Abstract
Little is known about the pre-biotic mechanisms that initiated the bioavailability of phosphorus, an element essential to life. A better understanding of phosphorus speciation in modern earth environments representative of early earth, may help to elucidate the origins of bioavailable phosphorus. This paper presents the first quantitative measurements of phosphite in a pristine geothermal pool representative of early earth. Phosphite and phosphate were initially identified and quantified in geothermal pool and stream samples at Hot Creek Gorge near Mammoth Lakes, California using suppressed conductivity ion chromatography. Results confirmed the presence of 0.06 ± 0.02 μM of phosphite and 0.05 ± 0.01 μM of phosphate in a geothermal pool. In the stream, phosphite concentrations were below detection limit (0.04 μM) and phosphate was measured at 1.06 ± 0.36 μM. The presence of phosphite in the geothermal pool was confirmed using both chemical oxidation and ion chromatography/mass spectrometry.
Introduction
Phosphorus, an element essential for life, is present in every living cell. Despite the ubiquity of this element in modern biological systems, little is known about how phosphorus initially became bioactive on early earth. The vast majority of elemental phosphorus on early planet earth was stored in the calcium phosphate mineral apatite, which remains the largest reservoir of phosphorus in the earth’s crust today (1–4). In this form, phosphorus is not readily water soluble, even though phosphate species in solution are most thermodynamically stable (5). Phosphite salts, from terrestrial or extraterrestrial sources (5), however, are approximately 1,000 times more soluble in water than calcium phosphate (6). A number of recent publications have postulated that environmental forces have transformed the fully-oxidized phosphorus in apatite into reduced phosphorus compounds, including gaseous phosphine (7,8), hypophosphite, and phosphite (6,9), and that these reduced forms of phosphorus may have had a significant role in biogenesis (5). Recent evidence supports this hypothesis. For example, microorganisms, such as Desulfotignum phosphitoxidans, have the ability to utilize the energy produced in the reduction of phosphite by sulfate for metabolism (10,11). This ability indicates that phosphite may have been present as a biologically active molecule on ancient earth.
Geothermal pools such as the one investigated in the current study, are windows to the reducing environment below the earth’s crust and are representative of the reducing environment of primitive earth. Extensive studies of the geothermal pools at Hot Creek Gorge near Mammoth Lakes, one of the largest sources of arsenic to the Los Angeles Aqueduct (12–14), have shown that naturally occurring arsenite (arsenic in the +III oxidation state) is rapidly oxidized to arsenate (+V oxidation state) once the geothermal water contacts the surface and even after it dilutes into the stream (15). Furthermore, the rapid oxidation is known to be bacterially mediated in both the pools and the stream. Arsenic and phosphorus belong to the same group on the period table and share similar chemical and biological properties. In fact, arsenate’s ability to mimic phosphate is thought to be the main mechanism by which arsenate interferes with cell activity and is toxic to cells. Also, the relative concentration of arsenite to arsenate (15,16) suggest measurements of water in geothermal pools of Hot Creek are indicative of a reducing environment.
This paper presents the first quantitative evidence of naturally occurring phosphite in water. This compound was measured in pristine geothermal pools collected from Hot Creek Gorge near Mammoth Lakes, California and supports the theory that elemental phosphorus was first incorporated into living organisms by cycling through phosphite.
