Abstract
Background
The mechanisms responsible for resistant or recurrent disease in childhood non-Hodgkin lymphoma (NHL) are not yet fully understood. A unique mechanism suggesting the role of the mitochondria as the key energy source responsible for residual cells has been assessed in the clinical setting on specimens from patients on therapy were found to have increased copies of mitochondrial DNA (mtDNA) associated with positive minimal residual disease and/or persistent disease (MRD/PD) status. The potential role of mtDNA in MRD/PD emphasizes queries into the contributions of relevant enzymatic pathways responsible for MRD/PD. This study hypothesized that in an in-vitro model, recovering or residual cells from chemotoxicity will exhibit an increase in both citrate synthase and isocitrate dehydrogenase expression and decrease in succinate dehydrogenase expression.
Procedure
Ramos cells (Burkitt lymphoma cell line) were exposed to varying concentrations of doxorubicin and vincristine for 1 hr; and allowing for recovery in culture over a 7-day period. cDNA was extracted on days 1 and 7 of the cell culture period to assess the relative expression of the aforementioned genes.
Results
Increase citrate synthase, increase isocitrate dehydrogenase and decrease succinate dehydrogenase expressions were found in recovering Ramos cells.
Conclusion
Recovering lymphoma cells appear to compensate by regulating enzymatic levels of appropriate genes in the Krebs Cycle suggesting an important role of the mitochondria in the presence of residual cells.
Introduction
Following the diagnosis and treatment of cancer, the probability or likelihood of relapse remains a lingering concern. Identifying patients at risk for relapse continues to be a challenge and in some diseases such as childhood acute lymphoblastic leukemia, presence of minimal residual disease/persistent disease (MRD/PD) at the end of therapy is a strong predictor for recurrent disease [1]. Thus, the significance of identifying patients with MRD/PD lies in possibly improving prognosis and disease free survival if appropriate interventions can be achieved [2].
While mechanisms hypothesizing how MRD/PD develops are diverse, the underlying principle remains the same: MRD/PD is based on residual cells ability to withstand and survive toxicity from chemotherapy [1]. Thus, one possible mechanism suggests that these residual cells could have recovered or survived as a result of a compensatory increase in energy metabolism through the mitochondria. We posit that the mitochondria may be important in establishing MRD/PD in some clinical situations. The human mitochondrial DNA (mtDNA) is a 16.6 kb circular-double stranded DNA molecule. When physiological conditions are modified, mtDNA copy number can be altered by the mitochondrial matrix to address the energy needs of the cell. Moreover, mtDNA copy number changes during cell growth, differentiation and after hormonal treatment or exercise [3]. It has been previously demonstrated that there is an increase in mtDNA copy number from thyroid tumor-derived cells when compared to corresponding normal controls [4]. It has also been demonstrated that there is an increase in mtDNA copy number with corresponding endometrial cancer development [5]. Taken together, these results suggest that the observed increases in mtDNA copy number may be a means in which malignant cells persist.
We recently reported that specimens from patients with non-Hodgkin lymphoma identified as MRD/PD-positive have increased mtDNA copy number/cell compared to controls and specimens that are MRD/PD-negative [6]. We further demonstrated that in a B-cell lymphoma culture model, surviving cells after chemotherapy exposure had higher mtDNA copy number [6]. The data suggested that increased copies of mtDNA per cell were found in tumor cells and residual or recovering cells. Mechanisms leading to increase in mtDNA copy number/cell, however, remain unclear. Because mitochondria are key players in ATP-related gene regulation, the current study was designed to investigate the role of relevant ATP-associated enzymes (citrate synthase, isocitrate dehydrogenase and succinate dehydrogenase) in order to further delineate the role of the mitochondria.
