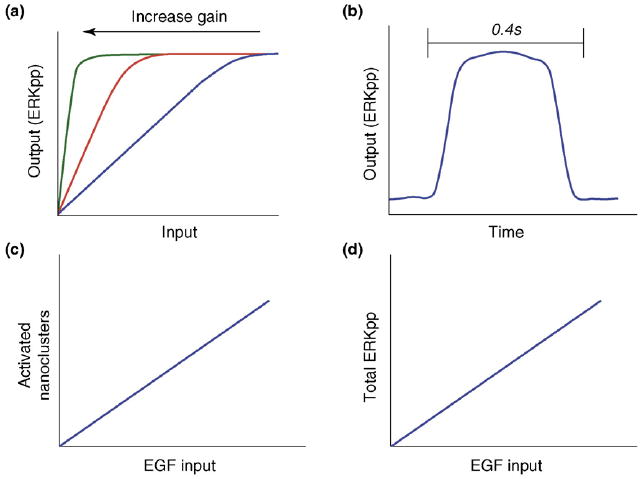
EGF signal transduction is digitized across the plasma membrane. (a) Ras nanoclusters operate as high-gain amplifiers generating high or maximal levels of ERKpp output over a wide range of Raf kinase inputs. The figure shows how signal output varies against input as amplifier gain is increased. At the highest level of gain –the level at which Ras nanoclusters operate – nanocluster output closely approximates a switch with a very low activation threshold. (b) Because Ras nanoclusters function as high-gain amplifiers, during their brief existence they generate maximal ERKpp output to generate a digital burst of ERKpp that is released into the cytosol. In this way, the plasma membrane functions as an analog-to-digital converter (ADC), with the digital bursts from nanoclusters operating as the discrete quantal units. (c) The analog EGF input present in the extracellular matrix (ECM) activates the EGF receptor, which triggers the formation of several Ras nanoclusters. This formation, which occurs through an unknown mechanism, is directly proportional to the external EGF concentration. (d) As the output of each nanocluster is dumped into the cytoplasm, the digital pulses from each nanocluster are summed to give a final cellular analog ERKpp output. In this way, the cytoplasm reverses the digital conversion that occurred at the plasma membrane, and thus it functions as a digital-to-analog converter (DAC). This linear, analog–digital–analog circuit relay generates the robust, high-fidelity signal transmission that occurs across the plasma membrane in vivo.
Ras nanoclusters are essential for signal transduction. In silico modeling shows that, as the number or size of Ras nanoclusters is decreased, the system responsiveness to EGF progressively fails [13]. If Ras nanoclusters disappear altogether and Ras proteins function exclusively as individual molecules, system output decreases to 3% of the maximum ERKpp output achievable with intact nanoclusters [13]. Importantly, these computer simulations precisely match biological experiments in which Ras signal output was progressively lost as Ras nanoclustering decreased [10]. Additional modeling reveals that one crucial role of nanoclusters is to increase the target area of the membrane available for Raf and KSR1–MEK–ERK recruitment. This area decreases if Ras nanoclustering is progressively diminished, and thus the probability of successful recruitment of the components of the MAPK module falls [13]. These combined data reveal that, without functional nanoclusters at the plasma membrane, cells cannot activate the MAPK cascade in response to EGF.