Experimental
Sampling
Water samples were collected from Hot Creek Gorge near Mammoth Lakes, California during four separate field campaigns conducted between September 25, 2004 and October 2, 2007. Four separate geothermal pools and the stream were sampled throughout the course of this study. This paper will focus on samples collected on January 13, 2007 and October 2, 2007 from the same active geothermal pool (Geothermal Pool #1) and five meters downstream from the pool in the creek. Measurements from previous campaigns are summarized in Supplementary Information Table S1. Runoff from several active geothermal pools, including Geothermal Pool #1, feed into Hot Creek, which is fed from pristine mountain snow-melt. Geothermal pool and stream samples were collected at a depth of 1 meter with a Teflon scooper (Waterra, UK) and transferred to 500 mL amber HDPE bottles. The scooper and bottles were rinsed on-site with water from the respective sampling site prior to sample collection. The samples were transported to a nearby field workstation and were filtered through 0.2 μM GTTP membrane filters (Millipore, Ireland) using polysulfone field filtration units (PN 673-126, Nalgene). The filtered samples were immediately transferred to 125 mL amber HDPE bottles and were stored continuously at 4°C prior to analysis. In the laboratory, all samples were transferred to 10 mL clear polystyrene sample vials (PN 055058, Dionex) prior to analysis and continuously stored in a refrigerated autosampler tray at 4°C. All samples were initially analyzed within six days of collection. Water (Barnstead; 18.2 MΩ) was generated immediately prior to analysis and was used as the laboratory blank sample.
Sample Analysis and Quality Assurance
All samples were analyzed with a suppressed conductivity ion chromatography system (Dionex, DX 600) configured with a CD25 suppressed conductivity detector using a method previously published by our group (17–19). The system was equipped with a GS50 gradient pump and an EG40 electrolytic eluent generator configured with a potassium hydroxide (KOH) eluent cartridge. The KOH gradient was as follows: 10 mM from 0 to 10 minutes; 10 mM – 30 mM from 10 to 11 minutes; and 30 mM from 11 to 18 minutes. Full-loop injections onto the high-capacity 4 mm alkanol quaternary ammonium columns (Dionex AS11 HC and AG11 HC) were performed using an autosampler (Dionex AS50) equipped with an 800 μL injection loop to enhance the sensitivity and enable trace analysis. Inherent in the use of large injection loops for quantitative studies is the risk of column overload that may compromise linear response of detectors or shift retention times. However, the added sensitivity made possible with this particular system configuration outweighed the restrictions imposed by the use of the large injection loop.
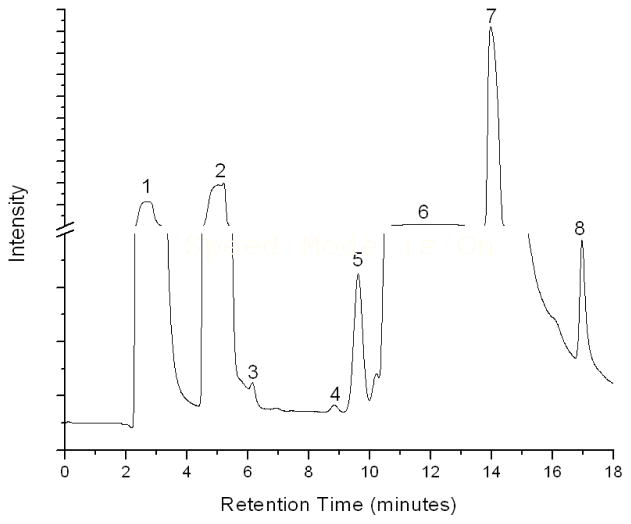
The preparation of standards and calibration procedures are detailed in previous work (18). Briefly, the instrument was calibrated for sodium phosphite dibasic pentahydrate (Riedel-deHaën, 98%) and sodium phosphate dibasic dihydrate (Riedel-deHaën, 99.5%) salts solvated in synthetic geothermal water prepared in the laboratory from sodium salts to replicate the anion concentrations of all major anions present in Hot Creek reported in the literature (13), and observed in preliminary investigations (data not shown). The concentrations of background anions in the synthetic geothermal water calibration solution were 0.5 mM fluoride, 6.0 mM chloride, 5.9 × 10−3 mM bromide, 4.1 × 10−5 mM nitrate, 8.6 mM hydrogen carbonate, and 1.00 mM sulfate. Six-point calibrations were performed in the range 0 to 0.80 μM for phosphite and phosphate. The phosphite calibration yielded a slope of 0.34 ± 0.03, intercept of 0.00 ± 0.01, and an r2 value of 0.97. The phosphate calibration yielded a slope of 0.38 ± 0.04, intercept of −0.02 ± 0.01, and an r2 value of 0.97. Figure 1 shows a typical chromatograph for phosphite and phosphate standards, each at concentrations of 0.40 μM, solvated in synthetic geothermal water. The peak assignments for the eight anions in this matrix were determined by running each compound alone with the same method used to acquire the data. The peak assignments are: (1) fluoride; (2) chloride; (3) bromide; (4) nitrate; (5) phosphite; (6) hydrogen carbonate; (7) sulfate; and (8) phosphate. A peak appears between the phosphite (peak 5) and hydrogen carbonate (peak 6) peaks in Figure 1. This unidentified peak appears consistently in natural geothermal pool, stream and synthetic geothermal water calibration standard samples.
