Abstract
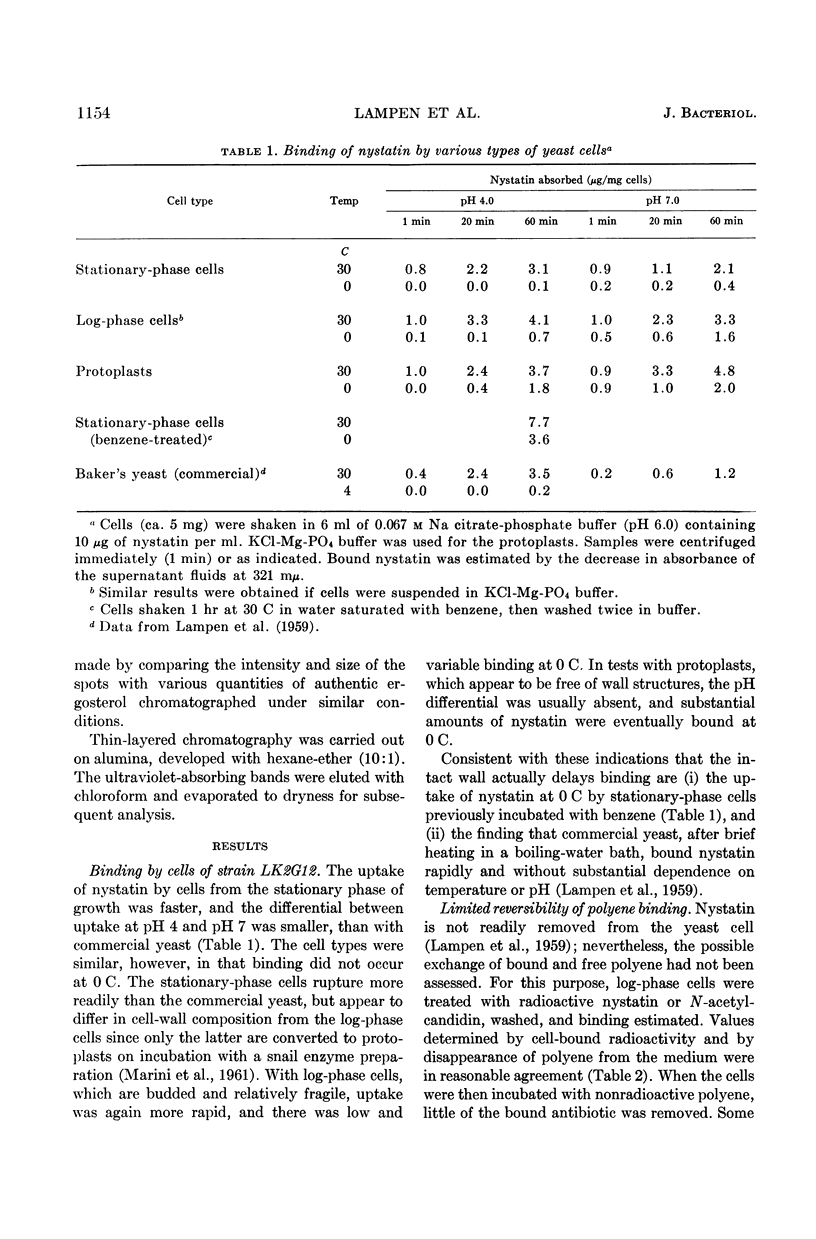
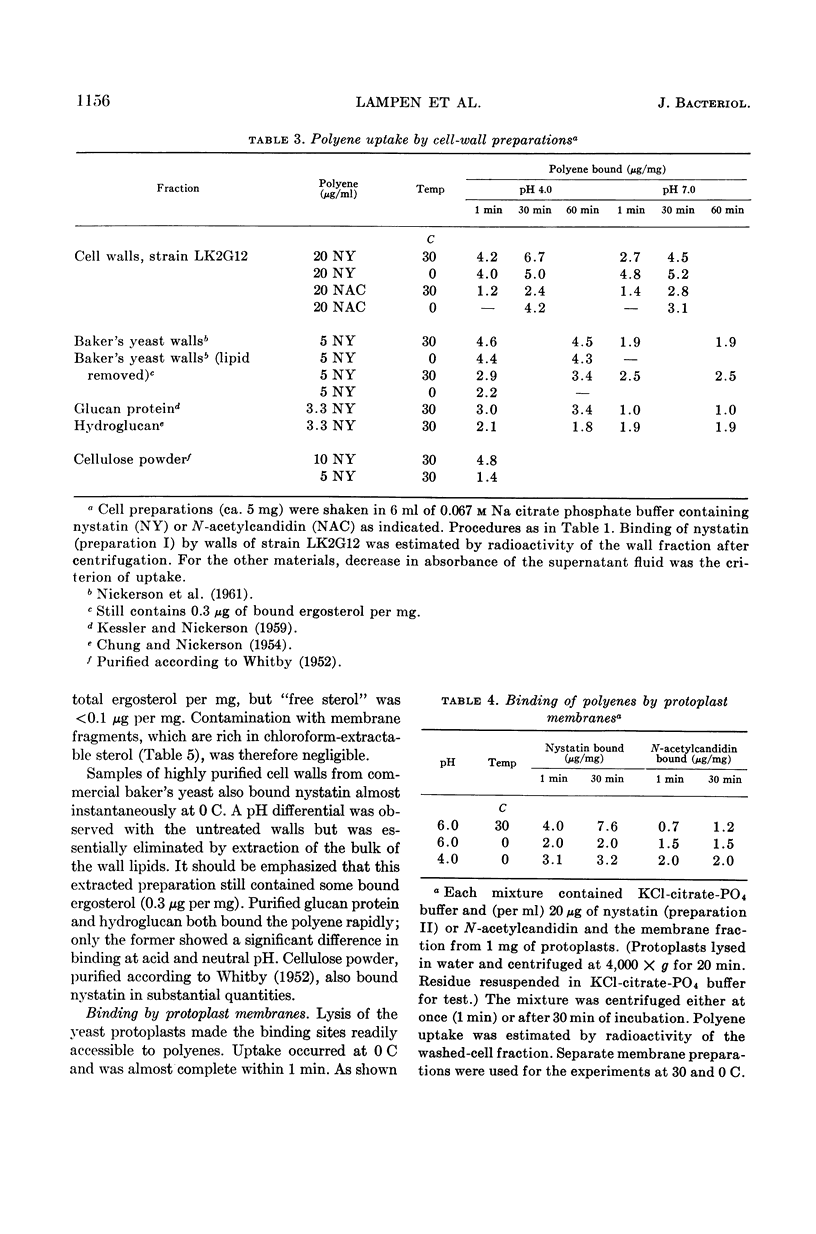
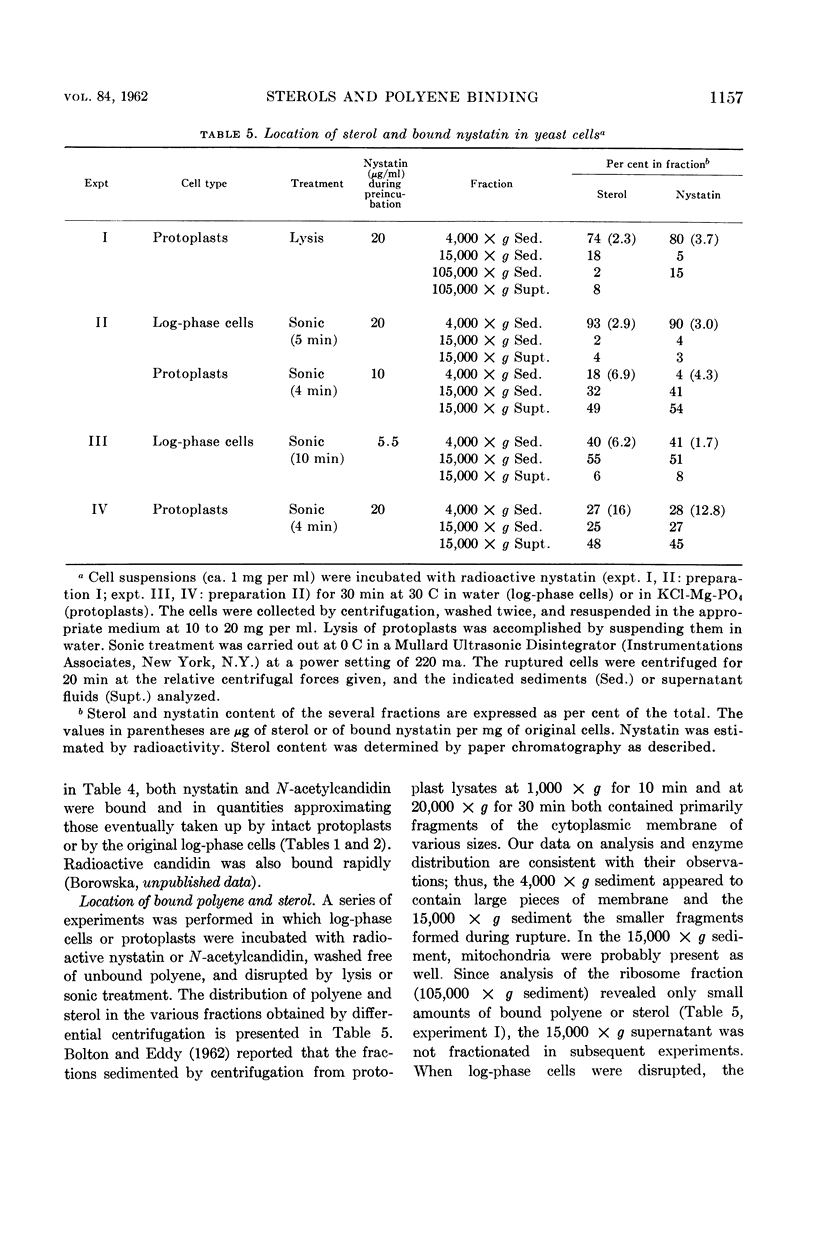
Lampen, J. O. (Rutgers, The State University, New Brunswick, N.J.), Peter M. Arnow, Zofia Borowska, and Allen I. Laskin. Location and role of sterol at nystatin-binding sites. J. Bacteriol. 84:1152–1160. 1962.—The polyene antifungal antibiotics nystatin and N-acetylcandidin were bound rapidly at 0 C by isolated cell walls and derived polysaccharides or by protoplast membranes from Saccharomyces cerevisiae strain LK2G12. These binding sites were relatively inaccessible or unreactive in the intact cell, since polyene uptake by protoplasts, log-phase cells, or stationary-phase cells was slow, especially at 0 C. Binding by the membrane appears to be the critical event in cell damage; thus, uptake of nystatin by the cell wall may actually be protective. Binding by all cell forms showed little reversibility. Bound radioactive nystatin or N-acetyl (1-C14) candidin was not displaced during incubation of log-phase yeast cells with a large excess of unlabeled polyene. Saturation of the polyenic moiety of nystatin to form the perhydro compound eliminated almost completely the affinity of the molecule for the yeast cell. Essentially all of the polyene bound by protoplasts was present on the