Abstract
Infections and inflammation trigger neutrophilias that are supported by a hematopoietic program of accelerated granulopoiesis known as emergency granulopoiesis. The intrinsic factors that drive reactive neutrophilias and emergency granulopoiesis have been inferred but not demonstrated. Here we show that alum can not elicit reactive neutrophilias in IL-1RI−/− mice whereas other inflammatory responses, including eosinophilia and antibody production, remain intact. Analysis of this specific impairment revealed an unanticipated role for IL-1RI in supporting increased proliferation by granulocyte/macrophage progenitors (GMP) and, surprisingly, multipotent progenitors (MPP) and hematopoietic stem cells (HSC). Indeed, HSC and MPP proliferative responses were most suppressed in IL-1RI−/− mice, suggesting a critical role for their proliferation in inflammatory granulopoiesis. Whereas IL-1 drives increased HSC proliferation directly in vitro, IL-1RI expression by radiation-resistant host cells was both necessary and sufficient for alum-induced HSC, MPP, and GMP proliferation and reactive neutrophilias in radiation chimeric mice. Thus, IL-1 plays a necessary, but indirect role in the support of alum-induced neutrophilias by expanding both pluripotent and myeloid progenitor compartments to accelerate granulopoiesis.
Keywords: Cell Differentiation, Cytokine Receptors, Hematopoiesis, Inflammation, Neutrophils
Introduction
Neutrophils are vital to innate immunity (1, 2). Normally, physiologic numbers of mature neutrophils are maintained by a “steady-state” granulopoietic pathway; acute infection or inflammation, however, trigger the mobilization of neutrophil stores from the bone marrow (BM) and blood into inflammatory sites (3, 4). The result of this mobilization is a reactive neutrophilia immediately followed by accelerated or “emergency” granulopoiesis in the BM (5, 6).
Neutrophils, like all other leukocytes, originate from self-renewing, long-term (LT-)4 hematopoietic stem cells (HSC). By asymmetrical division, LT-HSC give rise to short-term (ST-) HSC that possess a limited capacity for self-renewal (7) and finally develop into multipotent progenitors [MPP; (8)]. MPP produce lineage committed progenitors, including common myeloid progenitors (CMP) that differentiate into megakaryocyte/erythroid progenitors (MEP) or granulocyte/macrophage progenitors (GMP) (9). GMP produce neutrophils through a series of developmental stages, first as myelocytes and metamyelocytes, then band neutrophils, and finally, mature, segmented neutrophils (10).
Steady-state and “emergency” granulopoiesis can be distinguished by separate dependencies on the C/EBPα and C/EBPβ transcription factors [CCAAT enhancer binding proteins; (6, 11)]. Genetic disruption of C/EBPα abolishes steady-state granulopoiesis (11) whereas C/EBPβ deficiency leaves steady-state granulopoiesis intact but abrogates reactive neutrophilias (6).
It is generally thought that emergency granulopoiesis is activated by the increased expression of granulopoietic factors, IL-6, IL-3, G-CSF, and GM-CSF, that induce granulocytic progenitors to proliferate (6, 12). Indeed, microbial infections increase serum GM-CSF and G-CSF coincidentally with the number of myeloid progenitors in BM (13). In vitro, IL-6, IL-3, GM-CSF, and G-CSF promote proliferation and granulocytic differentiation by myeloid progenitors (14-18). Finally, neutrophilias elicited by over-expression of G-CSF, GM-CSF, or IL-3 are associated with increased C/EBPβ transcription in GMP, suggesting that C/EBPβ regulates granulopoiesis through GMP (6).
Despite these correlations, mice deficient for G-CSF, GM-CSF and IL-6, or G-CSF and GM-CSF mount reactive neutrophilias (5, 19), and both steady-state and emergency granulopoiesis are intact in mice deficient for the common β chain of IL-3R/GM-CSFR/IL-5R (20). Thus, IL-6, IL-3, GM-CSF, and G-CSF are dispensable for reactive neutrophilias and emergency granulopoiesis. To understand emergency granulopoiesis, identification of indispensable factors is required.
We have observed that alum promotes granulopoiesis at the expense of B lymphopoiesis, and have proposed that these lineages compete for developmental resources in the BM (10, 21). TNFα initiates these hematopoietic changes by lowering BM CXCL12 expression and mobilizing BM lymphocytes; TNFα alone, however, has little effect on granulopoiesis (21). Instead, TNFα and IL-1 synergize to reproduce alum’s effects on the BM (21) and that synergy implicated IL-1 as a central factor in emergency granulopoiesis.
Here we show that alum does not elicit reactive neutrophilias and emergency granulopoiesis in mice which lack functional receptors for IL-1. These defects are not due to generally diminished responses to alum’s inflammatory or adjuvant properties, as other responses, including eosinophilia and antibody production, remain intact or are enhanced. Alum does not mobilize neutrophils from the BM of IL-1RI−/− mice nor does it elicit emergency granulopoiesis as determined by accelerated granulopoietic output or proliferation by HSC, MPP, and GMP. These effects are indirect, as neutrophil mobilization and emergency granulopoiesis in bone marrow chimeric mice is determined by IL-1RI expression on radiation-resistant, non-hematopoietic cells in the host. We conclude that IL-1RI provides an indispensable signal that induces secondary factors to initiate and sustain reactive neutrophilias and emergency granulopoiesis.
Materials and Methods
Mice
C57BL/6 (CD45.2), congenic CD45.1 [B6.SJL-Ptprca Pep3b/BoyJ; (21)], and congenic IL-1RI−/− mice [B6.129S7-Il1r1tm1Imx/J (22)] were from Jackson Laboratories (Bar Harbor, ME). (C57BL/6 x C57BL/6.CD45.1)F1 mice were bred locally. Mice were housed in specific pathogen-free conditions at the Duke University Animal Care Facility and with sterile bedding, water, and food. All studies were approved by the Duke University Institutional Animal Care and Use Committee.
Immunization
Mice were immunized with one injection of NP8-CGG (60 μg) in alum (21).
Flow Cytometry
Cell suspensions from blood and BM were stained with FITC-, PE-, PE-Texas-Red, biotin-, allophycocyanin- (APC), APC-Cy7, PE-Cy5-, and PE-Cy7-conjugated mAb specific for mouse B220, IgM, CD11b, CD34, CD4, CD8, TER119, Gr-1, CD117, Sca-1, IL-7Rα, FcγRII/III, Flt3, or Ly-6G (BD Bioscience or eBioscience); and PE-conjugated 7/4 mAb (AbD Serotec). Streptavidin (SA) -PE-Texas-Red or SA-Texas-Red (BD Bioscience) were used to identify biotinylated mAb. Propidium iodide (Sigma) labeling identified dead cells. Labeled cells were analyzed or sorted with FACS Vantage SE™ or LSRII™ flow cytometers (BD Bioscience); data were analyzed with FlowJo software. LSK cells, Flt3− LSK cells, and Flt3+ LSK cells were sorted from BM suspensions following enrichment with CD117 microbeads (Miltenyi Biotec).