FIGURE 1.
Ion chromatograph of phosphite and phosphate standards, each at concentrations of 0.40 μM, solvated in synthetic geothermal water. The peak assignments are: (1) fluoride; (2) chloride; (3) bromide; (4) nitrate; (5) phosphite; (6) hydrogen carbonate; (7) sulfate; and (8) phosphate.
Ion Chromatography with Conductivity and Mass Spectrometer Detection
Samples for this experiment were collected from an active geothermal pool in Hot Creek Gorge on October 2, 2007. The samples were stored at 4°C prior to analysis on October 9, 2007. Full details of the ion chromatography/mass spectrometry (IC/MS) experimental methods can be found in Ivey and Foster (18). Highlights are presented here. Geothermal pool samples were treated with 1cc sulfonic acid cartridges (OnGuard II H cartridges, Dionex) to reduce the total amount of hydrogen carbonate prior to sample analysis. Treated samples were then injected into the IC (DX 600, Dionex) using a 700 μL injection loop. The IC was outfitted with an AS17 analytical column with guard and electrolytically generated KOH eluent. Conductivity detector data was collected for the entire 17 minute run time of the experiment. The effluent from the IC was pulsed into the mass spectrometer using an actuator valve at the rate of one pulse per second for short time intervals selected to catch phosphite and phosphate peaks without unnecessarily exposing the mass spectrometer to high concentrations of superfluous anions (i.e. chloride, hydrogen carbonate, and sulfate). Mass spectrometer data was acquired during retention time ranges of 8.0 – 9.5 minutes and 13.0 – 17.0 minutes.
The ion trap mass spectrometer (LCQ Deca, ThermoFinnigan) was configured with electrospray ionization operated with 8 kV on the needle, and the heated capillary tubing was held at 350 °C. Although volatile organic solvents such as methanol and acetonitrile are typically combined with water for electrospray ionization, 100% water was used in the current study. To accommodate for low volatility of the solvent, the electrospray nozzle was fully retracted, and the sheath gas was maintained at 80 (arbitrary units). Selection criteria for these parameters were based on previous studies (18). Mass spectra were collected using two consecutive single ion monitoring experiments at m/z = 81 ± 1 and m/z = 97 ± 1. The mass-to-charge ratio of 50 is the lowest that can be detected by the LCQ Deca mass spectrometer. Consequently, only singly charged phosphorus oxyanions were detected in this study, although about 50% of phosphite and phosphate are doubly charged at near neutral pH (17,19). Phosphite (H2PO3−), and phosphate (H2PO4−) were detected at m/z = 81, and m/z = 97, respectively.
