Abstract
This study investigates differences in the effects of comorbidities on survival in Medicare beneficiaries with prostate cancer. Medicare data were used to assemble a cohort of 65- to 76-year-old Black (n = 6,402) and White (n = 47,458) men with incident localized prostate cancer in 1999 who survived ≥1 year postdiagnosis. Comorbidities were more prevalent among Blacks than among Whites. For both races, greater comorbidity was associated with decreasing survival rates; however, the effect among Blacks was smaller than in Whites. After adjusting for age, socioeconomic status, and community characteristics, the association between increasing comorbidities and survival remained weaker for Blacks than for Whites, and racial disparity in survival decreased with increasing number of comorbidities. Differential effects of comorbidities on survival were also evident when examining different classes of comorbid conditions. Adjusting for treatment had little impact on these results, despite variation in the racial difference in receipt of prostatectomy with differing comorbidity levels.
Keywords: administrative data, comorbidity, disparities, Medicare, prostate cancer, social epidemiology, survival analysis
Introduction
Comorbid conditions increase the risk of mortality for individuals with many different diseases, including coronary heart disease, diabetes, infection, and cancer (Chirinos et al., 2007; Lesens et al., 2003; Monami et al., 2007; Ouellette, Small, & Termuhlen, 2004; Sachdev et al., 2004; Tammemagi, Neslund-Dudas, Simoff, & Kvale, 2004; Yancik et al., 1998). Comorbidity is also a strong predictor of mortality during an inpatient hospitalization and after a major operation (Alibhai et al., 2005; Ferrier, Spuesens, Le Cessie, & Baatenburg de Jong, 2005; Librero, Peiro, & Ordinana, 1999; Martins & Blais, 2006; Sundararajan et al., 2004). The effect of comorbidity on mortality varies by the type and severity of comorbidity, with larger increases in mortality seen for more serious conditions, such as congestive heart failure, than for less serious conditions, such as hypothyroidism (Elixhauser, Steiner, Harris, & Coffey, 1998).
The relationship between race and comorbidity is of potential clinical and public health importance. The prevalence of comorbidities varies by race in the United States, with Blacks having higher rates and earlier onset of comorbid conditions than Whites (McGee, Cooper, Liao, & Durazo-Arvizu, 1996). Racial differences in rates of comorbidities have been postulated to be an important cause of racial disparities in health and health care (Institute of Medicine of the National Academies, 2002), and several studies have examined the contribution of comorbidities to racial disparities in survival (Gomez, O'Malley, Stroup, Shema, & Satariano, 2007; Mayberry et al., 1995; Tammemagi, Nerenz, Neslund-Dudas, Feldkamp, & Nathanson, 2005). Although these studies have reached different conclusions about the relative contribution of comorbidities, they have generally shared the assumption that the effects of comorbidities on survival are the same in Black and White populations.
New Contribution
Despite the importance of comorbidities to outcomes, there have been relatively few prior studies examining racial differences in the relationship between comorbidity and outcome. One prior study found that the difference in mortality between Black and White men with prostate cancer disappeared as initial comorbidity increased, supporting the possibility that comorbidities have a greater effect on Whites than on Blacks (Freeman et al., 2004). However, another study demonstrated similar patterns of relative risks (RRs) for comorbidities among Black and White women with breast cancer (West, Satariano, Ragland, & Hiatt, 1996). In this article, we extend this work to investigate the differential effect of comorbidities on survival among a national cohort of elderly White and Black men with newly diagnosed prostate cancer.
Conceptual Model
The conceptual framework underlying this investigation is based on findings in three areas. First, demographic studies have demonstrated a narrowing mortality difference between Black and White men as they age (Johnson, 2000). Although Black men have nearly twice the mortality rate of White men prior to age 60, the difference narrows after age 60. For men between 75 and 79 years of age, Black all-cause mortality is 6,580 per 100,000, and White all-cause mortality is 5,066 per 100,000, a difference in risk of only 30% (Kung, Hoyert, Xu, & Murphy, 2008). These rates may reverse at older ages, with greater mortality among White than Black men aged more than 81 or more than 87 (Kestenbaum, 1992; Manton, Poss, & Wing, 1979). Increasing rates of chronic illness among Whites may contribute to this reversal; however, multiple studies have demonstrated that rates of comorbidity and disability remain higher among Blacks than Whites across the seventh and eighth decade of life (Dunlop, Song, Manheim, Daviglus, & Chang, 2007; Kelley-Moore & Ferraro, 2004; Miles & Bernard, 1992; Sudano & Baker, 2006). The possibility that racial differences in the effect of comorbidities on mortality among elderly individuals contribute to this crossover has received insufficient attention (Johnson, 2000). Second, because Blacks are diagnosed with and die from multiple chronic diseases at younger ages than Whites, elderly Blacks who are still alive with a chronic disease may have demonstrated their relative resilience to the effects of that disease on mortality compared with elderly Whites with the same disease. Third, the effect of comorbidity on mortality may be particularly important for individuals who are newly diagnosed with a disease, where there is uncertainty about the necessity of aggressive treatment and a relatively low case-fatality rate, such as prostate cancer (Wilt et al., 2008). In this setting, comorbidity may influence treatment decision making as well as mortality following treatment (Concato, Horwitz, Feinstein, Elmore, & Schiff, 1992; Fouad et al., 2004). These relationships are particularly interesting for prostate cancer where significant racial differences in treatment and mortality have been demonstrated and where expected life-expectancy is included in treatment guidelines (Godley et al., 2003; National Comprehensive Cancer Network, 2004; Polednak & Flannery, 1992; Schapira, McAuliffe, & Nattinger, 1995).
Methods
We conducted a retrospective cohort study of Black and White men aged more than 65, diagnosed with localized prostate cancer, in 270 U.S. metropolitan statistical areas (MSAs) between January 1 and December 31, 1999. The study was approved by the institutional review board of the University of Pennsylvania, Philadelphia.
Data Sources
Data from several sources were linked for this study. National Medicare claims files (MEDPAR, Part B, Outpatient, Denominator) were used to identify the study cohort and determine patient-level information on age, sex, principal and secondary diagnoses, comorbidities, procedures, and death status. National Medicare data provide a representative sample of the U.S. population; however, Medicare data alone do not provide information on cancer stage. Thus, SEER-Medicare files for prostate cancer (1991–1999) were used to develop and validate a predictive model for localized stage at presentation that was then applied to the national Medicare data. In addition, SEER-Medicare data were used in sensitivity analyses to determine if the relationships between comorbidity, race, and survival held in a smaller sample where stage information was available. Data from the 2000 census were used to determine MSA characteristics as well as create block group and census tract level measures of socioeconomic status (SES) that were used as proxies for individual level information.
Study Population
The initial sample included 1,021,919 beneficiaries identified by the Centers for Medicare and Medicaid Services as having an ICD9 code 185 (malignant neoplasm of prostate) in 1999. To ensure that men in the sample had an incident diagnosis of prostate cancer in 1999, we excluded men who had a code for prostate cancer, personal history of prostate cancer, or prostatectomy in the 3 years prior to the index year (n = 785,106) as well as men who did not have a code for a prostate biopsy, transurethral resection of the prostate, surgical pathology, or four or more codes for prostate cancer diagnosis in 1999 (n = 23,860). We then excluded men who did not live in a continental U.S. MSA (n = 52,299), were a race other than Black or White (n = 42,888), could not be linked to a census tract or block group (n = 1,636), were younger than 65 (n = 14,931), or had fewer than 3 months of data prior to January 1999 or were enrolled in an HMO between January 1999 and March of 2000 (n = 13,722). Because the benefit of active treatment for prostate cancer in elderly men is particularly controversial, we also excluded men who were more than 76 years of age (n = 20,769), giving us a final sample of 66,708 men.
Exclusion of Metastatic Cases
Because Medicare data contain no direct information on stage of disease at diagnosis (localized vs. metastatic), we imputed stage using a logistic regression model developed and validated from 1991 to 1999 SEER Medicare prostate cancer data. SEER-Medicare data were split into a training (n = 48,000) and validation set (n = 50,778). A logistic regression model was built on the training data; the outcome was localized stage at diagnosis (historic SEER Stage A, B, or C), and the predictor variables were 737 diagnostic codes selected by clinical experts as having a possible association with stage at diagnosis, along with age and the four geographic census regions. Because the goal was to create a model with the greatest discriminatory power, rather than the interpretation of any of the individual coefficients, all the variables were included in the final predictive model; the final model yielded a C-statistic of 0.852 (0.832 among Blacks and 0.854 among Whites). In the validation data, a cutoff of a predicted probability of localized disease less than 80% resulted in an overall prevalence of participants with localized disease greater than 95% (95.1%). After applying this model to the national Medicare data, 11,185 men with less than an 80% predicted probability of localized disease were excluded, yielding a sample of 55,523 men.