CFU-Complete Assay
Flt3− LSK cells (100) from naïve or immunized (d 2) mice were cultured in methylcellulose media (StemCell Technologies) with mouse SCF, IL-3, IL-6, and human erythropoietin. Later (8 d), colonies were counted and typed as CFU-GEMM, CFU-GM, BFU-E, CFU-M, and CFU-G by microscopy.
Limiting Dilution Assays
Flt3− LSK cells were sorted from BM pools from naïve (n=4) and immunized (d 2; n=4) mice. Cultures containing 1 (n=72), 2 (n=48), or 8 (n=12) Flt3− LSK cells were made by sorting onto OP9 stromal cell layers in 96-well plates with 10 ng/ml IL-7 and 10 ng/ml Flt3 ligand (R&D systems) (23). After 14 d, wells containing CD45+CD11b−B220+CD19+ cells (23) were scored positive for B-cell production.
BrdU Labeling
Mice were injected i.p. with 1 mg BrdU; 6 h after injection, BM cells were harvested and labeled to identify specific populations (Supplemental Fig. 2). Labeled BM cells were fixed, permeabilized, and treated with DNaseI to expose incorporated BrdU using a commercial kit (BD Bioscience); cells were then stained with FITC-labeled anti-BrdU for flow cytometric analysis. These preparations denatured the CD34 epitope (data not shown), preventing the discrimination of CMP and MEP (Supplemental Fig. 2).
HSC Culture
HSC, MPP, and GMP (n=500) were cultured with recombinant IL-1β (1 ng/ml; Peprotech) or GM-CSF (1 ng/ml; Peprotech) in X-VIVO15 serum-free medium (Cambrex) containing SCF (25 ng/ml; R&D systems) to promote HSC survival (24). Cultured cells were enumerated and characterized after 4 d.
Quantification of mRNA
mRNA from cells (104–105) was precipitated in Trizol (Invitrogen) and reverse transcribed with Superscript II (Invitrogen). Quantitative PCR amplifications of cDNA were performed (iCycler thermal cycler, Bio-Rad Laboratories) with SYBR® Green PCR Master Mix™ (Applied Biosystems) using primers specific for IL-1RI, GM-CSFR, and cDNA: IL-1RI forward, 5′-CTGAGGTCTTGGAGGGACAG-3′, and reverse, 5′-TCCTTCCTGGATGAGAGCAT-3′; GM-CSFR forward, 5′-GACACGAGGATGAAGCACTG-3′, and reverse, 5′-GAGGTCCTTCCTGAGGGTCT-3′; and β-actin forward, 5′-AGCCATGTACGTAGCCATCC-3′, and reverse, 5′-CTCTCAGCTGTGGTGGTGAA-3′. Amplification parameters: initial denaturation at 94°C for 10 m; amplification cycle, denaturing at 94°C for 10 s, anneal/extension at 60°C for 45 s. Relative gene expression was calculated by the comparative CT (threshold cycle) method of the manufacturer (Applied Biosystems) normalized to β-actin message; ΔCT values were determined by subtracting CT (target) from CT (β-actin). Expression levels relative to β-actin were defined as: 2−ΔCT.
Serum Ab measurements
NP-specific serum antibodies were quantified as described (25).
Adoptive reconstitutions
(C57BL/6 x C57BL/6.CD45.1)F1 mice were sublethally irradiated [600 rad, (26)] and reconstituted with equal numbers (5×106) of congenic C57BL/6.CD45.1 (IL-1RI+/+) and C57BL/6 (IL-1RI−/−) BM cells to generate mixed chimeric mice. Reciprocal chimeras were generated similarly [IL-1RI deficient C57BL/6→irradiated (C57BL/6.CD45.2/CD45.1)F1 and (C57BL/6.CD45.2/CD45.1)F1→into irradiated C57BL/6 (IL-1RI−/−)]. To control for any effects of hematopoietic reconstitution in these KO→WT and WT→KO chimeras, homologous [WT→WT and KO→KO] animals were created as well. Donor- and recipient cells in mixed and reciprocal chimeras were distinguished by CD45.1 and CD45.2 expression; chimerism was determined by the CD45.1:CD45.2 ratio of blood leukocytes.
Statistics
Paired data were analyzed by Student’s t test.
Results
Alum-induced neutrophilias require IL-1RI
IL-1 is an important component to inflammatory responses elicited by mineral salts (27) and synergizes with TNFα to increase neutrophil production in BM (21). To determine the role of IL-1RI in reactive neutrophilias, we injected C57BL/6 (BL/6) and congenic IL-1RI−/− mice with alum/antigen (21) and followed changes in blood leukocyte numbers over eight days (d).
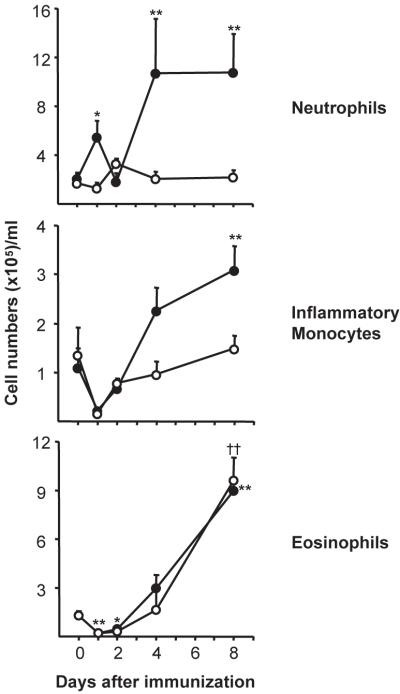
In BL/6 mice, alum elicited a biphasic neutrophilia; neutrophil (Supplemental Fig. 1) numbers rose (>2-fold) 1 d after immunization, returned to naïve levels on d 2, and again rose ≈5-fold above controls on d 4- and 8 post-immunization (Fig. 1). Alum also modulated the numbers of inflammatory monocytes (28) and eosinophils (Supplemental Fig. 1), with initial decreases 1 d after immunization, followed by steady increases that continued to d 8 (7-fold increase, Fig. 1).
Fig. 1. IL-1RI is required for alum-induced neutrophilia.
Peripheral blood cells of BL/6 and IL-1RI−/− mice were harvested after immunization (1–8 d) with NP8-CGG/alum. Neutrophils, inflammatory monocytes, and eosinophils were enumerated by flow cytometry (Supplemental Fig. 1). The mean (± SEM) numbers of cells/ml of blood from BL/6 (●; n=4–7) and IL-1RI−/− (○; d 1 and 2, n=2, others, n=4–5) mice are shown. Significant differences from naïve controls are indicated for BL/6 (*, P ≤ 0.05; **, P ≤ 0.01) and IL-1RI−/− (†, P ≤ 0.05;††, P ≤ 0.01) mice.