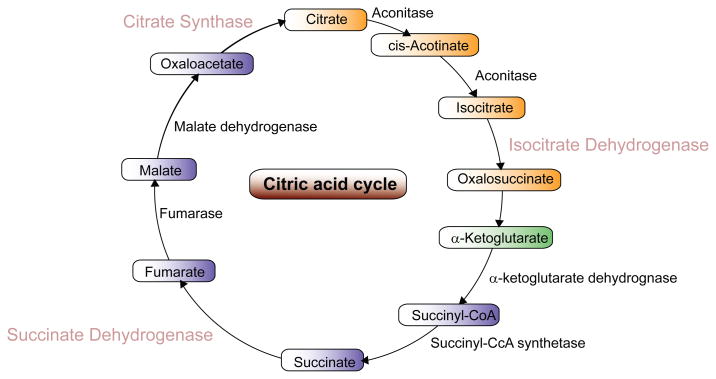
Citrate synthase is the pace-making enzyme involved in the first step of the Krebs cycle that is localized within the mitochondrial matrix and is synthesized by cytoplasmic ribosomes (Fig. 1). Because of its role in converting oxaloacetate to citrate, citrate synthase is both an indicator of ATP synthesis and mitochondrial content; specifically mtDNA [7]. As well, mitochondria proliferation is typically associated with an increase in citrate synthase expression and has also been utilized as an indicator of aerobic capacity and mitochondrial density [8].
Figure 1.
Schematic diagram of the Krebs cycle illustrating major components and enzymes. Citrate synthase is the primary enzyme converting oxaloacetate to citrate in the initial step of ATP synthesis.
Like citrate synthase, isocitrate dehydrogenase expression can also indicate mitochondrial resilience. Isocitrate dehydrogenase, specifically the mitochondrial NADP+-dependent enzyme class (IDPm), has been associated with the mitochondria’s response to oxidative stress by altering levels of mitochondrial NADPH [9]. It has been demonstrated that cell viability directly correlates with expression levels of IDPm after initial exposure to hydrogen peroxide [10]. Thus increased expression of IDPm under stressful conditions points to a compensatory response where IDPm activity can be utilized as a tool to assess the intracellular response to stressful factors acting on the cell.
Succinate dehydrogenase catalyzes the oxidation of succinate to fumarate in the Krebs cycle as well as feeds electrons to the respiratory chain. It has been observed that in the presence of ATP there is a decrease in electron flow signalling a decrease in succinate dehydrogenase expression suggesting an inverse relationship with ATP production [11]. As well it has also been observed that a complete lack of succinate dehydrogenase expression hampers electron flow, ultimately resulting in major oxidative stress known to promote tumor formation [12]. Moreover if normal succinate dehydrogenase activity resumed, there was a concurrent dissolution of the tumor [13]. Thus, the relative expression of succinate dehydrogenase can be utilized as a marker of oxidative damage with increased expression corresponding with a decrease in reactive oxygen species accumulation.
Assessing expression of these enzymes from residual cells could provide insight into how malignant cells are able to recover from chemotoxicity. We hypothesize that citrate synthase and isocitrate dehydrogenase expression levels will be higher and succinate dehydrogenase will be lower in the residual cells that survive the toxic effects of chemotherapy agents.
Materials and Methods
Cell lines and chemotherapy exposure
Ramos cells, a human Burkitt lymphoma (BL) cell line, were obtained from American Type Culture Collection (Manassas, VA) and maintained in RPMI 1640 1X (Mediatech Incorporated; Herndon, VA) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO), 2% sodium bicarbonate, 1% glucose, 1% sodium pyruvate, 1% HEPES, and 1% penicillin/streptomycin at 37 °C in a humidified atmosphere containing 5% CO2. Cells were exposed to vincristine sulfate (MP Biomedicals; Solon, OH) in concentrations of 3 and 6 nM; and doxorubicin-HCL (Biomol Internationals; Plymouth Meeting, PA) in concentrations of 100 and 500 nM; diluted in culture media. Drug concentrations used in the cultures were similar to clinically relevant plasma levels [14]. Prior to drug exposure, Ramos cells were centrifuged at 1,200 RPM for 10 minutes with the supernatant decanted. Cells were then treated with 10 ml Accumax (Innovate Cell Technologies; San Diego, CA) and incubated for 10 min at 37 °C. Cells (1.33 × 106 cells/ml) were then suspended in 1 mL of Ramos culture media containing 250 μL of the specific chemotherapy agent; in duplicate. After 1 hr drug exposure, cells were washed twice with fresh media; and resuspended in 1 mL of fresh media (5 × 105 cells/mL) and remained in culture for 7 days. Cell viability was assessed daily by Trypan blue exclusion method with cell viability being maintained between 72% and 97% over the course of the culture period, Figure 2A. Non-viable cells were removed from the culture on days 2, 4 and 6 by centrifugation at 500 RPM for 7 min and the remaining viable cells were resuspended in fresh media. Aliquots of the culture were obtained on days 1 and 7 to assess gene expression.