membrane. It was removed by treatment of the protoplasts with the sterol-complexing agent digitonin. A variety of evidence is offered that the binding site on the membrane contains a sterol. (The membrane sterol was mostly unesterified ergosterol.) This hypothesis is consistent with the ability of certain exogenous sterols to complex with polyenes and prevent their binding and growth-inhibiting action for fungi. The bound sterol of the wall structure may also participate in polyene binding, although a possible function of the wall polysaccharides cannot be excluded. The specific binding structure(s) appears to be absent from bacteria, since nystatin was not taken up even by heated or benzene-treated bacteria or by bacterial protoplasts. It should be noted that bacteria, which are insensitive to the polyene antibiotics, generally contain only traces of sterol, if any. Considerable quantities of sterol are present in fungi, algae, certain protozoa, and animal cells, all of which are sensitive in some degree.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHUNG C. W., NICKERSON W. J. Polysaccharide syntheses in growing yeasts. J Biol Chem. 1954 May;208(1):395–407. [PubMed] [Google Scholar]
- FIERTEL A., KLEIN H. P. On sterols in bacteria. J Bacteriol. 1959 Nov;78:738–739. doi: 10.1128/jb.78.5.738-739.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTLIEB D., CARTER H. E., SLONEKER J. H., AMMANN A. Protection of fungi against polyene antibiotics by sterols. Science. 1958 Aug 15;128(3320):361–361. doi: 10.1126/science.128.3320.361. [DOI] [PubMed] [Google Scholar]
- KESSLER G., NICKERSON W. J. Glucomannan-protein complexes from cell walls of yeasts. J Biol Chem. 1959 Sep;234:2281–2285. [PubMed] [Google Scholar]
- KINSKY S. C. Alterations in the permeability of Neurospora crassa due to polyene antibiotics. J Bacteriol. 1961 Dec;82:889–897. doi: 10.1128/jb.82.6.889-897.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMPEN J. O., ARNOW P. M., SAFFERMAN R. S. Mechanism of protection by sterols against polyene antibiotics. J Bacteriol. 1960 Aug;80:200–206. doi: 10.1128/jb.80.2.200-206.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMPEN J. O., ARNOW P. Significance of nystatin uptake for its antifungal action. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:792–797. doi: 10.3181/00379727-101-25098. [DOI] [PubMed] [Google Scholar]
- LAMPEN J. O., MORGAN E. R., SLOCUM A., ARNOW P. Absorption of nystatin by microorganisms. J Bacteriol. 1959 Aug;78:282–289. doi: 10.1128/jb.78.2.282-289.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARINI F., ARNOW P., LAMPEN J. O. The effect of monovalent cations on the inhibition of yeast metabolism by nystatin. J Gen Microbiol. 1961 Jan;24:51–62. doi: 10.1099/00221287-24-1-51. [DOI] [PubMed] [Google Scholar]