The blood of naive BL/6 and IL-1RI−/− mice contain identical numbers of neutrophils, inflammatory monocytes, and eosinophils (29) (Fig. 1). Alum did not elicit neutrophilia in IL-1RI−/− mice, as blood neutrophil numbers were not significantly changed at any time-point after immunization (Fig. 1). Inflammatory monocyte responses were also abrogated, but IL-1RI−/− mice did mount robust eosinophilias that matched BL/6 controls (Fig. 1). Thus, alum induces inflammatory neutrophilias and monocytoses via an IL-1RI dependent pathway, while induction of eosinophilia is IL-1RI independent.
Alum-induced inflammatory granulopoiesis is IL-1RI dependent
The absence of alum-induced neutrophilias in IL-1RI−/− mice implied a defect in emergency granulopoiesis (5, 6). To determine the role of IL-1RI in emergency granulopoiesis, we immunized BL/6 and IL-1RI−/− mice and followed the dynamics of HSC, MPP, CMP, and GMP (Supplemental Fig. 2) populations, as well as the primitive- and mature neutrophil compartments (Supplemental Fig. 1) in BM. To estimate any changes in proliferation rates, we injected mice i.p. with BrdU 6 hours before sacrifice and compared the frequencies of BrdU+ cells in each cell compartment from naïve and immunized mice (30).
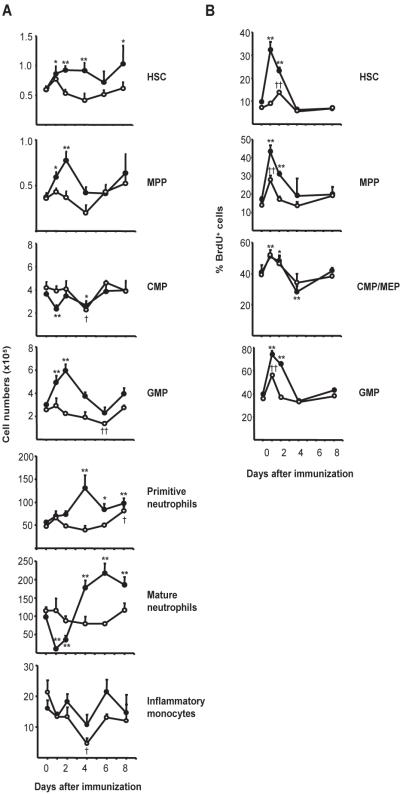
Immunization of BL/6 mice expanded the HSC [Flt3−Lin−Sca-1+c-Kit+ (Flt3− LSK)] and MPP (Flt3+ LSK) compartments 1 d after immunization. HSC [Supplemental Fig. 2; (7)] numbers rose to 150% of naïve controls, remained elevated through d 4, and then returned to naïve levels by d 6 (Fig. 2A). Similarly, MPP numbers [Supplemental Fig. 2; (8)] increased to 200% of controls 2 d after immunization and returned to normal by d 4 (Fig. 2A).
Fig. 2. IL-1RI is required for alum-induced emergency granulopoiesis.
(A) BM cells of BL/6 mice and IL-1RI−/− mice were harvested after immunization (1–8 d) and labeled to identify HSC, MPP, CMP, GMP, primitive neutrophils, and mature neutrophils (Supplemental Figs. 1 and 2). Average cell numbers (± SEM) from BL/6 (●) and IL-1RI−/− (○) mice are shown (n=3–11, each point). (B) BL/6 and IL-1RI−/− mice were immunized at various times then injected i.p. with 1 mg BrdU 6 h prior to tissue harvest. BM cells were labeled to identify hematopoietic progenitor compartments and then fixed, exposed to DNase, and stained with anti-BrdU mAb. Frequencies of BrdU+ cells in each progenitor compartment were determined by flow cytometry. Average frequencies (± SEM) of BrdU+ HSC, MPP, CMP/MEP, and GMP from BL/6 (●; n=3–9) and IL-1RI−/− (○; n=3–5) mice are shown. Significant differences from naïve controls are indicated for BL/6 (*, P ≤ 0.05; **, P ≤ 0.01) and IL-1RI−/− (†, P ≤ 0.05;††, P ≤ 0.01) mice.
Increased numbers of HSC and MPP were accompanied by increased BrdU uptake. Frequencies of BrdU+ HSC and MPP increased 3.5- and 2.5-fold, respectively, within 1 d of immunization and gradually returned to naïve levels by d 4 (Fig. 2B), indicating that alum induces HSC and MPP proliferation.
Alum immunization did not increase CMP numbers in BL/6 mice; rather, CMP numbers fell on d 1 but then recovered and remained at naïve levels (Fig. 2A). In contrast to the substantial increases in the frequencies of BrdU+ HSC and MPP, BrdU labeling of CMP/MEP (Lin−c-Kit+Sca-1−FcγRII/III−) rose only modestly and returned to normal by d 4 (Fig. 2B).
Unlike CMP, GMP numbers increased significantly 1- and 2 d after immunization and returned to normal by d 4 (Fig. 2A). Increased GMP numbers correlated with increased (2-fold) BrdU uptake, 1- and 2 d after immunization, indicating that GMP, like HSC and MPP, are targets of alum-induced proliferative signals (Fig. 2B).
Early proliferation by HSC, MPP, and GMP was followed by increased numbers of primitive neutrophils (myelocytes and metamyelocytes; Supplemental Fig. 1). This neutrophil compartment grew >2-fold by d 4 after immunization and remained significantly elevated through d 8 (Fig. 2A). The effect of alum on the mature neutrophil compartment in BM was more complex. Initially, mature neutrophils were mobilized from the BM by alum; 1 d after immunization, the numbers of mature, BM neutrophils fell by 85% (Fig. 2A), coincident with the first peak of neutrophilia (Fig. 1). This loss of mature neutrophils from the BM was soon reversed and followed by significant, 2-fold increases over naïve controls at days 4–8 (Fig. 2A). This increase in BM neutrophils coincided with the second and sustained wave of neutrophilia (Fig. 1).
We conclude that neutrophilic responses to alum are similar to those elicited by infection (3, 4); both begin with the mobilization of BM neutrophil stores and are sustained by inflammatory granulopoiesis. Unexpectedly, GMP were not the most primitive hematopoietic cells to proliferate in response to alum. HSC and MPP proliferation increased coincidentally with that of GMP, suggesting that all three compartments are sensitive to inflammatory signals.
In IL-1RI−/− mice, immunization with alum/antigen neither mobilized mature neutrophils nor expanded the HSC, MPP, or GMP compartments in BM. HSC numbers were not changed by immunization (Fig. 2A). The frequency of BrdU+ HSC did not rise 1 d after immunization, but did exhibit a transient increase on d 2 that was significantly attenuated compared to BL/6 controls (Fig. 2B). Likewise, MPP numbers in the BM of IL-1RI−/− mice were not affected by immunization (Fig. 2A). Frequencies of BrdU+ MPP transiently increased 1 d after immunization, but returned to naïve levels by day 2 (Fig. 2B). These observations indicate that alum-induced expansions of the HSC and MPP compartments in the BM are IL-1RI dependent.