GPI-receptor nanoclusters output digital bursts of Ins(1,4,5)P3 to generate an analog Ca2+ signal
A very elegant set of single-particle tracking (SPT) and SFVT studies has recently demonstrated that Ca2+ signaling by GPI-ARs exhibits a systems behavior nearly identical to that of the Ras–MAPK nanocluster system [25,26]. Kusumi and colleagues [25,26] activated the GPI-AR CD59 glycoprotein by clustering with antibodies conjugated to gold particles. This activation precisely matches physiological activation of the receptors with respect to both system output and the biophysical behavior of receptors clustered on the cell surface. SPT at high temporal resolution revealed that clusters of CD59 GPI-AR exhibit alternating periods of simple lateral diffusion and immobilization, with the periods of immobilization being dependent on both cholesterol and actin filaments [25,26]. Single molecules of green fluorescent protein (GFP)-tagged Lyn (an SFK) or GαI were observed simultaneously with GPI-AR clusters. Single molecules of green fluorescent protein (GFP)-tagged Lyn (an SFK) or Gαi are transiently recruited into the GPI-AR clusters; Lyn for periods of ~200 ms, and Gαi for somewhat shorter periods of ~133 ms [26]. Recruitment of Lyn does not correlate with periods of GPI-AR immobilization, but recruitment of Gαi to CD59 clusters occurs immediately before cluster immobilization. In combination, these observations indicate that CD59 cluster immobilization is a consequence of Lyn activation by means of Gαi recruitment, a conclusion supported by inhibitor experiments and the use of cell lines lacking SFKs [25,26]. The precise molecular mechanism that stops GPI-AR diffusion once the recruited Lyn kinase is activated by the arrival of a Gαi is unclear, but it is dependent on cholesterol and requires an intact actin cytoskeleton (Figure 1). The signaling cascade is completed by the recruitment and activation of phospholipase C gamma (PLCγ), which generates inositol (1,4,5)-triphosphate [ins(1,4,5)P3] by hydrolyzing phosphatidylinositol-bis(4,5)phosphate [PtdIns(4,5)P2] [25,26]. As with the signal output of ERKpp from Ras nanoclusters, all the steps in the GPI-AR pathway leading up to the formation of Ins(1,4,5)P3 are interdependent [25,26]. Signaling is triggered by the formation of GPI-AR clusters. Lyn is recruited to the clusters, and this is followed by recruitment and activation of Gαi and, in turn, Lyn activation [25,26]. Signal output from the GPI-AR cluster is a short, time-limited ‘digital’ pulse of Ins(1,4,5)P3 released into the cytosol [25,26]. Interestingly, the duration of the Ins(1,4,5)P3 pulse from each GPI-AR cluster is on the same time-scale as the output of ERKpp from a Ras nanocluster (Figure 1) [13,25,26]. The Ins(1,4,5)P3 output is digital, but the overall system response is continuous or analog, because the Ins(1,4,5)P3 outputs from the individual clusters are all summed in the cytosol [25,26]. Finally, the Ins(1,4,5)P3 signal is converted into an analog Ca2+ signal by activation of Ins(1,4,5)P3 receptors on intracellular calcium stores [25,26]. The similarity with the Ras system is striking. The GPI-AR system operates as an ADC by forming transient, digital signaling platforms (i.e. nanoswitches) that digitize the external anlog input signal for transmission across the plasma membrane. The cytosol then functions as a simple DAC by summing the individual Ins(1,4,5)P3 digital pulses to create a bulk analog Ca2+ output (Figure 1).
Small transient nanoclusters provide ideal building blocks for biological circuits
The evolution of the lipid-raft hypothesis from the original concept of stable pre-existing domains to one of more transient, dynamic platforms has been viewed with some skepticism because of the very short time- and length-scales recorded for nanodomains in living cells [30,31]. We take the opposite position and argue that it is precisely these properties that make nanoclusters and other types of nanodomains ideal components for building biological circuits. Analog systems are particularly vulnerable to degradation by ‘noise’ – noise equates to random disturbances or variation – when they are copied or amplified, such as occurs when transmitting an EGF signal across the plasma membrane. EGF signal transmission is robust in vivo, owing to the use of nanoclusters to build the transduction circuit [13,27,32]. By spatially dividing the transmission system into many transient modules, or nanoclusters, perturbations caused by intrinsic noise can be contained within individual modules [27]. The output from K-Ras nanoclusters – these function as high-gain amplifiers for Raf and MEK activity to closely approximate a switch-like ERKpp output (Figure 2) [13,27] – is inherently robust to perturbations in Raf and MEK activity [13,27]. Given that CD59 nanoclusters also generate a digital burst of output, it is likely that GPI-AR nanoclusters are similarly robust to perturbations in enzymatic activity. Thus, the crucial properties of modularity and high-gain amplification, which are inherent to nanoclusters, protect systems from signal degradation during transfer across the plasma membrane.
One remarkable feature of using nanoclusters to build analog–digital–analog relays is the high-fidelity signal transmission it affords for both low and high input [13]. The fidelity of ADCs is a function of both the sampling rate, which controls how many samples are taken per second, and the sampling precision, which controls how many quantization levels lie between the minimum and maximum inputs [27]. The higher these two factors are, the higher the fidelity of the system (Figure 3). Ras nanoclusters are highly dynamic and, given that nanoclusters turn over on average every 0.4 s, a ‘single’ nanocluster can effectively sample the external environment >100 times per minute [27]. Moreover, mammalian fibroblasts can generate ~40 000 nanoclusters on the plasma membrane [13,27]. Thus, the resolution of a signal between zero and maximum is effectively divided into 40 000 increments, affording the system extraordinarily high resolution [27]. Exactly the same argument is relevant to the GPI-AR signaling system. The combination of a fast sampling rate and a large sampling precision endows any nanocluster system with the high-fidelity signal transmission modeled in silico and observed in vivo [13].
