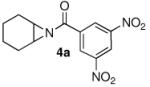
Abstract
 Conditions for the phosphoric acid-catalyzed highly enantioselective ring opening of meso-aziridines with a series of functionalized aromatic thiol nucleophiles are described. The procedure utilizes commercially available aromatic thiols, a series of meso-aziridines, and a catalytic amount of VAPOL Phosphoric acid to explore the substrate scope of this highly enantioselective reaction.
Conditions for the phosphoric acid-catalyzed highly enantioselective ring opening of meso-aziridines with a series of functionalized aromatic thiol nucleophiles are described. The procedure utilizes commercially available aromatic thiols, a series of meso-aziridines, and a catalytic amount of VAPOL Phosphoric acid to explore the substrate scope of this highly enantioselective reaction.
Aziridines are considered by many to be useful precursors for the production of advanced targets of interest primarily because of unique ring opening chemistry that can allow for direct access to a variety of chiral amines.1 In the case of nucleophilic ring opening of aziridines, a judicious choice of the nucleophile can allow for the preparation of a variety of functionalized products.1a
We believe many have the opinion that stereo-controlled chemistry would be vital for achieving a high degree of synthetic utility for such ring opening strategies. This control can be realized in cases where chiral catalysts allow for ring-opening desymmetrization reactions of meso-aziridines.2 Examples include the implementation of catalytic chiral metal complexes that can allow for a high enantioselectivity of the resulting ring opened product using primarily azide or cyanide based nucleophiles.3 As part of a recent study that has focused on chiral Brønsted acid-catalyzed additions using heteroatom nucleophiles4 we discovered unique reactivity whereby chiral phosphoric acids5 could activate aziridines for enantioselective ring-opening chemistry with trimethylsilyl azide (TMS-N3).6
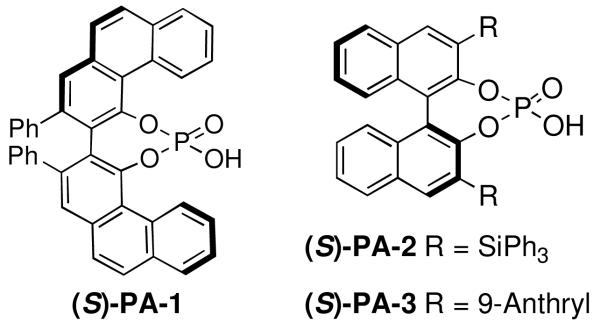
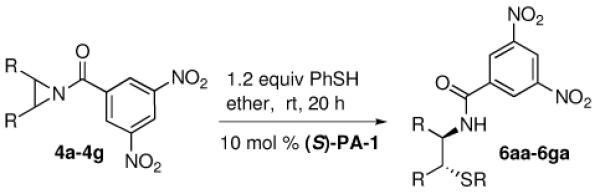
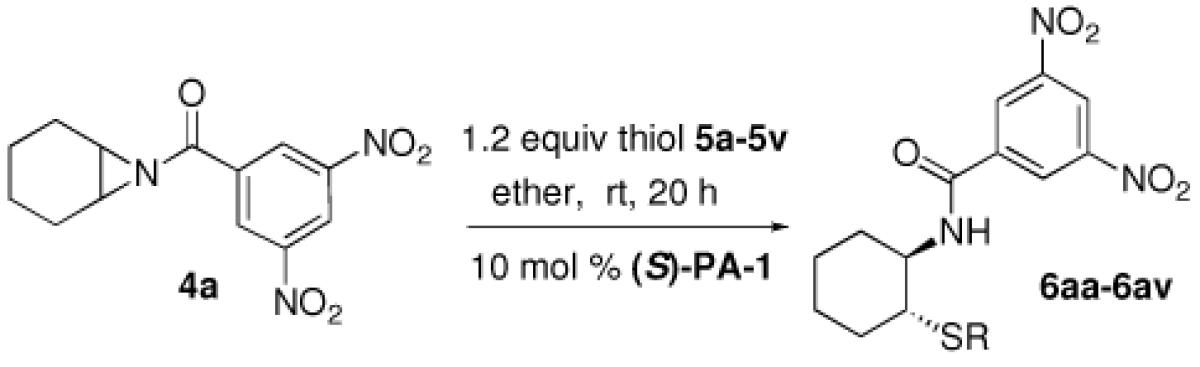
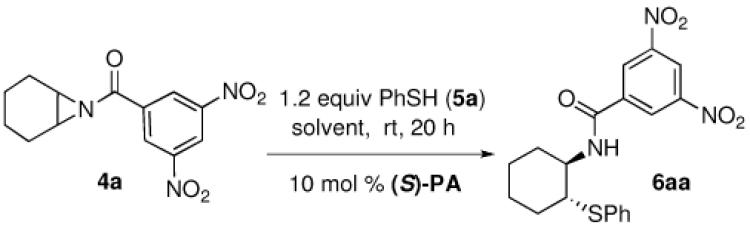
Our discovery prompted us to expand the scope of this chemistry by investigating the ring opening with sulfur-based nucleophiles. This expansion of substrate scope would allow for the preparation of interesting chiral β-aminothioethers.7 At the start of our study the previous methodology reporting catalytic asymmetric ring opening desymmetrization reactions utilizing thiols were rare, with low enantiomeric excesses being found.8 Late in our investigation using thiols a publication by Della Sala9 described an enantioselective ring opening of meso aziridines using (phenylthio)trimethylsilane (TMS-SPh) and the catalyst system we reported6 in our above azide chemistry. Interestingly, this report implied that silylated nucleophiles were required, and the authors explained their chemistry by invoking our previously postulated mechanism involving the importance of silicon being present on the nucleophile. However, in this communication we reveal that silylated thiols are not necessary as simple unsubstituted thiols can be used to obtain the same ring opened product with excellent yield and enantioselectivity using the same chiral VAPOL phosphoric acid catalyst PA-1 (Figure 1).