In contrast to BL/6 mice, GMP numbers in IL-1RI−/− mice did not increase after immunization; instead, GMP numbers gradually fell, becoming significantly depleted by d 6 (Fig. 2A). This decline was accompanied by reduced BrdU uptake, as BrdU+ GMP were increased only on d 1 and then returned to naïve levels (Fig. 2B). Thus, alum coincidentally elicits IL-1RI dependent proliferation in the HSC, MPP, and GMP compartments of BM.
The impaired proliferation by HSC, MPP, and GMP in immunized IL-1RI−/− mice was followed by almost no change in the numbers of neutrophils in BM (Fig. 2A). Whereas immunization eventually (d 8) produced a modest but significant increase in the numbers of primitive neutrophils, the mature neutrophil compartment of BM was neither depleted by mobilization nor expanded by proliferation (Fig. 2A). The absence of alum-induced neutrophilias in IL-1RI−/− mice results from defective neutrophil mobilization and abrogation of inflammatory granulopoiesis.
Alum’s failure to elicit reactive monocytoses in IL-1RI−/− mice (Fig. 1) suggested defective monocyte output; although immunizations did not significantly alter the numbers of inflammatory monocytes in the BM of BL/6 mice, in IL-1RI−/− mice the numbers of inflammatory monocytes fell to 25% of controls by day 4 before returning to normal (day 6; Fig. 2). IL-1RI controls, therefore, inflammatory myelopoietic pathways necessary for both emergency granulopoiesis and enhanced production of inflammatory monocytes.
Comparable HSC and MPP from naïve and immunized mice
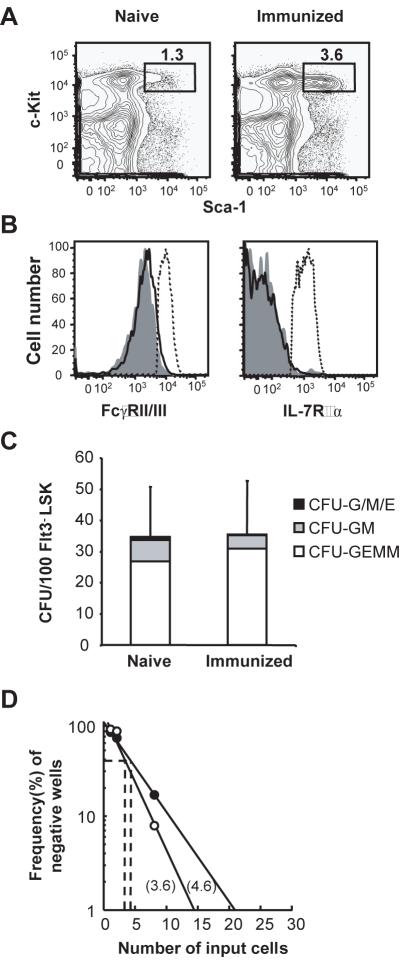
IFNγ and TNFα can induce Sca-1 expression by lineage-committed progenitors, causing them to mimic the LSK phenotype (31). Thus, the rapid increases in HSC and MPP numbers after alum immunization could be only apparent, an artifact of proliferation by committed progenitor cells induced to express Sca-1. To exclude this possibility, we analyzed LSK cells from naïve and immunized mice for expression of FcγRII/III and IL-7Rα, molecules not expressed by HSC and MPP but characteristic of GMP and CLP, respectively (9, 23). LSK from naïve and immunized (day 2) mice were phenotypically identical and neither expressed FcγRII/III or IL-7Rα (Fig. 3A and B), indicating that alum does not cause GMP or CLP to mimic the LSK phenotype.
Fig. 3. LSK cells from naive and immunized mice have equivalent lineage potential.
(A) Sca-1 and c-Kit staining of BM Lin− cells from naive and immunized (2 days after injection of NP8-CGG/alum) mice. (B) Analysis of LSK cells from naïve (shaded histograms) and immunized (open histograms) mice for FcγRII/III and IL-7Rα expression, compared to expression by GMP (PI−Lin−c-Kit+Sca-1−CD34+FcγRII/III+; broken lines, left histogram) and CLP (PI−Lin−IL-7Rα+c-KitlowSca-1low; broken lines, right histogram), respectively. (C) Erythroid and myeloid potential from single Flt3−LSK cells was determined by methylcellulose assay. Flt3−LSK cells (100 cells) were sorted from naïve and immunized (d 2) mice and seeded in methylcellulose medium containing SCF, IL-3, IL-6, and erythropoietin. The numbers and types of colonies were determined by optical microscopy 8 d later. The bars show the average numbers of colonies classified as CFU-GEMM (multilineage), CFU-GM (myeloid lineage), and the total number of CFU-G, -M, and -E (single lineage). The standard deviations of the total numbers of colonies are shown. (D) B-cell production by Flt3−LSK cells from naïve (●) and immunized (○) mice. Multiple wells of 1, 2, and 8 Flt3−LSK cells from naïve and immunized (d 2) mice were cultured on OP9 stromal cell layers in the presence of IL-7 and Flt3L. The frequencies of wells with B-cell growth were determined by the presence of CD45+CD11b−CD19+B220+ cells after 14 days in culture.
Next, we compared the ability of HSC (Flt3−LSK cells) from naïve and immunized mice to generate myeloid, erythroid, and multilineage colonies in methylcellulose cultures (32). We cultured 100 Flt3−LSK BM cells from naïve and immunized (day 2) mice with SCF, IL-3, IL-6, and erythropoietin (32). If inflammation induces myeloid-committed progenitors to mimic the LSK phenotype, then the Flt3−LSK compartment would contain a lower frequency of cells capable of generating multilineage colonies [CFU-granulocyte/erythroblast/macrophage/megakaryocyte (GEMM)] but a higher frequency that would produce myeloid colonies [CFU-granulocyte/macrophage)GM)] compared to naïve controls. After 8 d in culture, both Flt3−LSK cell cohorts produced identical numbers of multilineage colonies (Fig. 3C). Furthermore, the frequency of myeloid colonies arising from Flt3−LSK cells of immunized mice was actually lower than naïve controls (Fig. 3C). Flt3−LSK cells from both naïve and immunized mice produced virtually no single lineage colonies (<1 granulocyte, macrophage, or erythroid colonies/100 Flt3−LSK) (Fig. 3C). These results demonstrate that alum does not enrich the Flt3−LSK compartment with myeloid-committed progenitors.
We also compared the lymphoid potential of Flt3−LSK cells from naïve and immunized mice by determining their capacity to generate B-lineage cells in OP9 stromal-cell cultures containing IL-7 and Flt3 ligand (23). The frequencies of Flt3−LSK cells from immunized (1/3.6) and naïve (1/4.6) mice that supported B-lineage development were virtually identical (Fig. 3D); the equivalent lineage potentials of both Flt3−LSK cohorts identify these cells as HSC.
Alum retains robust inflammatory and adjuvant properties in IL-1RI−/− mice
IL-1 is a potent pro-inflammatory cytokine and IL-1RI is expressed by many cell types (33), raising the possibility that the absence of alum-induced neutrophilias in IL-1RI deficient mice is not a specific effect but a general suppression of all inflammatory responses.