Figure 3.
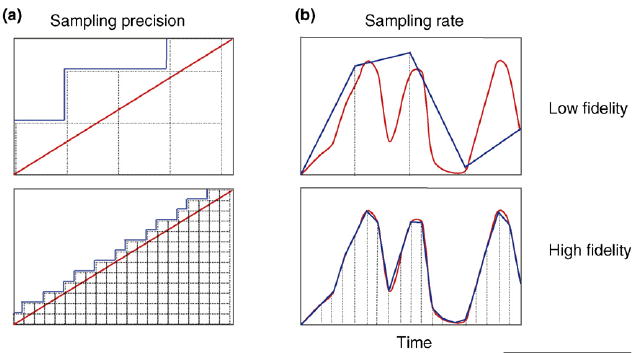
Nanoclusters make ideal components to build high-fidelity biological circuits. The fidelity of signal transfer in a linear ADC is a function of two variables: the sampling precision (i.e. the number of increments between zero and maxim) and the sampling rate (i.e. how many samples are taken over time). Panels (a) compare the fidelity of low versus high sampling-precision. With low sampling-precision [upper panel (a)] (sampling precision is represented by dotted lines), the output curve (blue) is a poor representation of the input signal (red curve). With higher sampling-precision, the output curve closely matches the input signal [lower panel (a)]. Some mammalian cells have up to 40 000 Ras nanoclusters on the inner leaflet of the plasma membrane, and thus the resolution between zero and maximal input for these systems is incredibly high in vivo. (b) The ability to accurately convey a continuous input over time is a function of sampling rate. The fidelity of signal output (blue curve) from a continuous input (red curve) for 1 min using a low sampling rate (4 times/min) is poor [upper panel (b)]. By increasing the sampling rate to 20 times/min [lower panel (b)], the fidelity of the output is significantly increased. Both Ras and GPI-AR nanoclusters sample their respective input signals >100 times/minute. Thus, it is the combination of high sampling-precision and high sampling-rates that enables biological circuits incorporating nanocluster components to generate high-fidelity signal transmission in vivo.
Computation shows that all other non-digital K-Ras nanocluster outputs generate non-linear, low-fidelity system outputs, with the loss of fidelity being proportional to the loss of digital output from the nanoclusters [13,27]. We speculate that these same properties will be shared by the GPI-AR nanocluster signaling system, although this remains to be experimentally validated. High-fidelity signal transfer therefore directly flows from the unique biophysical properties of nanoclusters, specifically their small size, digital output and transient nature (Figure 3).
The digital outputs of nanoclusters that dump fixed quanta of signal output into the cytosol also provide a simple solution to a fundamental problem of communications engineering [6,7,25]. It was previously believed that the recruitment and activation of each individual molecule lasted for a significant portion of the bulk duration of the signal [25]. Given that the bulk level of signal is the integration of many recruitment and activation events, the activation of the next molecule must be precisely synchronized with the inactivation of preceding signaling units. How cells might achieve such complicated integration over thousands of molecular complexes was difficult to explain. Using short-lived digital, or quantal, bursts renders such complicated computations unnecessary, because it is the simple addition of individual quantal pulses that makes up the bulk signal (Figure 4).
Figure 4.

Nanoclusters simplify signal transduction circuits. Many biological events take place very rapidly; for example, high-frequency oscillations (a). If the life-times of the individual signaling components are longer than the oscillatory frequency, then oscillations, or any other rapid change, are impossible to generate (b). The speed of the output change is therefore limited by the life-times of the individual signaling components. By contrast, if the signaling components are relatively short-lived, then their individual quantal pulses can be simply added up to generate the bulk, system output (c). The advantage of this system is that it eliminates the need for complex integration while allowing for the rapid changes of output. It is the elimination of complex integration from transduction circuits, the ability to transmit rapid change, the facility for high-fidelity signal transmission and the inherent robustness of nanoswitches that makes them ideal components for biological circuits.