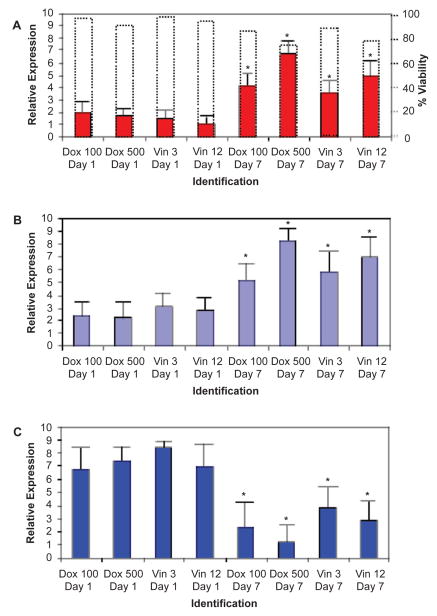
Figure 2.
Gene expression following exposure to either doxorubicin or vincristine on days 1 and 7 of cell culture. Drug exposures were performed in duplicate for both doxorubicin (100 and 500 nM) and vincristine (3 nM and 12 nM). A) Citrate synthase gene expression in Ramos cells on days 1 and 7 of cell culture following either doxorubicin or vincristine one-hour exposure. Respective dotted boxes represent percentage of viable cells recovered at each time points donated on the right Y-axis, ranging from 72%–97%. B) Isocitrate dehydrogenase expression in Ramos cells on days 1 and 7 of cell culture following either doxorubicin or vincristine one-hour exposure. C) Succinate dehydrogeanse expression in Ramos cells on days 1 and 7 of cell culture following either doxorubicin or vincristine one-hour exposure. Pair-Sample Student’s t-test used to assess significance. Error bars denote standard deviation. Colored bars indicated average of triplicate measurements. *indicates significance at the p < .05 level.
Nucleic acid extraction
RNA was extracted from aliquots of Ramos cells from days 1 and 7 as per the RNeasy Mini Protocol: Isolation of Total RNA from Animal Cells (Qiagen; Valencia, CA) with no additional sample preparations. This was followed by reverse transcription using Transcriptor First Strand cDNA Synthesis Kit: Standard RT-PCR Procedure (Roche Applied Science; Mannheim, Germany) with no additional sample preparation. Quality of cDNA was assessed by analysis of the 260/280 absorbance ratio using the ND-1000 Spectrophotometer (NanoDrop Technologies; Wilmington, DE) with an average total cDNA yield of approximately 50 ng.
Gene expression assessment
cDNA samples were assayed for citrate synthase, isocitrate dehydrogenase and succinate dehydrogenase gene expression using real-time PCR with a StepOne Plus System. (Applied Biosystems; Foster City, CA). Primers were constructed using Primer Express Software Version 2.0 (Applied Biosystems; Foster City, CA) The citrate synthase primers (forward: TCA CGA GCA TTG GGT GTA CTG; reverse: GAC CCT CTG TGC TCA TGG ACT T) amplified a region in the citrate synthase gene with a product of 185 base pairs. The isocitrate dehydrogenase primers (forward: GGT GGA GAG TGG AGC CAT CA; reverse: ACA TTG CTG AGG CCG TGA AT) amplified a region in the isocitrate dehydrogenase gene with a product of 208 base pairs. The succinate dehydrogenase primers (forward: CCC TGG ATG GGC TTG GA; reverse: CGC ATG CCA GGG AAG ACT AC) amplified a region in the succinate dehydrogenase gene with a product of 167 base pairs. The beta actin primers (forward: CCT GTA CGC CAA CAC AGT GC; reverse: ATA CTC CTG CTT GCT GAT CC) amplified a region in the beta actin gene with a product of 211 base pairs. Citrate synthase, isocitrate dehydrogenase and succinate dehydrogenase gene expression were quantified relative to beta actin, respectively. For the PCR setup, samples were run in triplicate with each reaction containing 2x iQ SYBR Green Supermix, 10 pmol forward primer, 10 pmol reverse primer in a final volume of 25 μL. The PCR cycling parameters were: 10-minute hot start at 95 °C; 35 cycles of 95 °C for 10 sec; 60 °C for 30 sec; 72 °C for 3 min; 95 °C for 15 sec; and 55 °C for 15 sec. At the conclusion of the PCR, melt curve data were collected by the StepOne Plus Software using a start temperature of 80 °C, with 0.5° incremental increase every 5 sec with a final temperature of 90 °C.