Variables
The primary outcome measures were survival and type of treatment. For the results presented here, the primary exposure variables were patient race and the number of comorbid conditions. Possible confounders included age, SES, geographic region, and community structure.
Outcome Measures: Death and Treatment
Date of death was determined from the Medicare Denominator files from 1999 to 2002. All other patients were censored as of December 31, 2002. Treatment was determined by ICD9 and HCPCS codes for prostatectomy or radiation in the MEDPAR, carrier, and outpatient files for the 12 months following diagnosis (see Appendix A; Begg et al., 2002). Patients with codes for both prostatectomy and radiation (n = 634, 1.14%) were categorized as having a prostatectomy because radiation was considered as given postoperatively.
Primary Exposures: Race and Comorbidities
Patient race was determined from the Medicare 1999 Denominator File and coded as Black or White. ICD9 diagnostic codes for comorbidities were identified in the Medicare MEDPAR, Outpatient, and Carrier files in the timeframe extending 90 days prior to diagnosis using the non-cancer-related categories described by Elixhauser et al. (1998) as well as an additional category, stroke, that was prevalent in our sample. We considered the impact of the comorbid burden, or comorbidity number, measured as the sum of these categories for each individual (reported as 0 to 4 and more than 4). Because a claim for a comorbidity could only occur if the patients had contact with the health care system before their date of diagnosis, we used claims from Part M and MEDPAR files to identify individuals who lacked a doctor's visit or hospitalization in the 90 days prior to diagnosis and in the year prior to diagnosis and conducted sensitivity analyses after excluding these individuals from the sample.
Confounders
Age at diagnosis was determined from the Medicare denominator file. Block group and census tract information were used as proxies for individual measures of SES. For men who could not be assigned to a block group (n = 7,236) or where there were insufficient data at the block group level, census tract by race measures were used; if census tract by race data were not available, overall census tract data were used. Socioeconomic status measures included in the models were median household income and percentage of males with less than a high school degree.
Community contextual factors such as geographic region and size, percentage living in poverty, racial composition, and level of segregation in the patient's city were also included. This information was obtained from the U.S. Census 2000 with region defined as North, South, West, and Midwest, and segregation was assessed using the isolation index (Massey & Denton, 1988).
Statistical Methods
The proportion of Blacks and Whites with individual comorbidity categories and with number of comorbidities for each was described, along with odds ratios (ORs) and the associated 95% confidence interval (CI). Fisher's exact test was used to determine whether each OR was different from 1.0. Racial differences in the distribution of number of comorbidities were assessed using a chi-square test. Survival rates were described using Kaplan-Meier plots.
Associations between survival times and either comorbidity number or individual Elixhauser categories were modeled using Cox regression. Initial analyses suggested that the assumption of proportional hazards for the Cox model was violated during the first year postdiagnosis; for this reason and because death after 1 year is more likely to reflect prostate cancer, we omitted 1,663 patients who died during the first year postdiagnosis and considered survival conditional on surviving for at least 1 year postdiagnosis. To maintain statistical power and to avoid fitting models where some strata have small numbers of deaths, patients with more than four comorbidities in the models using comorbidity number were pooled into a single category. For the models using individual Elixhauser comorbidities, four categories with fewer than 40 participants of either race (pulmonary circulation disease, HIV-AIDS, obesity, and drug abuse) were pooled into a single category. Results for this heterogeneous “other” category are not reported here.
Likelihood ratio tests were used to assess the strength of the overall interaction between race and comorbidities both in models that contained only race and comorbidity terms as well as in two models with adjustment for possible confounding. A partially adjusted model allowed for possible confounding by age, variables reflecting SES (income, proportion of males completing high school), and contextual characteristics (census region, MSA population, percentage of MSA living in poverty, proportion of MSA population that is Black, and level of MSA segregation) that were significantly associated with mortality in other analyses of the same data. The fully adjusted model also considered the effect of treatment (radiation, prostatectomy, or none) on survival. In addition to the main effect terms, we adjusted for possible confounding by interactions between race and treatment, and race and segregation. The same modeling procedure was used for individual Elixhauser categories, but only the results from the fully adjusted model are reported here. In addition, to ensure that the results were robust to concerns about the validity of the imputed measure of stage, the differential exposure of Blacks and Whites to the health care system prior to diagnosis, and the exclusion of individuals who died in the first year, we also ran the models in the SEER-Medicare data set (both using the SEER measure of localized stage and using the predicted measure of localized stage with the same cutoff point) with the sample of individuals who had a doctor's visit or hospitalization in the prior year and using 1-year mortality as the primary endpoint. Finally, logistic regression models were used to examine the effect of comorbidity number on three different outcomes related to treatment, that is, any versus no treatment, radiation versus no radiation, and prostatectomy versus no prostatectomy in both an unadjusted and adjusted fashion.
In addition to using the likelihood ratio test to provide an estimate of the overall strength of the interaction, we examined the results of individual Wald tests to better understand how specific comparisons of interest differed. For the models using comorbidity number, we tested the hypotheses that the hazard or OR for each number of comorbidities versus no comorbidities differed between Blacks and Whites. For each model, five comparisons were made, and the resulting p values (p) were adjusted for multiple comparisons using Hochberg's step-down approach to yield adjusted p values (pA; Hochberg, 1988).
Analyses were carried out using R 2.6. All hypothesis tests were two sided and used a Type I error rate of 0.05; 95% CIs are presented along with estimates of RR or odds of particular effects.
Results
Table 1 reports the sample demographics. Black and White men in the sample were of similar age at diagnosis, but Blacks tended to have lower SES, were more likely to live in the South and less likely to live in the West, and more likely to live in cities with higher levels of segregation. Compared with White men, Black men were less likely to undergo active treatment, including either prostatectomy or radiation.
Table 1.
Characteristics of the Sample
| Whites |
Blacks |
||
|---|---|---|---|
| Variable | (n = 47,458) | (n = 6,402) | P |
| Age (mean, range) | 70.4 (65.0–76.0) | 69.9 (65.0–76.0) | <.001 |
| SES measuresa | |||
| Income (median, IQR) thousands | 48.1 (37.0–64.0) | 32.2 (23.3–44.3) | <.001 |
| Percentage males < high school degree (median, range) | 26.2 (17.5–33.8) | 30.6 (24.5–35.7) | <.001 |
| Region (n, %) | |||
| Midwest | 12,107 (25.5) | 1,630 (25.5) | Overall <.001 |
| Northeast | 9,773 (20.6) | 1,336 (20.9) | |
| South | 17,887 (37.7) | 3,054 (47.7) | |
| West | 7,691 (16.2) | 382 (6.0) | |
| Isolation index (median, range) | 0.48 (0–0.83) | 0.65 (0.01–0.83) | <.001 |
| MSA percentage Black (median, IQR) | 11.6 (6.6–20.9) | 21.9 (14.6–27.1) | <.001 |
| MSA percentage in poverty (median, IQR) | 10.8 (9.5–13.0) | 10.8 (9.8–14.8) | <.001 |
| Treatment (n, %) | |||
| None | 21,858 (46.1) | 3,600 (56.2) | <.001 |
| Prostatectomy | 8,588 (18.1) | 775 (12.1) | <.001 |
| Radiation | 17,012 (35.9) | 2,027 (31.7) | <.001 |
| Comorbidities (n, %)b | |||
| None | 16,950 (35.7) | 1,658 (25.9) | Overall <.0001 |
| 1 | 14,322 (30.2) | 1,801 (28.1) | |
| 2 | 8,050 (16.9) | 1,269 (19.8) | |
| 3 | 4,073 (8.5) | 721 (11.3) | |
| 4 | 2,067 (4.4) | 416 (6.5) | |
| ≥4 | 1,996 (4.2) | 537 (8.4) | |
| Risk of death (n, %)c months | |||
| 0–12 | 244 (5.4) | 1,365 (3.8) | |
| 12–24 | 318 (9.1) | 1,566 (6.0) | |
| 24–36 | 348 (14.4) | 1,612 (9.3) | |
| 36–42 | 120 (16.7) | 666 (11.0) |
Note: SES = socioeconomic status; IQR = interquartile range; MSA = metropolitan statistical area.
Men were assigned to a block group and census tract to which SES measures from the Census 2000 SF3 were linked.
Odds of having specific number of comorbidities or more compared with fewer comorbidities, for example, greater than one versus none.
Number of deaths in each time interval with Kaplan-Meier estimate of cumulative risk of death.