One example that illustrates these features in a biological context is the use of the MAPK module to specify opposing cell fate decisions in rat pheochromocytoma PC-12 cells [3,4]. Activation of the MAPK pathway by EGF drives proliferation, whereas activation of the MAPK pathway by nerve growth factor (NGF) through activation of the TrkA receptor leads to differentiation [3]. The duration of ERK activation regulates these opposing biological outcomes [4]. EGF stimulates the rapid and transient activation of ERK that is required for proliferation. By contrast, NGF stimulates the sustained activation of ERK that is required for differentiation [4]. Immediate early gene products are stabilized through phosphorylation by ERK during prolonged ERK activation [33], providing a mechanism for cells to interpret and respond differently to transient and sustained ERK activation. It is a much simpler engineering feat to generate a brief signaling burst using short-lived quantal components than it is using long-lived platforms, because brief quantal bursts negate the need for complex predictive integrations within the system. Finally, oscillations are an important type of system output that underpin many key biological processes [34]. The frequency of biological oscillations used to drive biological outputs can be remarkably high [34]. Such high frequencies are simply impossible to generate with the use of long-lived signaling components. By contrast, the short quantal bursts generated from robust nanocluster components can be easily engineered to create robust, high-frequency oscillatory circuits (Figure 4).
Concluding remarks
Here, we have compared the spatial regulation of two signaling cascades – Ras-mediated activation of the MAPK module, and GPI-AR activation of PLC – to reveal the striking conservation of circuit engineering that exists between these two unrelated systems. Both the Ras and GPI systems use plasma membrane nanoclusters as the fundamental building blocks of their respective transduction circuits. Both types of nanoclusters are built using multiple components that are assembled from lipid–lipid, lipid–protein and protein–protein interactions. The two key features of both nanocluster systems are their transient nature and digital output. These crucial properties combine to generate a quantal signal burst from each nanocluster. There are many inherent engineering advantages in using a quantal output from digital nanoclusters to build biological circuits. First, the use of quantal components negates the need for complex integrations within signaling pathways, greatly simplifying data transmission. Second, digital nanoclusters enable high-fidelity signal transduction for both high and low input signals. Third, both long- and short-lived signal outputs can be easily engineered with the use of transient quantal bursts. Fourth, nanocluster outputs are inherently robust, which is a fundamental requirement enabling biological systems to function optimally in nature. Given that two unrelated signaling cascades both use functionally equivalent quantal components, and considering the inherent utility of this circuit design, we propose that the use of digital nanoclusters represents a general mechanism for biological signal transduction.
Table I.
Scaffolds spatially regulate activation of MAPK modules
| Scaffold | Location | Refs |
|---|---|---|
| Yeast | ||
| Ste5 | Plasma membrane | [60,61] |
| Pbs2p | Plasma membrane | [62,63] |
| Human | ||
| KSR1 | Plasma membrane | [64,65] |
| MP1-p14 | Endosome | [66] |
| Sef | Golgi | [67] |
| Paxillin | Focal adhesions | [68] |
| β-arrestin | Plasma membrane, endosome | [69] |
Scaffold proteins are recruited to specific sub-cellular locations to spatially and temporally restrict the activation of MAPK signaling cascades. In both yeast and higher eukaryotes, loss of scaffold spatial information results in loss of signaling [70,71], confirming that spatial regulation is a crucial function of scaffold proteins.
Acknowledgments
Work in the authors’ laboratories is supported by grants from the National Health and Medical Research Council (NHMRC), the Australian Research Council (ARC) and the National Institutes of Health (NIH) (GM066717). The Institute for Molecular Bioscience (IMB) is a Special Research Centre of the ARC.