- Rosenheim O. A specific colour reaction for ergosterol. Biochem J. 1929;23(1):47–53. doi: 10.1042/bj0230047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAFFNER C. P., BOROWSKI E. Biologically active N-acyl derivatives of polyene macrolide antifungal antibiotics. Antibiot Chemother (Northfield) 1961 Nov;11:724–732. [PubMed] [Google Scholar]
- SHOCKMAN G. D., LAMPEN J. O. Inhibition by antibiotics of the growth of bacterial and yeast protoplasts. J Bacteriol. 1962 Sep;84:508–512. doi: 10.1128/jb.84.3.508-512.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTTON D. D., ARNOW P. M., LAMPEN J. O. Effect of high concentrations of nystatin upon glycolysis and cellular permeability in yeast. Proc Soc Exp Biol Med. 1961 Oct;108:170–175. doi: 10.3181/00379727-108-26882. [DOI] [PubMed] [Google Scholar]
- TSUDA S., TATUM E. L. Intracellular crystalline ergosterol in Neurospora. J Biophys Biochem Cytol. 1961 Oct;11:171–177. doi: 10.1083/jcb.11.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITBY L. G. Riboflavinyl glucoside; a new derivative of riboflavin. Biochem J. 1952 Feb;50(4):433–438. doi: 10.1042/bj0500433. [DOI] [PMC free article] [PubMed] [Google Scholar]