Results and Discussion
Analysis of Geothermal Pool and Stream Samples
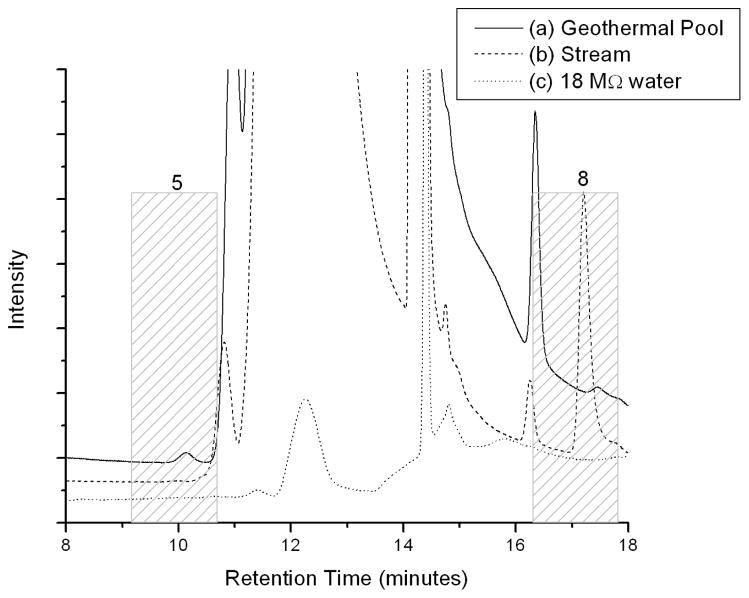
Supporting Information Table S1 summarizes the results from three field campaigns conducted September 25, 2004; August 7, 2006 and January 13, 2007. The geothermal pool and stream samples discussed here were collected on January 13, 2007. Representative chromatograms for samples from the geothermal pool, the stream, and pure 18.2 MΩ water are shown in Figure 2. The data in this figure are truncated to highlight differences in peak areas for peaks 5 and 8, which elute at 10.0 ± 0.8 minutes and 17.1 ± 0.8 minutes, respectively. The retention time error bars represent 3σ at a 99% confidence level for all 34 injections discussed here. These data include eleven geothermal pool, eleven stream and twelve calibration standard injections. The retention time errors are slightly larger than the typical range associated with ion chromatography. Active sites on the analytical column were prone to contamination because large volumes of partially saline (14) geothermal water were routinely injected onto the column. The geothermal pool sample was spiked with sodium phosphite dibasic pentahydrate and sodium phosphate dibasic dihydrate standards to identify peaks 5 and 8 as phosphite and phosphate, respectively (Supporting Information Figure S1). The phosphite and phosphate peaks of the spiked samples elude at 10.5 minutes and 17.2 minutes, respectively. A second peak appears in the peak 5 window on Figure S1 that eludes prior to the phosphite peak. Supporting Information Figure S2 presents standard additions of sodium phosphite dibasic pentahydrate and sodium phosphate dibasic dihydrate to 18.2 MΩ water. These data show the new peak in the peak 5 window is an unidentified impurity in the phosphate standard. For both samples, the retention times for each compound were well within the retention time windows described above.
FIGURE 2.
Representative chromatographs of (a) geothermal pool (solid line), (b) stream (dashed line), and (c) 18.2 MΩ water (dotted line). Data is offset for clarity.
Of the three samples presented in Figure 2, peak 5 is detectable only in geothermal pool water. Calibrations of the phosphite standard diluted in synthetic geothermal water show the peak area for peak 5 (phosphite) corresponds to a concentration of 0.06 ± 0.02 μM, where the error represents 3σ of the standard deviation for four repeat injections from the same processed sample, and the standard error of the slope generated from a linear regression fit to the calibration data. Note that phosphite is not a thermodynamically stable species (17), and will oxidize in the presence of molecular oxygen over time. Consequently, it is predicted that higher concentrations of phosphite would be measured in-situ and in grab samples with shorter storage times. Peak 5 is undetectable above the phosphite limit of detection (0.04 μM) in 18.2 MΩ laboratory water and stream samples. Peak 8 (phosphate) has a peak retention time of 17.5 minutes in the geothermal pool and 17.2 minutes in the stream. Geothermal pools in Hot Creek Gorge are more saline than the Hot Creek stream (14,20). Consequently, the tightly-retained phosphate peak would be predicted to elute later in the more concentrated geothermal water than in the stream water because of column overload conditions as observed. Peak 8, identified as phosphate, reflects concentrations of phosphate of 0.05 ± 0.01 μM and 1.06 ± 0.36 μM in geothermal pool and stream water samples, respectively. Note peak 8 is undetectable in the 18.2 MΩ water sample, indicating phosphate is below the phosphate detection limit (0.04 μM). Phosphate is typically measured in the micromolar range in pristine environments (21). Although the measured quantity of phosphate in the stream is well within the range of previous studies, the measured phosphate concentration in the geothermal pool is three times less than the lower limit of typical water samples (21). The relatively high phosphite and low phosphate concentrations measured in the geothermal pool with respect to the stream, suggest that the geothermal pool water is a source of phosphite and not phosphate to the stream water.