References
- 1.Ashe HL, Briscoe J. The interpretation of morphogen gradients. Development. 2006;133:385–394. doi: 10.1242/dev.02238. [DOI] [PubMed] [Google Scholar]
- 2.Stathopoulos A, Levine M. Dorsal gradient networks in the Drosophila embryo. Dev Biol. 2002;246:57–67. doi: 10.1006/dbio.2002.0652. [DOI] [PubMed] [Google Scholar]
- 3.Traverse S, et al. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J. 1992;288:351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 5.Kitano H. Towards a theory of biological robustness. Mol Syst Biol. 2007;3:137. doi: 10.1038/msb4100179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliver BM, et al. The philosophy of PCM. Proceedings of the I R E. 1948;36:1324–1331. [Google Scholar]
- 7.Schimizu TS, Bray D. Computational cell biology – the stochastic approach. In: Kitano H, editor. Foundations of Systems Biology. MIT Press; 2001. pp. 213–232. [Google Scholar]
- 8.Hancock JF. Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prior IA, et al. Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J Cell Biol. 2003;160:165–170. doi: 10.1083/jcb.200209091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plowman SJ, et al. H-Ras, K-Ras, and inner plasma membrane raft proteins operate in nanoclusters with differential dependence on the actin cytoskeleton. Proc Natl Acad Sci U S A. 2005;102:15500–15505. doi: 10.1073/pnas.0504114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hancock JF, Parton RG. Ras plasma membrane signaling platforms. Biochem J. 2005;389:1–11. doi: 10.1042/BJ20050231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murakoshi H, et al. Single-molecule imaging analysis of Ras activation in living cells. Proc Natl Acad Sci U S A. 2004;101:7317–7322. doi: 10.1073/pnas.0401354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian T, et al. Plasma membrane nanoswitches generate high-fidelity Ras signal transduction. Nat Cell Biol. 2007;9:905–914. doi: 10.1038/ncb1615. [DOI] [PubMed] [Google Scholar]
- 14.Marshall CJ. Ras effectors. Curr Opin Cell Biol. 1996;8:197–204. doi: 10.1016/s0955-0674(96)80066-4. [DOI] [PubMed] [Google Scholar]
- 15.Hibino K, et al. Single- and multiple-molecule dynamics of the signaling from H-Ras to cRaf-1 visualized on the plasma membrane of living cells. ChemPhysChem. 2003;4:748–753. doi: 10.1002/cphc.200300731. [DOI] [PubMed] [Google Scholar]
- 16.Plowman SJ, et al. Electrostatic interactions positively regulate K-Ras nanocluster formation and function. Mol Cell Biol. 28:4377–4385. doi: 10.1128/MCB.00050-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orlean P, Menon AK. Thematic review series: lipid posttranslational modifications. GPI anchoring of protein in yeast and mammalian cells, or: how we learned to stop worrying and love glycophospholipids. J Lipid Res. 2007;48:993–1011. doi: 10.1194/jlr.R700002-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Stefanova I, et al. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991;254:1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- 19.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 20.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 21.Brugger B, et al. The membrane domains occupied by glycosylphosphatidylinositol-anchored prion protein and Thy-1 differ in lipid composition. J Biol Chem. 2004;279:7530–7536. doi: 10.1074/jbc.M310207200. [DOI] [PubMed] [Google Scholar]
- 22.Gri G, et al. The inner side of T cell lipid rafts. Immunol Lett. 2004;94:247–252. doi: 10.1016/j.imlet.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Eisenberg S, Henis YI. Interactions of Ras proteins with the plasma membrane and their roles in signaling. Cell Signal. 2008;20:31–39. doi: 10.1016/j.cellsig.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Sharma P, et al. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell. 2004;116:577–589. doi: 10.1016/s0092-8674(04)00167-9. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki KG, et al. Dynamic recruitment of phospholipase C gamma at transiently immobilized GPI-anchored receptor clusters induces IP3–Ca2+ signaling: single-molecule tracking study 2. J Cell Biol. 2007;177:731–742. doi: 10.1083/jcb.200609175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki KG, et al. GPI-anchored receptor clusters transiently recruit Lyn and G alpha for temporary cluster immobilization and Lyn activation: single-molecule tracking study 1. J Cell Biol. 2007;177:717–730. doi: 10.1083/jcb.200609174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harding A, Hancock JF. Ras nanoclusters: combining digital and analog signaling. Cell Cycle. 2008;7:127–134. doi: 10.4161/cc.7.2.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeung K, et al. Mechanism of suppression of the Raf/MEK/ extracellular signal-regulated kinase pathway by the Raf kinase inhibitor protein. Mol Cell Biol. 2000;20:3079–3085. doi: 10.1128/mcb.20.9.3079-3085.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeung K, et al. Suppression of Raf-1 kinase activity and MAP kinase signaling by RKIP. Nature. 1999;401:173–177. doi: 10.1038/43686. [DOI] [PubMed] [Google Scholar]