Comorbidity Patterns
The overall burden of comorbidities differed among the races, with Blacks displaying a larger number of comorbidities compared with Whites (p < .0001): 35.7% of Whites had no comorbidities, compared with 25.9% of Blacks, whereas 8.5% of Whites had four or more comorbidities, compared with 14.7% of Blacks (Table 1). Table 2 shows that the prevalence of individual comorbidities ranged from 0.5% (paralysis) to 40.4% (hypertension) for Whites and between 0.9% (weight loss) and 58.4% (hypertension) for Blacks. The majority of comorbidities were more prevalent (OR = 1.15 to 2.86) in Blacks than in Whites. However, rates of coagulopathy, chronic obstructive pulmonary disease, and rheumatologic disease did not differ significantly among the two racial groups, while arrhythmia, depression, hypothyroidism, and valvular disease occurred less frequently in Blacks compared with Whites.
Table 2.
Prevalence, and Oddsa of Individual Comorbidities in Blacks Versus Whites
| Prevalence (n, %) |
|||
|---|---|---|---|
| Elixhauser Comorbidity | Whites | Blacks | OR (95% CI) |
| None | 16,950 (35.7) | 1,658 (25.9) | NA |
| Alcoholism | 241 (0.5) | 81 (1.3) | 2.51 (1.95, 3.23) |
| Anemia: blood loss | 4,087 (8.6) | 900 (14.1) | 1.74 (1.61, 1.88) |
| Anemia: deficiency | 3,895 (8.2) | 849 (13.3) | 1.71 (1.58, 1.85) |
| Arrhythmia | 4,619 (9.7) | 512 (8.0) | 0.81 (0.73, 0.89) |
| Coagulopathy | 1,001 (2.1) | 127 (2.0) | 0.94 (0.78, 1.13) |
| CHF | 1,685 (3.6) | 362 (5.7) | 1.63 (1.45, 1.83) |
| COPD | 6,103 (1.3) | 803 (12.5) | 0.97 (0.90, 1.05) |
| Depression | 789 (1.7) | 85 (1.3) | 0.80 (0.64, 1.00) |
| Diabetes: complicated | 288 (0.6) | 95 (1.5) | 2.47 (1.95, 3.12) |
| Diabetes: uncomplicated | 6,324 (13.3) | 1,397 (21.8) | 1.82 (1.70, 1.94) |
| Electrolyte abnormality | 1,021 (2.2) | 250 (3.9) | 1.85 (1.61, 2.13) |
| Hypertension | 19,193 (40.4) | 3,739 (58.4) | 2.07 (1.96, 2.18) |
| Hypothyroidism | 2,572 (5.4) | 214 (3.3) | 0.60 (0.52, 0.70) |
| Liver disease | 759 (1.6) | 131 (2.0) | 1.29 (1.07, 1.55) |
| Neurological disease | 873 (1.8) | 155 (2.4) | 1.32 (1.11, 1.57) |
| Paralysis | 246 (0.5) | 71 (1.1) | 2.15 (1.65, 2.81) |
| Peptic ulcer disease | 371 (0.8) | 75 (1.2) | 1.50 (1.17, 1.93) |
| Psychosis | 325 (0.7) | 124 (1.9) | 2.86 (2.33, 3.53) |
| PVD | 2,884 (6.1) | 445 (7.0) | 1.15 (1.04, 1.28) |
| Renal insufficiency | 795 (1.7) | 275 (4.3) | 2.63 (2.29, 3.03) |
| Rheumatologic disease | 915 (1.9) | 115 (1.8) | 0.93 (0.77, 1.13) |
| Stroke | 946 (2.0) | 268 (4.2) | 2.15 (1.87, 2.47) |
| Valvular disease | 2,452 (5.2) | 285 (4.5) | 0.86 (0.75, 0.97) |
| Weight loss | 111 (0.2) | 55 (0.9) | 3.70 (2.67, 5.11) |
Note: OR = odds ratio; CI = confidence interval; CHF = coronary heart failure; COPD = chronic obstructive pulmonary disease; PVD = peripheral vascular disease.
Odds ratio of individual comorbidity in Blacks versus Whites.
Relative Mortality Rates
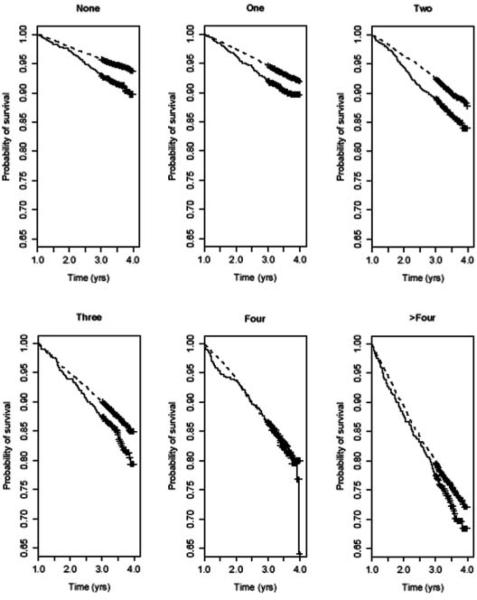
The Kaplan-Meier plots in Figure 1 demonstrate that the gap between mortality rates for Blacks and Whites narrows with increasing number of comorbidities. Table 3 shows that, in an unadjusted Cox model, increasing number of comorbidities was associated with increasing risk of mortality in both Whites and Blacks. However, the effect of increasing comorbidities differed between racial groups, with the death rates for Blacks increasing at a slower pace compared with that for Whites (overall p = .026). For example, Whites with more than four comorbidities had death rates that were 5.25-fold (95% CI = 4.70–5.86) greater than that for Whites with no comorbidities, but Blacks with more than four comorbidities had death rates that were 3.64-fold (95% CI = 2.90–4.58) greater than that for Blacks without comorbidities. These RRs were significantly different (pA = .040). Mortality among Blacks without comorbidities was 1.66-fold (95% CI = 1.39–1.98) greater than that for Whites without comorbidities, but Black mortality was only 1.06-fold (95% CI = 0.83–1.31) greater than White mortality for men with four comorbidities and 1.15-fold (95% CI = 0.96–1.38) greater for men with more than four comorbidities.
Figure 1. Kaplan-Meier Estimates of Survival Probability in Whites (dashed) and Blacks (solid) With None Through >4 Comorbidities.
Note: Censoring times are indicated by crosses.
Table 3.
Relative Risk of Death for Whites and Blacks for Differing Numbers of Comorbidities in Unadjusted and Adjusted Models
| Race-Specific Rates |
Comorbidity-Specific Rates: Relative Risk (95% CI), Black: Whitec | |||
|---|---|---|---|---|
| Number of Comorbidities | Relative Risk (95% CI) |
Hypothesis Tests p/pAb | ||
| Whitesa | Blacksa | |||
| Unadjusted model | ||||
| None | 1.00 | 1.00 | NA | 1.66 (1.39, 1.98) |
| 1 | 1.31 (1.19, 1.43) | 1.12 (0.89, 1.39) | .20/.40 | 1.42 (1.21, 1.67) |
| 2 | 1.93 (1.76, 2.13) | 1.66 (1.33, 2.07) | .21/.40 | 1.42 (1.21, 1.68) |
| 3 | 2.51 (2.26, 2.80) | 1.97 (1.54, 2.51) | .074/.222 | 1.30 (1.07, 1.59) |
| 4 | 3.48 (3.08, 3.93) | 2.21 (1.67, 2.93) | .004/.030 | 1.06 (0.83, 1.36) |
| ≥4 | 5.25 (4.70, 5.86) | 3.64 (2.90, 4.58) | .005/.040 | 1.15 (0.96, 1.38) |
| Overall p = .026d | ||||
| Partially adjusted modele | ||||
| None | 1.00 | 1.00 | NA | 1.57 (1.19, 2.08) |
| 1 | 1.25 (1.14, 1.36) | 1.05 (0.84, 1.31) | .17/.34 | 1.33 (1.01, 1.75) |
| 2 | 1.79 (1.63, 1.97) | 1.52 (1.22, 1.90) | .18/.34 | 1.34 (1.02, 1.76) |
| 3 | 2.31 (2.07, 2.57) | 1.79 (1.40, 2.29) | .066/.20 | 1.22 (0.90, 1.66) |
| 4 | 3.15 (2.78, 3.56) | 2.01 (1.52, 2.66) | .0041/.021 | 1.01 (0.72, 1.41) |
| ≥4 | 4.74 (4.24, 5.31) | 3.32 (2.63, 4.17) | .006/.024 | 1.10 (0.82, 1.48) |
| Overall p = .034d | ||||
| Fully adjusted modelf | ||||
| None | 1.00 | 1.00 | NA | 1.53 (1.14, 2.04) |
| 1 | 1.26 (1.12, 1.39) | 1.10 (0.88, 1.37) | .25/.58 | 1.33 (1.00, 1.76) |
| 2 | 1.77 (1.61, 1.95) | 1.57 (1.26, 1.96) | .33/.58 | 1.36 (0.68, 1.82) |
| 3 | 2.21 (1.99, 2.48) | 1.77 (1.38, 2.26) | .097/.36 | 1.22 (0.89, 1.67) |
| 4 | 2.99 (2.64, 3.38) | 1.93 (1.46, 2.56) | .005/.035 | 0.99 (0.70, 1.39) |
| ≥4 | 4.37 (3.91, 4.89) | 3.12 (2.48, 3.93) | .0098/.060 | 1.09 (0.81, 1.47) |
| Overall p = .039d | ||||
Note: CI = confidence interval.