Figure 1.
Chiral Phosphoric Acids
During our optimization process we quickly became aware that silylated nucleophiles were not necessary for the phosphoric acid-catalyzed thiophenol ring opening reactions of substituted N-benzoyl aziridine substrates. As in the chemistry by Della Sala we found that the 3,5 dinitro substitution was necessary to achieve the best enantioslectvity.9 Non-polar solvents like toluene provided moderate enantioselectivity for the reaction (entry 1), while dichloromethane (DCM) gave improved ee (entry 2). Polar solvents like EtOAc (entry 3), MeCN (entry 4), or tetrahydrofuran (THF) gave much lower enantioselectivity (entry 5), following a trend found in many other reactions involving chiral phosphoric acid-catalysis.4a,6 Both methyl t-butyl ether (entry 6) and diethyl ether (entry 7) were excellent solvents for the thiophenol ring opening, with the product found in these conditions having excellent yield and ee. Several additional catalysts were screened, but those evaluated gave poor selectivity results. For example, with catalyst PA-2 (entry 8) or PA-3 (entry 9) we found the enantioselectivities dropped to low levels. Unfortunately, when the catalyst loading was decreased to 5 mol % a lower yield and ee for 6aa was also found (entry 10).
Inspired by the high degree of catalytic activity and selectivity, we wanted to establish the generality of the reaction. In Table 2 we show the details of our investigations into varying the thiol substrate. We found that the reaction was very general for arene thiols. For example, we were able to perform the reaction with naphthyl-2-thiol (entry 2) and a variety of ortho, meta, or para substituted thiophenols (entries 3-7). Electron withdrawing substituents on the thiophenol were not detrimental to the reactivity and selectivity as chloro-, fluoro-, trifluoromethyl- groups were all tolerated (entries 8-11). Likewise, electron-donating substituents on the arene were shown to be compatible with phenoxy, methoxy, and methylsulfide groups in the para position (entries 12-14). Esters were shown to be compatible with the ring opening chemistry (entry 15), as was 2-methylfuran-3-thiol (entry 16), benzo[d]thiazole-2-thiol (entry 17), and 1-phenyl-1H-tetrazole-5-thiol (entry 18), albeit in moderate yield and selectivity. Unfortunately, the use of alkyl thiols also leads to lower enantioselectivities. For example, while benzyl thiols were moderately good substrates (entries 19-20), longer chain thiols like 2-phenylethanethiol (entry 21) and n-hexanethiol (entry 22) were relatively poor reaction partners with these conditions.
Table 2.
Enantioselective Ring Opening of 4a with Thols 5a-5v.