- 30.Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 31.Shaw AS. Lipid rafts: now you see them, now you don’t. Nat Immunol. 2006;7:1139–1142. doi: 10.1038/ni1405. [DOI] [PubMed] [Google Scholar]
- 32.Harding A, et al. Subcellular localization determines MAP kinase signal output. Curr Biol. 2005;15:869–873. doi: 10.1016/j.cub.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 33.Murphy LO, et al. Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol. 2002;4:556–564. doi: 10.1038/ncb822. [DOI] [PubMed] [Google Scholar]
- 34.Schuster S, et al. Modelling of simple and complex calcium oscillations. From single-cell responses to intercellular signaling. Eur J Biochem. 2002;269:1333–1355. doi: 10.1046/j.0014-2956.2001.02720.x. [DOI] [PubMed] [Google Scholar]
- 35.Abankwa D, et al. A novel switch region regulates H-Ras membrane orientation and signal output. EMBO J. 2008;27:727–735. doi: 10.1038/emboj.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorfe AA, et al. Structure and dynamics of the full-length lipid-modified H-Ras protein in a 1,2-dimyristoylglycero-3-phosphocholine bilayer. J Med Chem. 2007;50:674–684. doi: 10.1021/jm061053f. [DOI] [PubMed] [Google Scholar]
- 37.Rotblat B, et al. Three separable domains regulate GTP-dependent association of H-Ras with the plasma membrane. Mol Cell Biol. 2004;24:6799–6810. doi: 10.1128/MCB.24.15.6799-6810.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy S, et al. Individual palmitoyl residues serve distinct roles in H-Ras trafficking, microlocalization, and signaling. Mol Cell Biol. 2005;25:6722–6733. doi: 10.1128/MCB.25.15.6722-6733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abankwa D, et al. Ras nanoclusters: molecular structure and assembly. Semin Cell Dev Biol. 2007;18:599–607. doi: 10.1016/j.semcdb.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belanis L, et al. Galectin-1 is a novel structural component and a major regulator of H-Ras nanoclusters. Mol Biol Cell. 2008;19:1404–1414. doi: 10.1091/mbc.E07-10-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kusumi A, et al. Single-molecule tracking of membrane molecules: plasma membrane compartmentalization and dynamic assembly of raft-philic signaling molecules. Semin Immunol. 2005;17:3–21. doi: 10.1016/j.smim.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Kusumi A, et al. Molecular dynamics and interactions for creation of stimulation-induced stabilized rafts from small unstable steady-state rafts. Traffic. 2004;5:213–230. doi: 10.1111/j.1600-0854.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- 43.Kusumi A, et al. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct. 2005;34:351–378. doi: 10.1146/annurev.biophys.34.040204.144637. [DOI] [PubMed] [Google Scholar]
- 44.Mayor S, Rao M. Rafts: scale-dependent, active lipid organization at the cell surface. Traffic. 2004;5:231–240. doi: 10.1111/j.1600-0854.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- 45.Nicolau DV, Jr, et al. Identifying optimal lipid raft characteristics required to promote nanoscale protein–protein interactions on the plasma membrane. Mol Cell Biol. 2006;26:313–323. doi: 10.1128/MCB.26.1.313-323.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicolau DV, Jr, et al. Sources of anomalous diffusion on cell membranes: a Monte Carlo study. Biophys J. 2007;92:1975–1987. doi: 10.1529/biophysj.105.076869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 48.Ferrell JE, Jr, Machleder EM. The biochemical basis of an all-or-none cell fate switch in Xenopus oocytes. Science. 1998;280:895–898. doi: 10.1126/science.280.5365.895. [DOI] [PubMed] [Google Scholar]
- 49.Kholodenko BN. Negative feedback and ultrasensitivity can bring about oscillations in the mitogen-activated protein kinase cascades. Eur J Biochem. 2000;267:1583–1588. doi: 10.1046/j.1432-1327.2000.01197.x. [DOI] [PubMed] [Google Scholar]