Relative risk compared with no comorbidity for each race.
p Value (unadjusted/adjusted for multiple comparisons) comparing relative risk of death for each comorbidity number versus zero comorbidities in Blacks and Whites.
Relative risk for Blacks relative to Whites with the same number of comorbidities.
p Value for likelihood ratio test.
Adjusted for age, socioeconomic status, and contextual variables.
Adjusted for treatment in addition to variables in partially adjusted model.
Table 3 also shows that similar patterns were observed in the model (partially adjusted model) that included age, socioeconomic conditions (income, educational attainment), and contextual characteristics (geographic region, percentage living in poverty, percentage Black, isolation index). Increasing numbers of comorbidities remained associated with increased risk of death, with effects within each race that were somewhat smaller than in the unadjusted model. Increasing numbers of comorbidities continued to have a smaller effect on relative survival rates in Blacks than in Whites (overall p = .034). After partial adjustment, the RR of death in Blacks versus Whites with no comorbidities was only slightly smaller than that in the unadjusted model (RR = 1.57; 95% CI = 1.19–2.08); in contrast, the RR for Blacks versus Whites was only 1.01 (95% CI = 0.82–1.48) at four comorbidities and 1.10 (95% CI = 0.72–1.41) at more than four comorbidities. In this model, covariates that significantly (p < .05) increased risk of death included age, increasing proportion of Blacks in the MSA, income, and decreasing proportion of men with a high school degree. Although treatment with either radiation or prostatectomy was associated with better survival (p < .0001), adding treatment to the above model (Table 3, fully adjusted model) had only minor effects on the relationship between race, comorbidity, and survival. Two-way interactions between race and treatment (prostatectomy and radiation) and a three-way interaction between race, treatment, and comorbidity were not statistically significant (p = .17; p = .45, and p = .42).
Similar results were seen using SEER-Medicare data. In that sample of 96,986 individuals, the effect of comorbidity on survival differed significantly between Blacks and Whites, whether the model was estimated in the sample that excluded metastatic cases based on the SEER stage variable (n = 78,027, p value for interaction = .002) or the sample that excluded metastatic cases based on an 80% predicted probability of being localized from the predictive model (p value for interaction = .005). For example, in the sample using the SEER stage variable, the hazard ratio (HR) for four comorbidities compared with no comorbidities was 3.43 (95% CI = 3.14–3.75) among Whites and 2.74 (95% CI = 2.26–3.33) among Blacks. The same pattern was seen in Medicare data when the analysis was restricted to individuals who had a doctor's visit or a hospitalization in the past year (n = 51,527: 11.5% Black, 88.5% White). In this sample, the effect on comorbidities on survival among Whites was also greater than the effect among Blacks, although the statistical significance was slightly decreased given the smaller sample size (p value for interaction term = .003). For example, the HR for four comorbidities compared with no comorbidities was 2.88 (95% CI = 2.67–3.11) among Whites and 1.98 (95% CI = 1.65–2.37) among Blacks. When examining 1-year mortality (4.6% of Blacks, 2.9% of Whites), the effect of comorbidities on survival remained larger among Whites than among Blacks, although these differences were much smaller (e.g., for four comorbidities vs. no comorbidities, the HR was 1.41 [95% CI = 1.137–1.74] in Whites and 1.03 [95% CI = 0.64–1.64] in Blacks), and the interaction between comorbidity and race was not statistically significant.
Table 4 shows the risk of death among individuals with specific comorbidities relative to individuals of the same race without comorbidities as well as the risk of death in Blacks versus Whites for each comorbidity. The overall effect of individual comorbidities differed significantly among the races (overall p < .0001). When considering the effects of individual categories, we note that the statistical power to detect significance of specific effects varies with the number of participants for each race and for each category as well as the magnitude of the association with mortality. Because the number of participants with specific comorbidities varies, and for many categories is small (Table 2), lack of statistical significance for a particular category and comparisons among specific categories should be interpreted cautiously. Table 4 is divided on the basis of frequent (>5% in at least one race) and infrequent (<5% in both races) comorbidities. We focus on the question of whether a limited number of specific comorbidities are associated with pronounced differences in risk among races or whether the pattern displayed in Table 3 is broadly reflected across many individual comorbidities. Trends are described with the caveat that the results for specific categories often did not achieve statistical significance, as evidenced by 95% CIs that include 1.0. The nine more prevalent (>5%) comorbidities are described first. Relative to no comorbidities for the same race, six comorbidity categories were risk factors, while two (hypertension and hypothyroidism) were consistently protective for both races. In this model, Blacks without comorbidities had a 1.61-fold higher risk than Whites of death. Blacks also had a higher, although not necessarily statistically significant, risk of death than Whites in eight out of the nine more prevalent comorbidity categories (HRs ranging from 1.13 to 2.08); however, in six of these categories, the elevation in risk was less pronounced than for those with no comorbidities. A similar pattern occurred for the 15 less prevalent comorbidities. Except for complicated diabetes and rheumatologic disease, comorbidities were associated with elevated risk of mortality in both races compared with patients without comorbidities of the same race. With the exception of peptic ulcer disease, Blacks had a higher risk of death than Whites for these comorbidities (HRs ranging from 1.12 to 1.97); in 12 out of the 14 comorbidities that were risk factors, the elevation in risk for Blacks versus Whites was less pronounced than for patients without comorbidities.
Table 4.
Risk of Death (95% CI) in Fully Adjusted Model for Whites and Blacks With Specific Comorbidities
| Hazard Ratios (95% CI)a |
|||
|---|---|---|---|
| Elixhauser Comorbidity | Whitesb | Blacksc | Black:Whited |
| None | 1.00 | 1.00 | 1.61 (1.23, 2.09) |
| Prevalence >5% in one or both races | |||
| Anemia: blood loss | 1.04 (0.68, 1.59) | 1.35 (0.69, 2.63) | 2.08 (0.91, 4.76) |
| Anemia: deficiency | 1.23 (0.81, 1.89) | 0.87 (0.44, 1.71) | 1.13 (0.48, 2.62) |
| Arrhythmia | 1.16 (1.06, 1.27) | 1.01 (0.80, 1.27) | 1.40 (0.98, 2.00) |
| CHF | 1.86 (1.66, 2.09) | 2.22 (1.78, 2.78) | 1.92 (1.34, 2.74) |
| Diabetes: uncomplicated | 1.35 (1.25, 1.46) | 1.31 (1.11, 1.54) | 1.55 (1.15, 2.11) |
| Hypertension | 0.94 (0.88, 1.00) | 0.85 (0.73, 0.98) | 1.44 (1.10, 1.90) |
| Hypothyroidism | 0.92 (0.81, 1.04) | 0.53 (0.34, 0.84) | 0.93 (0.55, 1.59) |
| PVD | 1.51 (1.37, 1.66) | 1.19 (0.95, 1.50) | 1.27 (0.89, 1.81) |
| Valvular disease | 1.09 (0.97, 1.23) | 1.07 (0.81, 1.43) | 1.58 (1.05, 2.38) |
| Prevalence <5% in both races | |||
| Alcoholism | 1.54 (1.19, 2.00) | 1.51 (1.00, 2.28) | 1.57 (0.91, 2.70) |
| Coagulopathy | 1.13 (0.96, 1.34) | 1.03 (0.67, 1.58) | 1.46 (0.86, 2.47) |
| COPD | 1.83 (1.70, 1.97) | 1.27 (1.07, 1.53) | 1.12 (0.82, 1.54) |
| Depression | 1.20 (1.00, 1.43) | 1.46 (0.97, 2.21) | 1.96 (1.17, 3.30) |
| Diabetes: complicated | 0.97 (0.71, 1.31) | 0.96 (0.59, 1.57) | 1.59 (0.85, 3.01) |
| Electrolyte abnormality | 1.02 (0.87, 1.20) | 1.25 (0.95, 1.65) | 1.97 (1.31, 2.96) |
| Liver disease | 1.34 (1.11, 1.61) | 0.95 (0.62, 1.46) | 1.15 (0.66, 1.97) |
| Neurological disease | 1.48 (1.26, 1.74) | 1.63 (1.20, 2.22) | 1.77 (1.15, 2.72) |
| Paralysis | 1.06 (0.79, 1.43) | 1.06 (0.62, 1.80) | 1.60 (0.83, 3.08) |
| Peptic ulcer disease | 1.23 (0.94, 1.61) | 0.69 (0.36, 1.35) | 0.90 (0.42, 1.93) |
| Psychosis | 2.24 (1.83, 2.75) | 1.76 (1.26, 2.45) | 1.26 (0.79, 2.00) |
| Renal insufficiency | 1.63 (1.39, 1.91) | 1.33 (1.02, 1.73) | 1.31 (0.87, 1.97) |
| Rheumatologic disease | 1.05 (0.85, 1.28) | 0.95 (0.57, 1.57) | 1.45 (0.79, 2.66) |
| Stroke | 1.51 (1.30, 1.76) | 1.15 (0.86, 1.53) | 1.22 (0.81, 1.86) |
| Weight loss | 1.70 (1.20, 2.40) | 1.46 (0.93, 2.30) | 1.39 (0.75, 2.58) |
Note: CI = confidence interval; CHF = coronary heart failure; COPD = chronic obstructive pulmonary disease; PVD = peripheral vascular disease.