Statistical analyses
Paired two sample student t-test was used to assess statistical significance in gene expression over the culture period with the average of triplicate PCR measurements used in the analysis.
Results
The aim of this project was to examine expression of citrate synthase, isocitrate dehydrogenase and succinate dehydrogenase in Ramos cells after exposure to various concentrations of doxorubicin and vincristine. Gene expressions were assessed relative to the expression level of the reference/housekeeping gene (beta-actin). Following 1-hour exposure to the chemotherapy agents, Ramos cells recovered from the acute toxicity and respective gene expression from the recovered cells was assessed. As expected, for both doxorubicin-and vincristine-exposed Ramos cells, there was a statistically significant increase in citrate synthase and isocitrate dehydrogenase expression with increasing days in culture and increasing chemotherapy drug concentration (Fig. 2A, 2B). Conversely, there was also a concurrent statistically significant decrease in succinate dehydrogenase expression in both doxorubicin- and vincristine-exposed Ramos cells with increasing days in culture and increasing chemotherapy drug concentration (Fig. 2C). The most pronounced increases in citrate synthase and isocitrate dehydrogenase expressions were observed in cells recovered on the seventh day after the initial 1-hour exposure of the highest concentrations of doxorubicin and vincristine. Likewise, the most dramatic decrease in succinate dehydrogenase expression was observed in cells recovered on the seventh day after the 1-hour exposure to the highest concentrations of doxorubicin and vincristine (Fig. 2). Thus, the concomitant increases in citrate synthase and isocitrate dehydrogenase expressions and inverse decrease in succinate dehydrogenase expression are linked to both the number of days following chemotherapy exposure and concentration of chemotherapy agent.
Discussion
The aim of this study was to assess the expression of citrate synthase, isocitrate dehydrogenase and succinate dehydrogenase in residual cells that survived the toxic effects of chemotherapy agents. Previous studies showed that these surviving cells have higher mtDNA copies per cell compared to controls [6]. Our current study further demonstrated that Ramos cells recovering from chemotherapy had increase in both citrate synthase and isocitrate dehydrogenase expressions as well as decrease in succinate dehydrogenase expression with the most pronounced changes in the cells treated with the highest concentrations of chemotherapy agents. The data are consistent with mitochondrial involvement, specifically synthesis of ATP and expression of antioxidant pathways, in establishing a survival phenotype for residual malignant cells recovering from chemotherapy treatment.
Previous work demonstrated that chemotherapy agents have a direct inhibiting effect on ATP synthesis within the mitochondrial matrix [15]. By assessing the relative gene expression of citrate synthase, the mechanism by which this effect occurs can be elucidated. It was found that there was lower citrate synthase expression on Day 1 following the initial chemotherapy exposure suggesting that the chemotherapy agents had a quick and potent effect on ATP synthesis and mitochondrial functioning. After the residual cells were allowed to recover, though, there was an observed increase in citrate synthase expression and likewise ATP synthesis suggesting that these cells exhibited a survival phenotype.
Additionally, immediately following chemotherapy exposure, the neoplastic cells treated with the highest concentration of chemotherapy agents had lower expression of citrate synthase when compared to those cells treated with the lowest concentration of doxorubicin and vincristine, respectively. As the surviving residual cells were allowed to recover over the course of the one-week period, however, citrate expression was highest in cells exposed to the highest concentration of chemotherapy agents. This suggested that the initial chemotherapy exposure either killed the weaker neoplastic cells or cellular resistance developed after exposure to a higher concentration of chemotherapy agents. Therefore, we posit that the higher concentration chemotherapy agents initially eradicated a higher percentage of neoplastic cells but resulted in surviving cells that may be more difficult to eliminate.