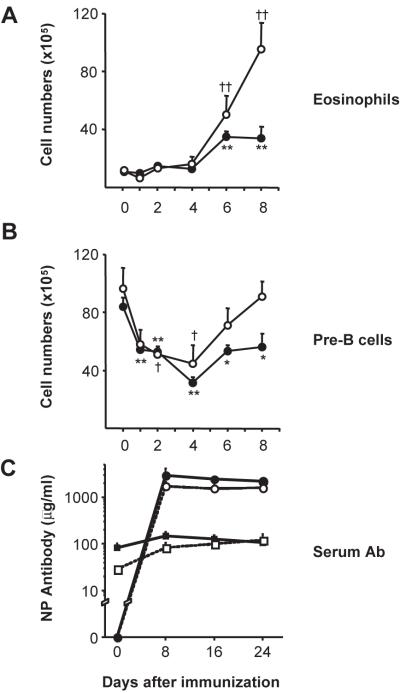
We believe this not to be the case. First, alum elicits eosinophilias in IL-1RI−/− mice (Fig. 1) that are equal to or greater than the eosinophilic responses of BL/6 mice (Fig. 4A). Second, alum mobilizes pre-B cells from the BM of BL/6 and IL-1RI−/− mice with equal efficiency (21). In immunized BL/6 and IL-1RI−/− mice, pre-B-cell numbers in BM fell >50% by day 4 (Fig. 4B). Recovery of these inflammatory losses was more rapid in IL-1RI−/− mice, perhaps due to the lack of competition for growth resources in the absence of expanded granulopoiesis (10) (Fig. 4B). Finally, IL-1RI−/− mice immunized with NP-CGG in alum mounted characteristic and robust serum Ab responses (Fig. 4C).
Fig. 4. Robust inflammatory responses in IL-1RI−/− mice.
BM cells of BL/6 mice and IL-1RI−/− mice were harvested after immunization (1–8 d) and labeled with mAbs to identify eosinophils (Supplemental Fig. 1) and pre-B cells [ref (10)]. Average numbers (± SEM) of eosinophils (A) and pre-B cells (B) from BL/6 (●) and IL-1RI−/− (○) mice are shown (n=3–11, each point). Significant differences from naïve controls are indicated for BL/6 (*, P ≤ 0.05; **, P ≤ 0.01) and IL-1RI−/− (†, P ≤ 0.05; ††, P ≤ 0.01) mice. (C) NP16-BSA binding λ+ and κ+ serum antibody was determined in BL/6 (closed symbols) and IL-1RI−/− (open) mice (n=2, each point) before and after immunization with 60 μg NP8-CGG/alum. NP-specific serum λ (circles) and κ (squares) antibodies were quantified (mean±SD) by ELISA.
Given that alum induces strong eosinophilic responses, mobilizes BM pre-B cells, and acts as a potent adjuvant in IL-1RI−/− mice, we conclude that the absence of inflammatory neutrophilias and monocytoses in IL-1RI−/− mice represents specific effects on neutrophils and monocytes.
IL-1RI−/− HSC, MPP, and GMP proliferate in immunized BL/6 hosts
Although LSK cells express IL-1RI and proliferate in response to IL-1β in vitro (Supplemental Fig. 3), IL-1 could modulate hematopoiesis indirectly (33). To determine if IL-1RI expression by HSC, MPP, and GMP is required for their alum-induced proliferation, we generated chimeric mice by reconstituting irradiated (C57BL/6 x C57BL/6.CD45.1)F1 mice with equal numbers of BM cells from C57BL/6.CD45.1 (WT) and IL-1RI−/− C57BL/6 (KO; CD45.2) donors. Donor and host hematopoietic cells were identified by CD45 allelism. We determined BrdU incorporation by donor HSC, MPP, and GMP 2 d after immunization, when IL-1RI-dependent proliferative defects are maximal (Fig. 2B). If IL-1RI expression by HSC, MPP, or GMP is required for their proliferation in response to alum, then WT progenitor cells should respond to immunization but KO cells should not.
We determined the frequencies of WT and KO hematopoietic cells in the blood of mixed and reciprocal chimeric mice four wks after reconstitution. All chimeras exhibited a modest excess of KO leukocytes (51 ± 6% KO vs. 40 ± 7% WT; P ≤0.05); all chimeras had similar numbers of blood leukocytes (data not shown).
In naïve chimeras, the frequencies of BrdU+ WT- and KO HSC, MPP, and GMP were equivalent but labeling of HSC and MPP was elevated compared to naive BL/6 and IL-1RI−/− controls (P ≤ 0.05; compare Figs. 2 and 5). Increased frequencies of BrdU+ HSC and MPP in chimeras may reflect ongoing hematopoietic replenishment. Immunization of chimeric mice equally increased (≈150%) BrdU labeling of WT- and KO HSC, MPP, and GMP (Fig. 5), and these increases matched those of BL/6 controls (Fig. 2). Robust proliferative responses by IL-1RI deficient hematopoietic cells demonstrates an intermediate signal(s) that determines HSC, MPP, and GMP proliferation. This intermediate, trans-acting signal could be produced by the IL-1RI+ hematopoietic compartment of mixed chimeras or, as IL-1RI is widely expressed (33), could come from radiation resistant host cells of hematopoietic or non-hematopoietic origin.
Fig. 5. Alum induces IL-1RI deficient HSC, MPP, and GMP to proliferate in chimeric mice.
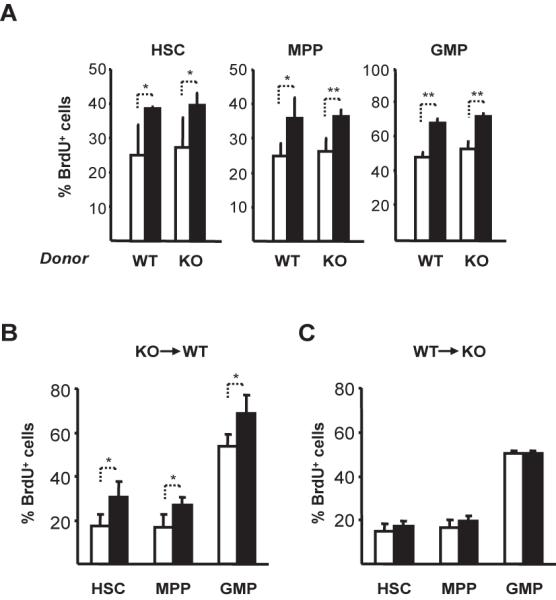
(A) Irradiated (C57BL/6 x C57BL/6.CD45.1)F1 mice were reconstituted with equal numbers (5 × 106) of C57BL/6.CD45.1 (WT/CD45.2) and IL-1RI−/− (KO/CD45.2) BM cells. After (4 wks), chimeras were immunized with NP8-CGG/alum and 2 d later, injected i.p. with 1 mg BrdU. Six h after BrdU injection, BM cells were harvested and the frequencies of BrdU+ HSC, MPP, and GMP (Supplemental Fig. 2) determined by flow cytometry. Average frequencies (±SD) of BrdU+ WT-(CD45.1) and KO (CD45.2) HSC, MPP, and GMP from naïve (open symbols; n=4) and immunized (closed; n=3) mice are shown. To generate reciprocal chimeras, (B) irradiated (C57BL/6 x C57BL/6.CD45.1)F1 mice were reconstituted with KO (C57BL/6.IL-1RI−/−) BM cells (KO→WT) or (C) irradiated C57BL/6 IL-1RI−/− mice were reconstituted with (C57BL/6 x C57BL/6.CD45.1)F1 BM cells (WT→KO). Four wks later, chimeric mice were immunized and analyzed as for (A). Average (±SD) frequencies of BrdU+ HSC, MPP, and GMP from naïve (open; n=4) and immunized (closed; n=4) mice are shown. Significant differences between groups are indicated: *, P ≤ 0.05; **, P ≤ 0.01.