Risk of death in fully adjusted model relative to patients with no comorbidities for individual Elixhauser comorbidity categories.
Hazard ratios (HR + 95% CIs) for Whites relative to Whites with no comorbidities.
Hazard ratios (HR + 95% CIs) for Blacks relative to Blacks with no comorbidities.
Hazard ratios (HR + 95% CIs) for Blacks relative to Whites with the same comorbidity.
Relative Treatment Rates
We investigated the possibility that patterns in the association of comorbidity burden with treatment and mortality were similar (Appendix B). After adjustment for age, SES, and contextual characteristics, having one or two comorbidities increased the chances of receiving treatment compared with having no comorbidities for both Blacks and Whites. Beyond two comorbidities, there was a decreasing trend toward receiving treatment for both races. Although the overall test of significance for an interaction between comorbidity number and race did not achieve significance (overall p = .110), the racial difference in the odds of receipt of treatment at two versus zero comorbidities differed significantly among the races (pA = .040). For individuals without comorbidities, Blacks were less likely than Whites to receive treatment (OR = 0.65; 95% CI = 0.54–0.78); this ratio was slightly higher for one (OR = 0.77; 95% CI = 0.64–0.92) and two comorbidities (OR = 0.80; 95% CI = 0.66–0.98). Examination of the patterns of treatment with radiation or prostatectomy suggested that the differences seen in any versus no treatment were largely a reflection of differences in prostatectomy rates among Black and White men. Here, the overall test of significance for an interaction between comorbidity number and race was significant (overall p = .027). The odds of a Black man with one and two comorbidities receiving prostatectomy were 1.46 (95% CI = 1.20–1.78) and 1.32 (95% CI = 1.06–1.65), respectively, compared with a Black man with no comorbidities. In contrast, the comparable ORs for White men were 1.13 (95% CI = 1.06–1.19) and 0.91 (95% CI = 0.85–0.98). Differences between the ORs were either significant or approached significance (pA = .056 and .010, respectively). Black men with no comorbidities had an odds of receiving prostatectomy that was 0.48-fold (95% CI = 0.36–0.63) that of White men with no comorbidities. The racial difference in odds of prostatectomy was smaller among men with one (OR = 0.62; pA = .052) or two comorbidities (OR = 0.80; pA = .009) but not among men with higher numbers of comorbidities. In contrast to prostatectomy rates, Black and White men had very similar rates of receiving radiation across all numbers of comorbidities.
Survival Patterns
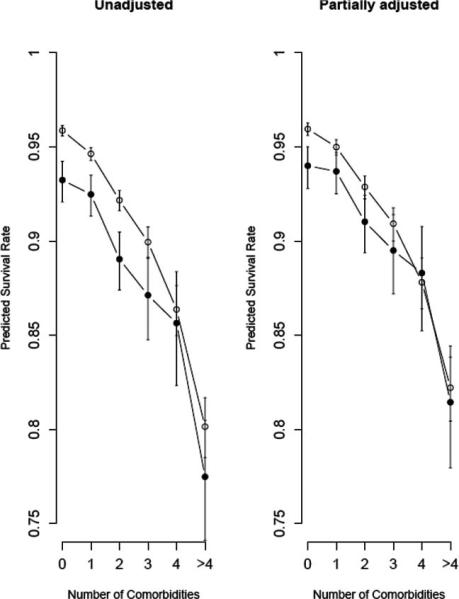
In addition to estimating RR of failure, we used the Cox models to illustrate estimated 4-year survival rates for the unadjusted and partially adjusted models from Table 3 (Figure 2). In the adjusted models, age was set to 70 years, region to South, and the values for the other variables were set to the medians over all patients in the study (percentage poverty of 10.8%, MSA population of 1.3 million, isolation index of 0.50, median household income of $46,351, and percentage of males graduating high school = 73.2%). Figure 2 shows that predicted 4-year survival in Blacks (93.2%) was around 3% lower than in Whites without comorbidities (95.9%), but the gap narrowed to less than 1% for Blacks with four comorbidities (85.6%) compared with Whites with four comorbidities (86.4%). The partially adjusted model predicted that Whites without comorbidities survived at rates that were 2% higher than that for Blacks (96% vs. 94%), while Whites with four or more than four comorbidities survived at rates that were 0.5% lower than that for Blacks (88.3% for Blacks vs. 87.8% for Whites).
Figure 2. Survival Probabilities With 95% Confidence Intervals for Whites (open) and Blacks (solid) Based on the Unadjusted Model or the Model Adjusted for Age, Socioeconomic Status, and Community Structure.
Note: Estimates for the partially adjusted model are for a 70-year-old man living in the south at the median level of all variables in the model.
Discussion
This article demonstrates that an increasing burden of comorbidity tends to have less of an effect on Black than on White survival among elderly men with prostate cancer. Although 3-year mortality rates are relatively low for all groups, the absolute differences in survival between Black and White men are largest for men without comorbidities and narrow with increasing number of comorbidities. This pattern was most pronounced when examining counts of comorbidities but was also seen for individual comorbidities. Although racial differences in receipt of prostatectomy vary by comorbidity burden, differences in treatment do not explain racial differences in the effect of comorbidities on survival.
Despite the importance of comorbidities to outcomes, there have been relatively few prior studies examining racial differences in the relationship between comorbidity and outcome. One analysis of a North Carolina cohort found that the presence of hypertension or diabetes had a smaller impact on the development of disability among Blacks than among Whites (Kelley-Moore & Ferraro, 2004). Another study demonstrated similar patterns of association between the Charlson comorbidity index and mortality in Black and White women with newly diagnosed breast cancer (West et al., 1996). Interestingly, Freeman et al. (2004) in a study of prostate cancer patients also demonstrated a racial difference in the effect of comorbidity on survival. The study included 864 prostate cancer patients from a single city, and the authors collected data using chart review rather than administrative claims. Results demonstrated that racial differences in mortality were greatest for men with no comorbidities (HR = 1.75; 95% CI = 1.33–2.31) and disappeared for men with five or more comorbidities (HR = 0.90; 95% CI = 0.59–1.29), a pattern remarkably similar to that seen in our data (Freeman et al., 2004). Our study extends these findings to a national cohort of more than 50,000 men with localized disease, demonstrating a smaller racial disparity in survival among men with more comorbidities, a smaller effect of comorbidity on survival among Black men, and racial differences in the relationship between comorbidity and the use of prostatectomy.
What are the potential explanations for this finding? Several studies suggest that Blacks develop chronic illnesses at an earlier age than Whites (Dunlop et al., 2007). Thus, it is possible that Black men with chronic illnesses who are alive in their 60s and 70s are less “susceptible” to the impact of the illness because individuals who were at high risk of dying from their illness have died at an earlier age (Feinglass et al., 2007). This type of survivor bias would select for more resilient individuals among Blacks and could explain a lower effect of comorbidity on mortality among elderly Blacks. Another possible explanation relates to differences in severity of prostate cancer. If Black men have more aggressive or advanced prostate cancer than White men, they may be more likely to die from prostate cancer and less likely to die from other causes. A larger competing risk of death from prostate cancer among Black men than White men could result in a smaller effect of comorbidities on overall survival. Although unmeasured severity of prostate cancer among Blacks may explain some of the results, its contribution is likely to be relatively small given the focus on individuals with localized disease. Furthermore, the same effect was seen in the study by Freeman et al. (2004) that included more extensive information on stage and grade taken from clinical charts. Another possible explanation is that being diagnosed with a chronic illness is a marker of better access to or quality of care among Blacks. If this were true, the higher level of health care access and quality among Blacks with more comorbidities would counterbalance the increased impact of comorbidity on survival. Finally, it is possible that the difference in effect relates to racial differences in the severity of the comorbidities. For the great majority of comorbidities (except diabetes), we have information only on the presence or absence of the comorbidity, not the severity of the disease itself. If Whites had greater severity of disease for any given comorbidity (e.g., worse coronary heart failure), this may lead to a greater impact of the comorbidity on outcome among Whites. However, most evidence suggests that the severity of common comorbidities (e.g., diabetes, coronary heart failure, renal failure) is greater among Blacks than among Whites, suggesting that this level of unmeasured severity is more likely to have minimized than exaggerated the observed effect.