- 50.Markevich NI, et al. Long-range signaling by phosphoprotein waves arising from bistability in protein kinase cascades. Mol Syst Biol. 2006;2:61. doi: 10.1038/msb4100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwacke JH, Voit EO. The potential for signal integration and processing in interacting MAP kinase cascades. J Theor Biol. 2007;246:604–620. doi: 10.1016/j.jtbi.2006.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wittinghofer A, Pai EF. The structure of Ras protein: a model for a universal molecular switch. Trends Biochem Sci. 1991;16:382–387. doi: 10.1016/0968-0004(91)90156-p. [DOI] [PubMed] [Google Scholar]
- 53.Morrison DK, Cutler RE. The complexity of Raf-1 regulation. Curr Opin Cell Biol. 1997;9:174–179. doi: 10.1016/s0955-0674(97)80060-9. [DOI] [PubMed] [Google Scholar]
- 54.Fukuda M, et al. A novel regulatory mechanism in the mitogen-activated protein (MAP) kinase cascade. Role of nuclear export signal of MAP kinase kinase. J Biol Chem. 1997;272:32642–32648. doi: 10.1074/jbc.272.51.32642. [DOI] [PubMed] [Google Scholar]
- 55.Fukuda M, et al. Interaction of MAP kinase with MAP kinase kinase: its possible role in the control of nucleocytoplasmic transport of MAP kinase. EMBO J. 1997;16:1901–1908. doi: 10.1093/emboj/16.8.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 57.Harding A, et al. Identification of residues and domains of Raf important for function in vivo and in vitro. J Biol Chem. 2003;278:45519–45527. doi: 10.1074/jbc.M303106200. [DOI] [PubMed] [Google Scholar]
- 58.Zhu J, et al. Identification of Raf-1 S471 as a novel phosphorylation site critical for Raf-1 and B-Raf kinase activities and for MEK binding. Mol Biol Cell. 2005;16:4733–4744. doi: 10.1091/mbc.E05-02-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Terai K, Matsuda M. Ras binding opens c-Raf to expose the docking site for mitogen-activated protein kinase kinase. EMBO Rep. 2005;6:251–255. doi: 10.1038/sj.embor.7400349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng Y, et al. Functional binding between Gbeta and the LIM domain of Ste5 is required to activate the MEKK Ste11. Curr Biol. 1998;8:267–278. doi: 10.1016/s0960-9822(98)70108-3. [DOI] [PubMed] [Google Scholar]
- 61.Inouye C, et al. Ste5 RING-H2 domain: role in Ste4-promoted oligomerization for yeast pheromone signaling. Science. 1997;278:103–106. doi: 10.1126/science.278.5335.103. [DOI] [PubMed] [Google Scholar]
- 62.Maeda T, et al. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science. 1995;269:554–558. doi: 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- 63.Reiser V, et al. Polarized localization of yeast Pbs2 depends on osmostress, the membrane protein Sho1 and Cdc42. Nat Cell Biol. 2000;2:620–627. doi: 10.1038/35023568. [DOI] [PubMed] [Google Scholar]
- 64.Cacace AM, et al. Identification of constitutive and Ras-inducible phosphorylation sites of KSR: implications for 14-3-3 binding, mitogen-activated protein kinase binding, and KSR overexpression. Mol Cell Biol. 1999;19:229–240. doi: 10.1128/mcb.19.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muller J, et al. C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold. KSR1 Mol Cell. 2001;8:983–993. doi: 10.1016/s1097-2765(01)00383-5. [DOI] [PubMed] [Google Scholar]
- 66.Teis D, et al. Localization of the MP1-MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev Cell. 2002;3:803–814. doi: 10.1016/s1534-5807(02)00364-7. [DOI] [PubMed] [Google Scholar]
- 67.Torii S, et al. Sef is a spatial regulator for Ras/MAP kinase signaling. Dev Cell. 2004;7:33–44. doi: 10.1016/j.devcel.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 68.Turner CE, et al. Paxillin: a new vinculin-binding protein present in focal adhesions. J Cell Biol. 1990;111:1059–1068. doi: 10.1083/jcb.111.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luttrell LM, et al. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci U S A. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pryciak PM, Huntress FA. Membrane recruitment of the kinase cascade scaffold protein Ste5 by the Gbetagamma complex underlies activation of the yeast pheromone response pathway. Genes Dev. 1998;12:2684–2697. doi: 10.1101/gad.12.17.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou M, et al. Solution structure and functional analysis of the cysteine-rich C1 domain of kinase suppressor of Ras (KSR) J Mol Biol. 2002;315:435–446. doi: 10.1006/jmbi.2001.5263. [DOI] [PubMed] [Google Scholar]