To determine the origin of this proliferation signal, we generated KO→WT and WT→KO BM chimeras. Four weeks after reconstitution, ~60% of blood leukocytes were derived from donor cells in both chimera types. Chimeric mice were immunized and the frequencies of BrdU+ HSC, MPP, and GMP were measured 2 d later. In KO→WT chimeras, immunization significantly increased BrdU labeling of progenitors compared to naïve chimera controls (Fig. 5B). In contrast, the frequencies of BrdU+ HSC, MPP, and GMP did not increase after immunization of WT→KO chimeras (Fig. 5C). We conclude that it is IL-1RI expression/activity in a radiation-resistant, host compartment(s) that determines the reactive proliferation of HSC, MPP, and GMP.
Discussion
Infections elicit neutrophilias that are sustained by a distinct, emergency granulopoietic program that is activated by ill defined intrinsic signals (6). We previously noted that reactive neutrophilias induced by alum (Fig. 1) are mimicked by the co-administration of TNFα and IL-1β (10, 21). More recently, alum has been shown to be a potent inducer of IL-1β secretion by activation of the Nalp3 inflammasome (34). Taken together, these observations suggested that IL-1β might be the intrinsic activating signal for emergency granulopoiesis.
Whereas myeloid cell numbers in naïve IL-1RI−/− and C57BL/6 mice are indistinguishable (Figs. 1, 2), alum/antigen elicited neither the pronounced reactive neutrophilias and inflammatory monocytic responses nor the emergency granulopoiesis in IL-1RI−/− mice (Fig. 2) that characterized C57BL/6 controls. To our knowledge, this is the first demonstration of a single, indispensable cytokine/receptor pathway for emergency granulopoiesis (5, 20). A modest increase in the number of primitive neutrophils was observed in the BM of IL-1RI−/− mice 8 days after immunization (Fig. 2A). In contrast to the rapid induction of eosinophilia and mobilization of BM lymphocytes (Fig. 4), this late neutrophilic response comes well after reactive neutrophilias of congenic controls, and may represent another (chronic?) pathway for increased neutrophil production.
Alum, a common vaccine adjuvant, promotes inflammation and immunogenicity via the Nalp3 inflammasome and the production of the proinflammatory cytokines IL-1β, IL-18, and IL-33 (34, 35). Nonetheless, abrogation of neutrophilia and emergency granulopoiesis in IL-1RI−/− mice was a specific defect, as other inflammatory responses, reactive eosinophilia (Figs. 1, 4A), mobilization of BM pre-B cells (Fig. 4B), and robust antibody production (Fig. 4C), remained intact. IL-3 and IL-5 promote eosinophilia (36) and mobilization of BM B-lineage cells depends on TNFα (21). Thus, other alum-induced inflammatory responses are fully active in IL-1RI−/− mice.
Emergency granulopoiesis is generally thought to represent increased GMP proliferation (6), and we observed significant increases in GMP numbers 1- and 2 d after immunization accompanied by increased BrdU uptake (Fig. 2). In addition, however, we found that in C57BL/6 mice, alum simultaneously induced proliferation by HSC and MPP (Fig. 2); HSC and MPP are themselves the immediate targets of inflammatory signals.
In contrast to control mice, alum elicited no significant increases in HSC, MPP, and GMP numbers in IL-1RI−/− mice (Fig. 2A) and significantly attenuated proliferative responses by these compartments (Fig. 2B). The lack of increases in HSC, MPP, and GMP numbers in IL-1RI−/− mice after immunization (Fig. 2A), despite modest but significant increases in proliferation (Fig. 2B), raises the possibility that IL-1RI-dependent signals act not only to sustain high levels of progenitor proliferation but also to promote progenitor survival. These observations indicate an unexpected role for HSC and MPP proliferation in the induction of emergency granulopoiesis.
Curiously, proliferation and expansion by the HSC and MPP compartments in immunized C57BL/6 mice are associated with increased numbers of GMP but not CMP (Fig. 2A). If GMP arise from CMP, why do we not detect an expansion in this compartment as well? One possibility is that inflammation accelerates CMP differentiation, such that the increased CMP production by MPP is obscured by a very rapid CMP to GMP differentiation. Alternatively, inflammation may cause some fraction of MPP to differentiate into GMP directly, a phenomenon suggested by Adolfsson et al. (8). The latter hypothesis is consistent with our failure to observe increased numbers of MEP and CLP after alum immunization (data not shown). Inflammatory signals elicited by alum appear to accelerate myelopoiesis specifically [(10, 21), Figs. 2, 4A, 4B]. Given that immunization did not alter the lineage potential of Flt3−LSK HSC (Figs. 3C, D), this hematopoietic specialization likely represents alteration of external cues that influence hematopoietic lineage decisions (10, 21).
Although HSC and MPP express IL-1RI and proliferate in response to IL-1 in vitro (Supplemental Fig. 3), IL-1RI expression by HSC, MPP, and GMP is not required for their proliferation in response to alum (Fig. 5). In mixed chimeras, proliferation by IL-1RI−/− HSC, MPP, and GMP was identical to that of IL-1RI-bearing cells (Fig. 5). IL-1/IL-1RI signals are, therefore, necessary for the induction of reactive neutrophilias and emergency granulopoiesis by alum, but they must act indirectly to drive proliferation in the HSC, MPP, and GMP compartments.
IL-1 induces the production of many growth factors and inflammatory mediators (33); among these, IL-3, IL-6, G-CSF, and GM-CSF have been implicated as agents of emergency granulopoiesis (6, 12). However, these cytokines are dispensable for robust granulopoietic responses to inflammation (5, 20). In consequence, these dispensable cytokines can not be the intermediate cues that drive IL-1RI dependent emergency granulopoiesis in alum immunized mice. Given that HSC, MPP, and GMP appear to be the immediate targets of alum-induced, IL-1RI dependent proliferation (Fig. 2), IL-1RI signals may act through intermediate hematopoietic factors that induce proliferation by both HSC and committed progenitors, such as IL-11 (37, 38) or thrombopoietin (39).