Interestingly, we also find a racial difference in the effect of comorbidity on the use of prostatectomy, although these differences do not mediate the relationship between race, comorbidity, and survival. Here the pattern is not linear, with the greatest racial differences seen in patients with no comorbidities and the smallest differences seen in patients with one or two comorbidities. This pattern may reflect the combined effect of several forces: Having one or two comorbidities may be a marker for better access to care and a more “level playing field” for Black and White men. Above this number of comorbidities, the overall burden of disease may overwhelm the equalizing effect of access to care, and prostatectomy is likely to become a relatively rare intervention, with greater use among individuals with greater access and resources.
Individual comorbidities were heterogeneous in terms of their association with race and the absolute risk of mortality. Despite this heterogeneity, examination of the patterns across individual comorbidities suggested that the results seen for the comorbidity count largely reflected a broad underlying pattern among the individual comorbidities rather than a pronounced impact of a small set of individual comorbidities. With the exception of hypothyroidism, hypertension, and complicated diabetes, individual comorbidities tended to be associated with increased risk of mortality for both Blacks and Whites. In general, the risk for Blacks versus Whites with a particular comorbidity tended to be smaller than for Blacks versus Whites without comorbidities, although the CIs for these risk estimates were broadly overlapping. Beyond this finding, the results for the individual comorbidities did not provide any particular insight on the mechanism for the underlying pathway. We caution that because the sample sizes for individual comorbidities, particularly for Blacks, were considerably smaller than for the comorbidity count, these comparisons had more limited power, and for this reason, we focused on broad patterns rather than specific categories.
The finding that the effect of comorbidities varies by race has important implications for studies of racial disparities in health and health care. Many studies adjust for comorbidity when examining racial differences, but few, if any, consider the interaction between comorbidity and race. We have previously demonstrated that racial differences in physician trust vary substantially by geographic location and socio-demographic characteristics (Armstrong, Ravenell, McMurphy, & Putt, 2007). It seems likely that this type of variation in disparity is more the rule than the exception—a reality that has important consequences for efforts to reduce disparities. If disparities are not uniform within a specific clinical situation but vary by the characteristics of the patient or even of their city of residence, it becomes critically important to understand which groups are at greatest risk before developing interventions to reduce disparities. For example, although programs to reduce racial disparities in prostate cancer survival might naturally focus on Black and White men with the greatest contact with the health care system, those men are also most likely to have multiple comorbidities and, as demonstrated in this study, the smallest disparity in survival. Similarly, we have previously demonstrated that disparities in prostate cancer treatment may vary widely between hospitals or geographic regions (Asch & Armstrong, 2007). Given the recent evidence that relatively little progress has been made in reducing cancer disparities over the last decade (Gross, Smith, Wolf, & Andersen, 2008), it is possible that an inadequate appreciation of the complex variation within any given disparity has limited the effectiveness of our efforts to reduce these disparities. It is hoped that increasing our understanding of this variation can lead to more effective interventions and a different story for the next decade.
This study has several limitations. We used administrative data to define the cohort and assess comorbidities, treatment, and outcome. Administrative data have limited accuracy in some settings, although considerable support exists for their use in defining surgical treatment, comorbidities, and survival (Fisher et al., 1992). The ability to identify prostate cancer patients from administrative data is less certain; however, we triangulated several approaches to maximize the accuracy of the case ascertainment. For several states, we were also able to compare the number of cases in our data with the number in the state SEER registries and found very similar numbers of cases. We studied a single disease, and the relationship between race, comorbidity, and survival in prostate cancer may not be generalizable to other diseases. As mentioned earlier, the number of Blacks in our study, while large, was substantially smaller than the number of Whites, so our ability to detect effects in Blacks was smaller than in Whites. Although the use of comorbidity number in addition to individual comorbidities reduced analytical issues with small numbers, comorbidity number is an imperfect estimate of comorbidity burden because all comorbidities are weighted similarly. Our study focused on men diagnosed with localized prostate cancer, and the generalizability of the finding to men with metastatic disease is unknown.
In summary, comorbid conditions have differential effects on survival after a diagnosis of localized prostate cancer between Black and White men. Racial disparities are most pronounced between Black and White men with no or few comorbidities and are not evident at higher levels of comorbidity.
Acknowledgments
This research was sponsored by the Center for Population Health and Health Disparities at the University of Pennsylvania under Public Health Services Grant P50-CA105641. The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; and the Research Data Assistance Center (ResDAC), University of Minnesota.
Appendix A Treatment Codes
Prostatectomy
Prostatectomy
60.3 Suprapubic prostatectomy
60.5 Radical prostatectomy
60.6 Other prostatectomy
60.69 Other prostatectomy
Prostatectomy: Subtotal
55801 Prostatectomy, perineal, subtotal (including control of postoperative bleeding,vasectomy, meatotomy, urethral calibration and/or dilation, and internal urethrotomy)
55821 Prostatectomy (including control of postoperative bleeding, vasectomy, meatotomy, urethral calibration and/or dilation, and internal urethrotomy); suprapubic, subtotal, one or two stages
55831 Prostatectomy (including control of postoperative bleeding, vasectomy, meatotomy, urethral calibration and/or dilation, and internal urethrotomy); retropubic, subtotal
Prostatectomy: Perineal
60.62 Perineal prostatectomy
55810 Prostatectomy, perineal radical;
55812 Prostatectomy, perineal radical; with lymph node biopsy(s) (limited pelvic lymph adenectomy)
55815 Prostatectomy, perineal radical; with bilateral pelvic lymphadenectomy, including external iliac, hypogastric and obturator nodes
Prostatectomy: Retropubic
60.4 Retropubic prostatectomy
55840 Prostatectomy, retropubic radical, with or without nerve sparing;
55842 Prostatectomy, retropubic radical, with or without nerve sparing; with lymph node biopsy(s) (limited pelvic lymphadenectomy)
55845 Prostatectomy, retropubic radical, with or without nerve sparing; with bilateral pelvic lymphadenectomy, including external iliac, hypogastric, and obturator nodes
55866 Laparoscopy, surgical prostatectomy, retropubic radical, including nerve sparing