It was found that isocitrate dehydrogenase expression paralleled citrate synthase expression in neoplastic cells treated with chemotherapy agents. That is, initially following chemotherapy treatment residual neoplastic cells expressed low levels of isocitrate dehydrogenase most likely due to the chemotherapy administration. As the cells were allowed to recover, there is a marked increase in the expression of isocitrate dehydrogenase. One possible explanation for this observation could be the residual cells response to the toxic ROS effects of chemotoxicity which ultimately leads to a survival phenotype possibly via a compensatory mechanism [6].
Immediately following chemotherapy exposure the neoplastic cells treated with the highest concentration of chemotherapy agents had lower expression of isocitrate dehydrogenase when compared to those cells treated with lowest concentrations of vincristine and doxorubicin, respectively. As the surviving residual cells were allowed to recover over the course of the one-week period, however, isocitrate dehydrogenase expression was highest in cells exposed to the highest concentration of chemotherapy agents. This suggested that even though the initial chemotherapy treatment may have reduced ATP synthesis as measured by lowered citrate synthase activity, it did not result in the accumulation of reactive oxygen species as isocitrate dehydrogenase levels were also lowered. On the last day of the culture, though, there is an accumulation of reactive oxygen species as isocitrate dehydrogenase gene expression levels increase. This could imply that there is a build up of oxidative radicals in recovering cells with the highest frequency in those residual cells initially treated with the highest concentration of chemotherapy agents.
In contrast to the two aforementioned genes, expression of succinate dehydrogenase decreased with increasing days in culture. The elevated succinate dehydrogenase expression on day one following exposure suggests that oxidative damage is minimal after induction chemotherapy treatment. As surviving cells are allowed to recover, however, it appears that there is an increase in oxidative damage as confirmed by the decreased expression of succinate dehydrogenase. The observation suggests that residual cells may confer survival by increasing oxidative stress to a point where the naturally occurring apoptotic mechanism of the mitochondria cannot function properly. Expression of succinate dehydrogenase on day seven of culture was the lowest in cells exposed to the highest concentrations of doxorubicin and vincristine; suggesting that cells exposed to higher concentrations of chemotherapy agents exhibited more oxidative damage. Therefore it appears that while the higher concentration chemotherapy agents are more potent, this does not necessarily correlate with a decreased risk of relapse and more effective treatment.
The objective of the study was to demonstrate relative expression of citrate synthase, isocitrate dehydrogenase and succinate dehydrogenase in residual cells following chemotherapy exposure to vincristine or doxorubicin. After observing increased mtDNA copy number/cell in residual BL cells following chemotherapy exposure, we aimed to assess the expression of relevant Krebs cycle genes. The increased citrate synthase and isocitrate dehydrogenase expressions occurred with decreased succinate dehydrogenase expression; and suggest a relationship with the increased mtDNA copy numbers previously observed in residual cells. Further detailed studies relating enzyme expression to mtDNA copies are necessary to delineate the relationship. Moreover, assessing the relative expression of key Krebs cycle genes highlights the roles of ATP synthesis and antioxidant pathways as it pertains to the presence of residual cells following chemotherapy exposure. We acknowledge that the data should be interpreted with the limitations of the study in which there was a lack of assessing gene expression in control cells as a point of reference, which are planned in subsequent studies. Future studies are planned to implement gene silencing in order to assess the individual contribution of each gene of interest as it pertains to the presence of residual cells, as well as gene expression of control cells and gene assessment in cells over longer culture periods. In conclusion, our study suggests that lymphoma cells that recover from chemotoxity have a phenotype in which they appear to compensate by regulating enzymatic levels of appropriate genes in the Krebs cycle. This mechanism suggests an important role of the mitochondria in residual cells.
Acknowledgments
The was supported in part by NIH/NCI Grant #CA121955.
Footnotes
Disclosure
The authors report no conflicts of interest.