Alternatively, the emergency granulopoiesis induced by alum could be driven by a density-dependent feedback between BM neutrophils and their progenitors, i.e., BM neutrophils suppress the proliferation and differentiation of hematopoietic progenitor cells. Inflammatory mobilization of mature neutrophils from the BM would relax this suppression, activating HSC, MPP, and GMP proliferation. A similar hypothesis was offered previously to explain the stability of steady-state neutrophil pools (40). If so, abrogation of emergency granulopoiesis in IL-1RI−/− mice could be the result of defective mobilization by BM neutrophils and the continuation of suppression (Fig. 2A). Consistent with this feedback hypothesis, alum neither mobilized BM neutrophils (data not shown) nor induced HSC, MPP, and GMP proliferation in WT→KO chimeras (Fig. 5C). In contrast, all chimeras generated in IL-1RI-sufficient hosts (WT+KO→WT, KO→WT, Fig. 5A, B) exhibited robust neutrophil mobilization (data not shown) and progenitor proliferation after immunization with alum.
The linkage of neutrophil mobilization and progenitor proliferation points out a caveat to interpreting our and earlier studies of emergency granulopoiesis. All previous reports relied solely on blood neutrophil counts to define emergency granulopoiesis (5, 6); our observations highlight an intrinsic limitation to this type of analysis, as it fails to discriminate between mobilization and emergency granulopoietic output.
Earlier, we proposed that developing neutrophils and B cells compete for specific resources in the BM such that the expansion of one lineage results in a compensatory diminution of the other (10). Alum mobilizes B-lineage cells from the BM and affects a central lymphopenia that coincides with the period of emergency granulopoiesis (10). In IL-1RI−/− mice, alum depleted the BM of pre-B cells but the recovery of this compartment was significantly faster than in control mice (Fig. 4B). As alum does not expand granulopoiesis in IL-1RI−/− mice, our competition model predicts that vacated niches/resources normally occupied by GMP and primitive neutrophils might be available to accelerate the restoration of the BM’s B-lineage compartments.
The increased availability of developmental niches in the BM of immunized IL-1RI−/− mice may also explain the enhanced production of eosinophils compared to immunized C57BL/6 mice (days 6 and 8; Fig. 4A). On the other hand, neutrophils likely mitigate alum-induced inflammation, and impaired neutrophilic responses in immunized IL-1RI−/− mice may prolong inflammatory responses and enhance eosinophilias. That IL-1RI−/− mice are capable of mounting inflammatory eosinophilias despite attenuated proliferation of HSC, MPP, and GMP implies that a more differentiated progenitor responds to inflammatory signals that increase eosinophil production. This observation is consistent with the work of Iwasaki and colleagues, who showed that eosinophilias elicited by helminth infections are supported by expansions of a committed eosinophil progenitor compartment but not CMP or GMP (41).
The requirement for IL-1RI in alum-induced neutrophilias and emergency granulopoiesis emphasizes the central role of IL-1 in innate immune responses (27, 42, 43). Alum drives IL-1β and IL-18 secretion by activating the Nalp3 inflammasome (27, 34, 35) and these cytokines have been proposed to be the agents of alum’s several inflammatory properties including adjuvanticity (34). Instead, our work indicates that the effects of secreted IL-1β may be focused on neutrophilic responses, as alum-induced eosinophilia, pre-B cell mobilization, and antibody responses remain intact in IL-1RI−/− mice (Fig. 4). Indeed, our findings are consistent with the seemingly paradoxical findings by Eisenbarth et al. (34), showing that the alum’s adjuvanticity is abrogated in mice defective for components of the Nalp3 inflammasome, but intact in animals lacking MyD88, a crucial signaling component of the IL-1 and IL-18 receptors (44). Thus, alum must stimulate humoral responses independently of the cytokines currently associated with the Nalp3 inflammasome.
Supplementary Material
Acknowledgements
We thank T. M. Holl, D. Liao, and A. Patel for assistance.
Footnotes
- BL/6
- C57BL/6
- CGG
- chicken γ-globulin
- CLP
- common lymphoid progenitor
- CMP
- common myeloid progenitor
- d
- day
- GM-CSFR
- granulocyte/macrophage colony stimulating factor receptor
- GMP
- granulocyte/macrophage progenitor
- HSC
- hematopoietic stem cell
- IL-1RI
- interleukin-1 receptor I
- LT
- long-term
- MEP
- megakaryocyte/erythroid progenitor
- MPP
- multipotent progenitor
- NP
- (4-hydroxy-3-nitrophenyl)acetyl
- SA
- streptavidin
- SCF
- stem cell factor
- ST
- short-term
References
- 1.Engle WD, Rosenfeld CR. Neutropenia in high-risk neonates. J Pediatr. 1984;105:982–986. doi: 10.1016/s0022-3476(84)80095-5. [DOI] [PubMed] [Google Scholar]
- 2.Gessler P, Luders R, Konig S, Haas N, Lasch P, Kachel W. Neonatal neutropenia in low birthweight premature infants. Am J Perinatol. 1995;12:34–38. doi: 10.1055/s-2007-994396. [DOI] [PubMed] [Google Scholar]
- 3.Quie PG. The phagocytic system in host defense. Scand J Infect Dis Suppl. 1980;(Suppl 24):30–32. [PubMed] [Google Scholar]
- 4.Burg ND, Pillinger MH. The neutrophil: function and regulation in innate and humoral immunity. Clin Immunol. 2001;99:7–17. doi: 10.1006/clim.2001.5007. [DOI] [PubMed] [Google Scholar]
- 5.Basu S, Hodgson G, Zhang HH, Katz M, Quilici C, Dunn AR. “Emergency” granulopoiesis in G-CSF-deficient mice in response to Candida albicans infection. Blood. 2000;95:3725–3733. [PubMed] [Google Scholar]
- 6.Hirai H, Zhang P, Dayaram T, Hetherington CJ, Mizuno S, Imanishi J, Akashi K, Tenen DG. C/EBPbeta is required for ‘emergency’ granulopoiesis. Nat Immunol. 2006;7:732–739. doi: 10.1038/ni1354. [DOI] [PubMed] [Google Scholar]
- 7.Yang L, Bryder D, Adolfsson J, Nygren J, Mansson R, Sigvardsson M, Jacobsen SE. Identification of Lin(−)Sca1(+)kit(+)CD34(+)Flt3- short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105:2717–2723. doi: 10.1182/blood-2004-06-2159. [DOI] [PubMed] [Google Scholar]
- 8.Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SE. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 10.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang DE, Zhang P, Wang ND, Hetherington CJ, Darlington GJ, Tenen DG. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice. Proc Natl Acad Sci U S A. 1997;94:569–574. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker F, Zhang HH, Matthews V, Weinstock J, Nice EC, Ernst M, Rose-John S, Burgess AW. IL6/sIL6R complex contributes to emergency granulopoietic responses in G-CSF- and GM-CSF-deficient mice. Blood. 2008;111:3978–3985. doi: 10.1182/blood-2007-10-119636. [DOI] [PubMed] [Google Scholar]