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | thiol | yield, %a | ee, %b | entry | thiol | yield, %a | ee, %b |
| 1 |  |
97 | 96 | 12 |  |
81 | 89 |
| 2 |  |
99 | 95 | 13 | 99 | >99 | |
| 3 |  |
94 | 93 | 14 | 78 | 90 | |
| 4 | 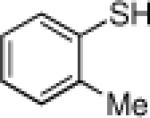 |
88 | 94 | 15 | 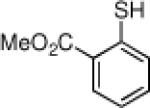 |
72 | 74 |
| 5 |  |
85 | 93 | 16 | 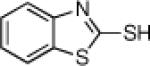 |
75 | 55 |
| 6 | 99 | 93 | 17 |  |
71 | 82 | |
| 7 | 96 | 93 | 18 | 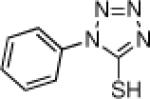 |
78 | 61 | |
| 8 | 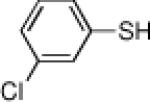 |
87 | 96 | 19 | 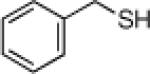 |
86 | 59 |
| 9 | 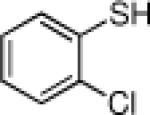 |
89 | 98 | 20 |  |
42 | 62 |
| 10 |  |
85 | 91 | 21 | 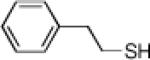 |
40 | 32 |
| 11 | 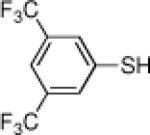 |
70 | 84 | 22 | HS–n-hexyl | 15 | 18 |
Isolated yields
Ee values were determined by chiral-HPLC (see Supporting Information).
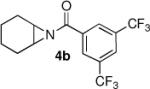
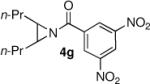
We were also pleased to find that the aziridine substrate could be varied with little compromise in yield or enantioselectivity (Table 3). Our previous study with TMSN3 ring openings utilized aziridines with nitrogen protected by a 3,5-trifluoromethyl substituted benzoyl group.6 However, in these thiol studies this substrate, while reactive, gave a much lower enantioselectivity for the ring opened product (entry 2). Substitution in the para position of the benzoyl group on the aziridine proved to be detrimental to the selectivity, with the ring opened product being obtained in a nearly racemic form (entry 3).
Table 3.
Enantioselective Ring Opening of Aziridines 4a-4g with thiophenol.
Isolated yields
Ee values were determined by chiral-HPLC (see Supporting Information).
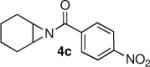
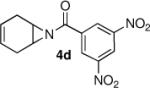
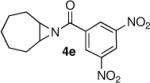
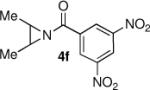
We continued the study by investigating the reaction with two additional meso aziridines with fused ring systems and two substrates with acyclic substituents. The fused cyclohexene based substrate 4d was shown to be an excellent reaction partner with thiophenol (entry 4). A fused cycloheptane 4e was also a suitable substrate for the reaction (entry 5). Both methyl (entry 6) and n-propyl (entry 7) 1,2-disubstituted meso aziridines could be used in the ring opening chemistry, providing high yield and enantioselectivity of the respective products using PA-1 as the catalyst.
Our discovery that simple, non-silylated aromatic thiols can be used for these chiral phosphoric acid-catalyzed additions strongly suggests that a more simple hydrogen-bond activation of the aziridine N-acyl moiety and the incoming thiol could be invoked to explain the mechanism.10 This is in contrast to the previous phosphoric acid catalyzed methodology for aziridine ring openings that may be following a mechanism based on silicon catalysis.6,9
In conclusion, we have discovered a catalytic enantioselective process for the ring opening of meso N-acyl aziridines in a high yielding, enantioselective manner. In comparison to known methods,8,9 we believe our procedure is attractive due to the reactions procedural simplicity. Finally, the reaction is the first of its type to tolerate a wide range of aromatic and heteroaromatic thiols.
Supplementary Material
Table 1.
Optimization of the Aziridine Ring-Opening with PhSH
 | ||||
|---|---|---|---|---|
| entry | catalyst (S) | solvent | yield, %a | ee, %b |
| 1 | PA-1 | toluene | 74 | 70 |
| 2 | PA-1 | DCM | 91 | 86 |
| 3 | PA-1 | EtOAc | 89 | 31 |
| 4 | PA-1 | MeCN | 82 | 40 |
| 5 | PA-1 | THF | 95 | 12 |
| 6 | PA-1 | MTBE | 99 | 96 |
| 7 | PA-1c | ether | 95 | 97 |
| 8 | PA-2 | ether | 78 | 0 |
| 9 | PA-3 | ether | 64 | 11 |
| 10 | PA-1d | ether | 65 | 89 |
Isolated yields
Ee values were determined by chiral-HPLC (see Supporting Information).
With (R)-PA-1 as the catalyst a 96 % yield and 96 % ee favoring the opposing enantiomer was found for product 6aa.
Reaction with 5 mol % of catalyst (S)-PA-1.