Radiation Therapy
Radiation Therapy: General
V58.1 Radiotherapy
V67.1 Radiotherapy follow-up examination
V66.1 Convalescence following radiotherapy
77432 Stereotactic radiation treatment management of cerebral lesion(s) (complete course of treatment consisting of one session)
77470 Special treatment procedure (eg, total body irradiation, hemibody radiation, per oral, endocavitary or intraoperative cone irradiation)
77499 Unlisted procedure, therapeutic radiology treatment management
Radiation Therapy: External Beam
92.21 Superficial radiation
92.22 Orthovoltage radiation
92.23 Radioisotopic teleradiotherapy
92.24 Teleradiotherapy using protons
92.25 Teleradiotherapy using electrons
92.26 Teleradiotherapy of other particulate radiation
92.29 Other radiotherapeutic procedure
77261 Therapeutic radiology treatment planning; simple
77262 Therapeutic radiology treatment planning; intermediate
77263 Therapeutic radiology treatment planning; complex
77280 Therapeutic radiology simulation-aided field setting; simple
77285 Therapeutic radiology simulation-aided field setting; intermediate
77290 Therapeutic radiology simulation-aided field setting; complex
77295 Therapeutic radiology simulation-aided field setting; three-dimensional
77299 Unlisted procedure, therapeutic radiology clinical treatment planning
77300 Basic radiation dosimetry calculation, central axis depth dose calculation, TDF, NSD, gap calculation, off axis factor, tissue inhomogeneity factors, calculation of nonionizing radiation surface and depth dose, as required during course of treatment, only when prescribed by the treating physician
77305 Teletherapy, isodose plan (whether hand or computer calculated); simple (one or two parallel opposed unmodified ports directed to a single area of interest)
77310 Teletherapy, isodose plan (whether hand or computer calculated); intermediate (three or more treatment ports directed to a single area of interest)
77315 Teletherapy, isodose plan (whether hand or computer calculated); complex (mantle or inverted Y, tangential ports, the use of wedges, compensators, complex blocking, rotational beam, or special beam considerations)
77321 Special teletherapy port plan, particles, hemibody, total body
77332 Treatment devices, design and construction; simple (simple block, simple bolus)
77333 Treatment devices, design and construction; intermediate (multiple blocks, stents, bite blocks, special bolus)
77334 Treatment devices, design and construction; complex (irregular blocks, special shields, compensators, wedges, molds or casts)
77336 Continuing medical physics consultation, including assessment of treatment parameters, quality assurance of dose delivery, and review of patient treatment documentation in support of the radiation oncologist, reported per week of therapy
77370 Special medical radiation physics consultation
77401–77431 radiation treatment delivery OR management
77520–77525 Proton beam treatment delivery
Radiation Therapy: Brachytherapy
92.2 Therapeutic radiology and nuclear medicine (includes 92.27 Implantation or insertion of radioactive elements)
92.27 Implantation or insertion of radioactive elements
55860 Exposure of prostate, any approach, for insertion of radioactive substance;
55865 Exposure of prostate, any approach, for insertion of radioactive substance; with bilateral pelvic lymphadenectomy, including external iliac, hypogastric and obturator nodes
55862 Exposure of prostate, any approach, for insertion of radioactive substance; with lymph node biopsy(s) (limited pelvic lymphadenectomy)
55859 Transperineal placement of needles or catheters into prostate for interstitial radioelement application, with or without cystoscopy
77326 Brachytherapy isodose plan; simple (calculation made from single plane, one to four sources/ribbon application, remote afterloading brachytherapy, 1 to 8 sources)
77327 Brachytherapy isodose plan; intermediate (multiplane dosage calculations, application involving 5 to 10 sources/ribbons, remote afterloading brachytherapy, 9 to 12 sources)
77328 Brachytherapy isodose plan; complex (multiplane isodose plan, volume implant calculations, over 10 sources/ribbons used, special spatial reconstruction, remote afterloading brachytherapy, over 12 sources)
77331 Special dosimetry (e.g., TLD, microdosimetry) (specify), only when prescribed by the treating physician
77750–77799 Clinical brachytherapy
C1715 Brachytherapy needle
C1716 Brachytherapy source, gold 198, per source
C1717 Brachytherapy source, high dose rate iridium 192, per source
C1718 Brachytherapy source, iodine 125, per source
C1719 Brachytherapy source, non-high dose rate iridium 192, per source
C1720 Brachytherapy source, palladium 103, per source
C1728 Catheter, brachytherapy seed administration
C2632 Brachytherapy solution, Iodine-125, per mci
C2633 Brachytherapy source, cesium-131, per source
C2634 Brachytherapy source, high activity, iodine 125, per source
C2635 Brachytherapy source, high activity, palladium 103, per source
C2636 Brachytherapy linear source, palladium 103, per 1 mm
Q3001 Radioelements for brachytherapy, any type, each
Appendix B Odds Ratios (95% CI) of Treatment Versus No Treatment, Prostatectomy Versus No Prostatectomy, and Radiation Versus No Radiation for Whites and Blacks for Differing Numbers of Comorbidities in Model Adjusted for Age, Socioeconomic Status, and Contextual Variablesa
| Race-Specific Rates |
Comorbidity-Specific Rates Relative Risk (95% CI) Black:Whitec | |||
|---|---|---|---|---|
| Number of Comorbidities | Odds Ratios (95% CI) |
Hypothesis Tests |
||
| Whitesa | Blacksa | p/pAb | ||
| Any versus no treatment | ||||
| None | 1.00 | 1.00 | NA | 0.65 (0.54, 0.78) |
| 1 | 1.14 (1.09, 1.19) | 1.35 (1.18, 1.54) | .20/.40 | 0.77 (0.64, 0.92) |
| 2 | 1.02 (0.96, 1.07) | 1.26 (1.08, 1.46) | .21/.40 | 0.80 (0.66, 0.98) |
| 3 | 0.81 (0.75, 0.87) | 0.97 (0.81, 1.16) | .074/.222 | 0.78 (0.62, 0.98) |
| 4 | 0.72 (0.66, 0.79) | 0.78 (0.63, 0.98) | .004/.030 | 0.71 (0.54, 0.93) |
| ≥4 | 0.52 (0.47, 0.57) | 0.60 (0.49, 0.74) | .005/.040 | 0.76 (0.58, 0.99) |
| Overall p = .110d | ||||
| Radiation versus no treatment or prostatectomy | ||||
| None | 1 | 1 | NA | 0.97 (0.80, 1.18) |
| 1 | 1.07 (1.02, 1.12) | 1.15 (1.00, 1.33) | .36/.88 | 1.04 (0.86, 1.26) |
| 2 | 1.09 (1.03, 1.15) | 1.13 (0.96, 1.32) | .64/.88 | 1.01 (0.82, 1.24) |
| 3 | 0.95 (0.88, 1.02) | 1.01 (0.84, 1.23) | .54/.88 | 1.04 (0.81, 1.31) |
| 4 | 0.86 (0.78, 0.95) | 0.88 (0.69, 1.11) | .88/.88 | 0.99 (0.74, 1.32) |
| ≥4 | 0.67 (0.46, 0.74) | 0.73 (0.58, 0.91) | .52/.88: | 1.05 (0.80, 1.39) |
| Overall p = .96d | ||||
| Prostatectomy versus no treatment or radiation | ||||
| None | 1.00 | 1.00 | NA | 0.48 (0.36, 0.63) |
| 1 | 1.13 (1.06, 1.19) | 1.46 (1.20, 1.78) | .014/.056 | 0.62 (0.47, 0.81) |
| 2 | 0.91 (0.85, 0.98) | 1.32 (1.06, 1.65) | .002/.010 | 0.69 (0.51, 0.92) |
| 3 | 0.72 (0.65, 0.80) | 0.87 (0.65, 1.18) | .227/.681 | 0.58 (0.40, 0.83) |
| 4 | 0.66 (0.57, 0.76) | 0.67 (0.45, 1.01) | .942/.912 | 0.48 (0.30, 0.77) |
| ≥4 | 0.46 (0.38, 0.55) | 0.45 (0.29, 0.68) | .876/.942 | 0.46 (0.28, 0.75) |
| Overall p = .027d | ||||
Relative risk presented compared with no comorbidity for each race.
p Value for test of the hypothesis that the odds of death for Blacks with a specific number of comorbidities versus Blacks with no comorbidities is identical to the odds for Whites for the same comorbidities. Values shown are unadjusted/adjusted for multiple comparisons.
Relative risk for Blacks relative to Whites with the same number of comorbidities.
p Value for likelihood ratio test.