- 13.Cheers C, Haigh AM, Kelso A, Metcalf D, Stanley ER, Young AM. Production of colony-stimulating factors (CSFs) during infection: separate determinations of macrophage-, granulocyte-, granulocyte-macrophage-, and multi-CSFs. Infect Immun. 1988;56:247–251. doi: 10.1128/iai.56.1.247-251.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metcalf D. In vitro cloning of hemopoietic cells. Bull Cancer. 1978;65:417–419. [PubMed] [Google Scholar]
- 15.Koike K, Stanley ER, Ihle JN, Ogawa M. Macrophage colony formation supported by purified CSF-1 and/or interleukin 3 in serum-free culture: evidence for hierarchical difference in macrophage colony-forming cells. Blood. 1986;67:859–864. [PubMed] [Google Scholar]
- 16.Souza LM, Boone TC, Gabrilove J, Lai PH, Zsebo KM, Murdock DC, Chazin VR, Bruszewski J, Lu H, Chen KK, et al. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986;232:61–65. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- 17.Donahue RE, Seehra J, Metzger M, Lefebvre D, Rock B, Carbone S, Nathan DG, Garnick M, Sehgal PK, Laston D, et al. Human IL-3 and GM-CSF act synergistically in stimulating hematopoiesis in primates. Science. 1988;241:1820–1823. doi: 10.1126/science.3051378. [DOI] [PubMed] [Google Scholar]
- 18.Caracciolo D, Clark SC, Rovera G. Human interleukin-6 supports granulocytic differentiation of hematopoietic progenitor cells and acts synergistically with GM-CSF. Blood. 1989;73:666–670. [PubMed] [Google Scholar]
- 19.Hibbs ML, Quilici C, Kountouri N, Seymour JF, Armes JE, Burgess AW, Dunn AR. Mice lacking three myeloid colony-stimulating factors (G-CSF, GM-CSF, and M-CSF) still produce macrophages and granulocytes and mount an inflammatory response in a sterile model of peritonitis. J Immunol. 2007;178:6435–6443. doi: 10.4049/jimmunol.178.10.6435. [DOI] [PubMed] [Google Scholar]
- 20.Nishinakamura R, Miyajima A, Mee PJ, Tybulewicz VL, Murray R. Hematopoiesis in mice lacking the entire granulocyte-macrophage colony-stimulating factor/interleukin-3/interleukin-5 functions. Blood. 1996;88:2458–2464. [PubMed] [Google Scholar]
- 21.Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. Inflammation Controls B Lymphopoiesis by Regulating Chemokine CXCL12 Expression. J Exp Med. 2004;199:47–58. doi: 10.1084/jem.20031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glaccum MB, Stocking KL, Charrier K, Smith JL, Willis CR, Maliszewski C, Livingston DJ, Peschon JJ, Morrissey PJ. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol. 1997;159:3364–3371. [PubMed] [Google Scholar]
- 23.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 24.Domen J, Weissman IL. Hematopoietic stem cells need two signals to prevent apoptosis; BCL-2 can provide one of these, Kitl/c-Kit signaling the other. J Exp Med. 2000;192:1707–1718. doi: 10.1084/jem.192.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z, Koralov SB, Gendelman M, Carroll MC, Kelsoe G. Humoral immune responses in Cr2−/− mice: enhanced affinity maturation but impaired antibody persistence. J Immunol. 2000;164:4522–4532. doi: 10.4049/jimmunol.164.9.4522. [DOI] [PubMed] [Google Scholar]
- 26.Congdon CC, Gengozian N, Makinodan T. Agglutinin production in normal, sublethally irradiated, and lethally irradiated mice treated with mouse bone marrow. J Immunol. 1956;77:250–256. [PubMed] [Google Scholar]
- 27.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henderson RB, Hobbs JA, Mathies M, Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood. 2003;102:328–335. doi: 10.1182/blood-2002-10-3228. [DOI] [PubMed] [Google Scholar]
- 29.Labow M, Shuster D, Zetterstrom M, Nunes P, Terry R, Cullinan EB, Bartfai T, Solorzano C, Moldawer LL, Chizzonite R, McIntyre KW. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J Immunol. 1997;159:2452–2461. [PubMed] [Google Scholar]
- 30.Schittek B, Rajewsky K, Forster I. Dividing cells in bone marrow and spleen incorporate bromodeoxyuridine with high efficiency. Eur J Immunol. 1991;21:235–238. doi: 10.1002/eji.1830210136. [DOI] [PubMed] [Google Scholar]
- 31.Zhang P, Nelson S, Bagby GJ, Siggins R, 2nd, Shellito JE, Welsh DA. Lineage-c-kit+Sca-1+ Cell Response to Escherichia Coli Bacteremia in Balb/c Mice. Stem Cells. 2008 doi: 10.1634/stemcells.2007-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ling KW, Ottersbach K, van Hamburg JP, Oziemlak A, Tsai FY, Orkin SH, Ploemacher R, Hendriks RW, Dzierzak E. GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J Exp Med. 2004;200:871–882. doi: 10.1084/jem.20031556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 34.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008 doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Nookala S, Re F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J Immunol. 2007;178:5271–5276. doi: 10.4049/jimmunol.178.8.5271. [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi Y, Suda T, Suda J, Eguchi M, Miura Y, Harada N, Tominaga A, Takatsu K. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J Exp Med. 1988;167:43–56. doi: 10.1084/jem.167.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hangoc G, Yin T, Cooper S, Schendel P, Yang YC, Broxmeyer HE. In vivo effects of recombinant interleukin-11 on myelopoiesis in mice. Blood. 1993;81:965–972. [PubMed] [Google Scholar]
- 38.Lemoli RM, Fogli M, Fortuna A, Motta MR, Rizzi S, Benini C, Tura S. Interleukin-11 stimulates the proliferation of human hematopoietic CD34+ and CD34+CD33-DR- cells and synergizes with stem cell factor, interleukin-3, and granulocyte-macrophage colony-stimulating factor. Exp Hematol. 1993;21:1668–1672. [PubMed] [Google Scholar]
- 39.Young JC, Bruno E, Luens KM, Wu S, Backer M, Murray LJ. Thrombopoietin stimulates megakaryocytopoiesis, myelopoiesis, and expansion of CD34+ progenitor cells from single CD34+Thy-1+Lin- primitive progenitor cells. Blood. 1996;88:1619–1631. [PubMed] [Google Scholar]
- 40.Layton JE, Hockman H, Sheridan WP, Morstyn G. Evidence for a novel in vivo control mechanism of granulopoiesis: mature cell-related control of a regulatory growth factor. Blood. 1989;74:1303–1307. [PubMed] [Google Scholar]
- 41.Iwasaki H, Mizuno S, Mayfield R, Shigematsu H, Arinobu Y, Seed B, Gurish MF, Takatsu K, Akashi K. Identification of eosinophil lineage-committed progenitors in the murine bone marrow. J Exp Med. 2005;201:1891–1897. doi: 10.1084/jem.20050548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 43.Chen CJ, Shi Y, Hearn A, Fitzgerald K, Golenbock D, Reed G, Akira S, Rock KL. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest. 2006;116:2262–2271. doi: 10.1172/JCI28075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.