Acknowledgment
We thank the Petroleum Research Fund (PRF 45899-G1) and the National Institutes of Health (NIH GM-082935) for financial support. We thank Dr. Emily B. Rowland and Dr. Gerald B. Rowland for the preparation of important starting materials and for helpful discussions.
Footnotes
Supporting Information Available. Please see for experimental procedures, characterization, chiral HPLC conditions, and spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.a Yudin AK. Aziridines and Epoxides in Organic Synthesis. Wiley-VCH; Weinheim: 2006. [Google Scholar]; b Masahiko H, Ono K, Hoshimi H, Oguni Nobuki. Tetrahedron. 1996;52:7817. [Google Scholar]
- 2.For a review on the desymmetrization of meso aziridines please see: Schneider C. Angew. Chem., Int. Ed. 2009;48:2082. doi: 10.1002/anie.200805542.
- 3.a Li Z, Fernandez M, Jacobsen EN. Org. Lett. 1999;1:1611. doi: 10.1021/ol990992h. [DOI] [PubMed] [Google Scholar]; b Mita T, Fujimori I, Wada R, Wen J, Kanai M, Shibasaki M. J. Am. Chem. Soc. 2005;127:11252. doi: 10.1021/ja053486y. [DOI] [PubMed] [Google Scholar]; c Fukuta Y, Mita T, Fukuda N, Kanai M, Shibasaki M. J. Am. Chem. Soc. 2006;128:6312. doi: 10.1021/ja061696k. [DOI] [PubMed] [Google Scholar]; d Fujimori I, Mita T, Maki K, Shiro M, Saro A, Furusho S, Kanai M, Shibasaki M. Tetrahedron. 2007;63:5820. [Google Scholar]; e Wu B, Gallucci JC, Parquette JR, RajanBabu TV. Angew. Chem., Int. Ed. 2009;48:1126. doi: 10.1002/anie.200804415. [DOI] [PubMed] [Google Scholar]
- 4.a Rowland GB, Zhang H, Rowland EB, Chennamadhavuni S, Wang Y, Antilla JC. J. Am. Chem. Soc. 2005;127:15696. doi: 10.1021/ja0533085. [DOI] [PubMed] [Google Scholar]; b Liang Y, Rowland EB, Rowland GB, Perman JA, Antilla JC. Chem. Commun. 2007:4477. doi: 10.1039/b709276h. [DOI] [PubMed] [Google Scholar]; c Li G, Fronczek FR, Antilla JC. J. Am. Chem. Soc. 2008;130:12216. doi: 10.1021/ja8033334. [DOI] [PubMed] [Google Scholar]
- 5.For reviews with considerable chiral phosphoric acid coverage see: Akiyama T, Itoh J, Fuchibe K. Adv. Synth. Catal. 2006;348:999.Connon SJ. Angew. Chem., Int. Ed. 2006;45:3909. doi: 10.1002/anie.200600529.Akiyama T. Chem. Rev. 2007;107:5744. doi: 10.1021/cr068374j.Terada M. Chem. Commun. 2008:4097. doi: 10.1039/b807577h.Adair G, Mukherjee S, List B. Aldrichchimica Acta. 2008;41:31.
- 6.Rowland EB, Rowland GB, Rivera-Otero E, Antilla JC. J. Am. Chem. Soc. 2007;129:12084. doi: 10.1021/ja0751779. [DOI] [PubMed] [Google Scholar]
- 7.a Mellah M, Voituriez A, Schulz E. Chem. Rev. 2007;107:5133. doi: 10.1021/cr068440h. [DOI] [PubMed] [Google Scholar]; b Pellissier H. Tetrahedron. 2007;63:1297. [Google Scholar]
- 8.a Hayashi M, Ono K, Oshimi H, Oguni N. Tetrahedron. 1996;52:7817. [Google Scholar]; b Luo Z–B, Hou X–L, Dai L–X. Tetrahedron; Asymmetry. 2007;18:443. [Google Scholar]; c Wang Z, Sun X, Ye S, Wang W, Wang B, Wu J. Tetrahedron; Asymmetry. 2008;19:964. [Google Scholar]; d Lattanzi A, Della Sala G. Eur. J. Org. Chem. 2009:1845. [Google Scholar]
- 9.Della Sala G, Lattanzi A. Org. Lett. 2009;11:3330. doi: 10.1021/ol901209n. [DOI] [PubMed] [Google Scholar]
- 10.For a description of this type of mechanism with regard to imine activations see a “three point contact model” description in: Simon L, Goodman JM. J. Am. Chem. Soc. 2008;130:8741. doi: 10.1021/ja800793t.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.