References
- Alibhai SM, Leach M, Tomlinson G, Krahn MD, Fleshner N, Holowaty E, et al. 30-Day mortality and major complications after radical prostatectomy: Influence of age and comorbidity. Journal of the National Cancer Institute. 2005;97:1525–1532. doi: 10.1093/jnci/dji313. [DOI] [PubMed] [Google Scholar]
- Armstrong K, Ravenell KL, McMurphy S, Putt M. Racial/ethnic differences in physician distrust in the United States. American Journal of Public Health. 2007;97:1283–1289. doi: 10.2105/AJPH.2005.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch DA, Armstrong K. Aggregating and partitioning populations in health care disparities research: Differences in perspective. Journal of Clinical Oncology. 2007;25:2117–2121. doi: 10.1200/JCO.2006.09.3336. [DOI] [PubMed] [Google Scholar]
- Begg CB, Riedel ER, Bach PB, Kattan MW, Schrag D, Warren JL, et al. Variations in morbidity after radical prostatectomy. New England Journal of Medicine. 2002;346:1138–1144. doi: 10.1056/NEJMsa011788. [DOI] [PubMed] [Google Scholar]
- Chirinos JA, Veerani A, Zambrano JP, Schob A, Perez G, Mendez AJ, et al. Evaluation of comorbidity scores to predict all-cause mortality in patients with established coronary artery disease. International Journal of Cardiology. 2007;117:97–102. doi: 10.1016/j.ijcard.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Concato J, Horwitz RI, Feinstein AR, Elmore JG, Schiff SF. Problems of comorbidity in mortality after prostatectomy. JAMA: The Journal of the American Medical Association. 1992;267:1077–1082. [PubMed] [Google Scholar]
- Dunlop DD, Song J, Manheim LM, Daviglus ML, Chang RW. Racial/ethnic differences in the development of disability among older adults. American Journal of Public Health. 2007;97:2209–2215. doi: 10.2105/AJPH.2006.106047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Medical Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- Feinglass J, Lin S, Thompson J, Sudano J, Dunlop D, Song J, et al. Baseline health, socioeconomic status, and 10-year mortality among older middle-aged Americans: Findings from the Health and Retirement Study, 1992 2002. Journal of Gerontology. Series B, Psychological Sciences and Social Sciences. 2007;62:S209–S217. doi: 10.1093/geronb/62.4.s209. [DOI] [PubMed] [Google Scholar]
- Ferrier MB, Spuesens EB, Le Cessie S, Baatenburg de Jong RJ. Comorbidity as a major risk factor for mortality and complications in head and neck surgery. Archives of Otolaryngology—Head and Neck Surgery. 2005;131:27–32. doi: 10.1001/archotol.131.1.27. [DOI] [PubMed] [Google Scholar]
- Fisher ES, Whaley FS, Krushat WM, Malenka DJ, Fleming C, Baron JA, et al. The accuracy of Medicare's hospital claims data: Progress has been made, but problems remain. American Journal of Public Health. 1992;82:243–248. doi: 10.2105/ajph.82.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad MN, Mayo CP, Funkhouser EM, Irene Hall H, Urban DA, Kiefe CI. Comorbidity independently predicted death in older prostate cancer patients, more of whom died with than from their disease. Journal of Clinical Epidemiology. 2004;57:721–729. doi: 10.1016/j.jclinepi.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Freeman VL, Durazo-Arvizu R, Keys LC, Johnson MP, Schafernak K, Patel VK. Racial differences in survival among men with prostate cancer and comorbidity at time of diagnosis. American Journal of Public Health. 2004;94:803–808. doi: 10.2105/ajph.94.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godley PA, Schenck AP, Amamoo MA, Schoenbach VJ, Peacock S, Manning M, et al. Racial differences in mortality among Medicare recipients after treatment for localized prostate cancer. Journal of the National Cancer Institute. 2003;95:1702–1710. doi: 10.1093/jnci/djg094. [DOI] [PubMed] [Google Scholar]
- Gomez SL, O'Malley CD, Stroup A, Shema SJ, Satariano WA. Longitudinal, population-based study of racial/ethnic differences in colorectal cancer survival: Impact of neighborhood socioeconomic status, treatment and comorbidity. BMC Cancer. 2007;7:193. doi: 10.1186/1471-2407-7-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CP, Smith BD, Wolf E, Andersen M. Racial disparities in cancer therapy: Did the gap narrow between 1992 and 2002? Cancer. 2008;112:900–908. doi: 10.1002/cncr.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–803. [Google Scholar]
- Institute of Medicine of the National Academies Unequal treatment: Confronting racial and ethnic disparities in health care. 2002 Retrieved March 13, 2009, from http://www.iom.edu/?id=16740. [PMC free article] [PubMed]
- Johnson NE. The racial crossover in comorbidity, disability, and mortality. Demography. 2000;37:267–283. [PubMed] [Google Scholar]
- Kelley-Moore JA, Ferraro KF. The Black/White disability gap: Persistent inequality in later life? Journal of Gerontology. Series B, Psychological Sciences and Social Sciences. 2004;59:S34–S43. doi: 10.1093/geronb/59.1.s34. [DOI] [PubMed] [Google Scholar]
- Kestenbaum B. A description of the extreme aged population based on improved Medicare enrollment data. Demography. 1992;29:565–580. [PubMed] [Google Scholar]
- Kung H-C, Hoyert DL, Xu J, Murphy SL. Table 3. Number of deaths and death rates by age, race, and sex: United States, 2005. National Vital Statistics Reports. 2008 April 24;56(10) Retrieved January 17, 2008, from http://www.cdc.gov/nchs/data/nvsr/nvsr56/nvsr56_10.pdf. [Google Scholar]
- Lesens O, Methlin C, Hansmann Y, Remy V, Martinot M, Bergin C, et al. Role of comorbidity in mortality related to Staphylococcus aureus bacteremia: A prospective study using the Charlson weighted index of comorbidity. Infection Control and Hospital Epidemiology. 2003;24:890–896. doi: 10.1086/502156. [DOI] [PubMed] [Google Scholar]
- Librero J, Peiro S, Ordinana R. Chronic comorbidity and outcomes of hospital care: Length of stay, mortality, and readmission at 30 and 365 days. Journal of Clinical Epidemiology. 1999;52:171–179. doi: 10.1016/s0895-4356(98)00160-7. [DOI] [PubMed] [Google Scholar]
- Manton KG, Poss SS, Wing S. The Black/White mortality crossover: Investigation from the perspective of the components of aging. Gerontologist. 1979;19:291–300. doi: 10.1093/geront/19.3.291. [DOI] [PubMed] [Google Scholar]
- Martins M, Blais R. Evaluation of comorbidity indices for inpatient mortality prediction models. Journal of Clinical Epidemiology. 2006;59:665–669. doi: 10.1016/j.jclinepi.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Massey D, Denton N. The dimensions of residential segregation. Social Forces. 1988;67:281–315. [Google Scholar]
- Mayberry RM, Coates RJ, Hill HA, Click LA, Chen VW, Austin DF, et al. Determinants of Black/White differences in colon cancer survival. Journal of the National Cancer Institute. 1995;87:1686–1693. doi: 10.1093/jnci/87.22.1686. [DOI] [PubMed] [Google Scholar]
- McGee D, Cooper R, Liao Y, Durazo-Arvizu R. Patterns of comorbidity and mortality risk in Blacks and Whites. Annals of Epidemiology. 1996;6:381–385. doi: 10.1016/s1047-2797(96)00058-0. [DOI] [PubMed] [Google Scholar]
- Miles TP, Bernard MA. Morbidity, disability, and health status of Black American elderly: A new look at the oldest-old. Journal of the American Geriatrics Society. 1992;40:1047–1054. doi: 10.1111/j.1532-5415.1992.tb04485.x. [DOI] [PubMed] [Google Scholar]
- Monami M, Lambertucci L, Lamanna C, Lotti E, Marsili A, Masotti G, et al. Are comorbidity indices useful in predicting all-cause mortality in Type 2 diabetic patients? Comparison between Charlson index and disease count. Aging Clinical and Experimental Research. 2007;19:492–496. doi: 10.1007/BF03324736. [DOI] [PubMed] [Google Scholar]
- The National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology: Prostate cancer. 2004 doi: 10.6004/jnccn.2010.0012. Retrieved March 13, 2009, from http://www.nccn.org/professionals/physician_gls/PDF/prostate.pdf. [DOI] [PubMed]
- Ouellette JR, Small DG, Termuhlen PM. Evaluation of Charlson-age comorbidity index as predictor of morbidity and mortality in patients with colorectal carcinoma. Journal of Gastrointestinal Surgery. 2004;8:1061–1067. doi: 10.1016/j.gassur.2004.09.045. [DOI] [PubMed] [Google Scholar]
- Polednak AP, Flannery JT. Black versus White racial differences in clinical stage at diagnosis and treatment of prostatic cancer in Connecticut. Cancer. 1992;70:2152–2158. doi: 10.1002/1097-0142(19921015)70:8<2152::aid-cncr2820700824>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Sachdev M, Sun JL, Tsiatis AA, Nelson CL, Mark DB, Jollis JG. The prognostic importance of comorbidity for mortality in patients with stable coronary artery disease. Journal of the American College of Cardiology. 2004;43:576–582. doi: 10.1016/j.jacc.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Schapira MM, McAuliffe TL, Nattinger AB. Treatment of localized prostate cancer in African-American compared with Caucasian men. Less use of aggressive therapy for comparable disease. Medical Care. 1995;33:1079–1088. doi: 10.1097/00005650-199511000-00002. [DOI] [PubMed] [Google Scholar]
- Sudano JJ, Baker DW. Explaining US racial/ethnic disparities in health declines and mortality in late middle age: The roles of socioeconomic status, health behaviors, and health insurance. Social Science & Medicine. 2006;62:909–922. doi: 10.1016/j.socscimed.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. Journal of Clinical Epidemiology. 2004;57:1288–1294. doi: 10.1016/j.jclinepi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among Black and White patients with breast cancer. JAMA: The Journal of the American Medical Association. 2005;294:1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. In lung cancer patients, age, race-ethnicity, gender and smoking predict adverse comorbidity, which in turn predicts treatment and survival. Journal of Clinical Epidemiology. 2004;57:597–609. doi: 10.1016/j.jclinepi.2003.11.002. [DOI] [PubMed] [Google Scholar]
- West DW, Satariano WA, Ragland DR, Hiatt RA. Comorbidity and breast cancer survival: A comparison between Black and White women. Annals of Epidemiology. 1996;6:413–419. doi: 10.1016/s1047-2797(96)00096-8. [DOI] [PubMed] [Google Scholar]
- Wilt TJ, Macdonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: The comparative effectiveness and harms of treatments for clinically localized prostate cancer. Annals of Internal Medicine. 2008;148:435–448. doi: 10.7326/0003-4819-148-6-200803180-00209. [DOI] [PubMed] [Google Scholar]
- Yancik R, Wesley MN, Ries LA, Havlik RJ, Long S, Edwards BK, et al. Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: A population-based study. Cancer. 1998;82:2123–2134. [PubMed] [Google Scholar]