References
- 1.Cave H, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. European Organization for Research and Treatment of Cancer—Childhood Leukemia Cooperative Group. N Engl J Med. 1998;339(9):591–8. doi: 10.1056/NEJM199808273390904. [DOI] [PubMed] [Google Scholar]
- 2.Attarbaschi A, et al. Minimal residual disease values discriminate between low and high relapse risk in children with B-cell precursor acute lymphoblastic leukemia and an intrachromosomal amplification of chromosome 21: the Austrian and German acute lymphoblastic leukemia Berlin-Frankfurt-Munster (ALL-BFM) trials. J Clin Oncol. 2008;26(18):3046–50. doi: 10.1200/JCO.2008.16.1117. [DOI] [PubMed] [Google Scholar]
- 3.Conley KE, et al. Mitochondrial dysfunction: impact on exercise performance and cellular aging. Exerc Sport Sci Rev. 2007;35(2):43–9. doi: 10.1249/JES.0b013e31803e88e9. [DOI] [PubMed] [Google Scholar]
- 4.Kim MM, et al. Mitochondrial DNA quantity increases with histopathologic grade in premalignant and malignant head and neck lesions. Clin Cancer Res. 2004;10(24):8512–5. doi: 10.1158/1078-0432.CCR-04-0734. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, et al. Mitochondrial genetic polymorphisms and pancreatic cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1455–9. doi: 10.1158/1055-9965.EPI-07-0119. [DOI] [PubMed] [Google Scholar]
- 6.Kusao I, et al. Chemotoxicity recovery of mitochondria in non-Hodgkin lymphoma resulting in minimal residual disease. Pediatr Blood Cancer. 2008;51(2):193–7. doi: 10.1002/pbc.21545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leek BT, et al. Effect of acute exercise on citrate synthase activity in untrained and trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2001;280(2):R441–7. doi: 10.1152/ajpregu.2001.280.2.R441. [DOI] [PubMed] [Google Scholar]
- 8.Renner K, Kofler R, Gnaiger E. Mitochondrial function in glucocorticoid triggered T-ALL cells with transgenic bcl-2 expression. Mol Biol Rep. 2002;29(1–2):97–101. doi: 10.1023/a:1020335221341. [DOI] [PubMed] [Google Scholar]
- 9.Cupp JR, McAlister-Henn L. NAD(+),-dependent isocitrate dehydrogenase. Cloning, nucleotide sequence, and disruption of the IDH2 gene from Saccharomyces cerevisiae. J Biol Chem. 1991;266(33):22199–205. [PubMed] [Google Scholar]
- 10.Jo SH, et al. Control of mitochondrial redox balance and cellular defense against oxidative damage by mitochondrial NADP+-dependent isocitrate dehydrogenase. J Biol Chem. 2001;276(19):16168–76. doi: 10.1074/jbc.M010120200. [DOI] [PubMed] [Google Scholar]
- 11.Gutman M. Modulation of mitochondrial succinate dehydrogenase activity, mechanism and function. Mol Cell Biochem. 1978;20(1):41–60. doi: 10.1007/BF00229453. [DOI] [PubMed] [Google Scholar]
- 12.Ishii N, et al. A mutation in succinate dehydrogenase cytochrome b causes oxidative stress and ageing in nematodes. Nature. 1998;394(6694):694–7. doi: 10.1038/29331. [DOI] [PubMed] [Google Scholar]
- 13.Bourgeron T, et al. Mutation of a nuclear succinate dehydrogenase gene results in mitochondrial respiratory chain deficiency. Nat Genet. 1995;11(2):144–9. doi: 10.1038/ng1095-144. [DOI] [PubMed] [Google Scholar]
- 14.Ozgen U, et al. Degradation of vincristine by my eloper oxidase and hypochlorous acid in children with acute lymphoblastic leukemia. Leuk Res. 2003;27(12):1109–13. doi: 10.1016/s0145-2126(03)00098-5. [DOI] [PubMed] [Google Scholar]
- 15.Chiaratti MR, Meirelles FV. Increase in mitochondrial DNA quantity and impairment of oxidative phosphorylation in bovine fibroblast cells treated with ethidium bromide for 15 passages in culture. Genet Mol Res. 2006;5(1):55–62. [PubMed] [Google Scholar]