Oxidation of Geothermal Pool and Stream Samples
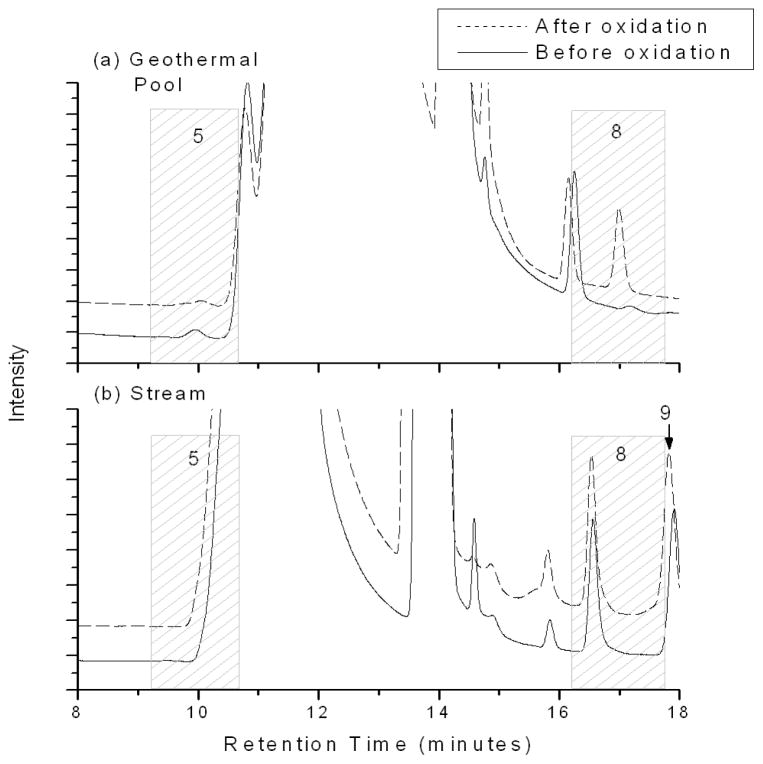
The assignment of unknown peaks based on matching retention times observed with analytical standards is not enough evidence for absolute identification, especially when an indiscriminate, universal detector such as the conductivity detector used in the current study is employed. Geothermal water and stream water samples were oxidized with a highly effective iodate solution (22) in order to provide a secondary confirmation of the preliminary assignment of peak 5 as phosphite. Panel (a) of Figure 3 presents representative chromatographs of geothermal pool water prior to oxidation (solid line) and 96 minutes after the addition of the iodate oxidation solution. Although there appears to be a visual difference in the peak area of peak 5 before and after oxidation, there was no statistical difference between the phosphite concentrations measured before and after oxidation.
FIGURE 3.
Iodate oxidation results. Panel (a) presents typical chromatographs of geothermal pool water prior to oxidation (solid line) and 96 minutes after the addition of iodate oxidation solution (dashed line). Panel (b) presents typical chromatographs of stream water prior to oxidation (solid line) and 45 minutes after the addition of iodate oxidation solution (dashed line). Peak 9 is arsenate. Data are offset for clarity.
Iodate solutions were run separately to determine if the oxidation solution contained peaks in the retention time ranges used to identify phosphite and phosphate (data not shown). These data show that there are no additional peaks in the retention time range for peak 5, and that there is a small reproducible peak in the retention time range for peak 8. Consequently, the peak area in the retention time range for peak 8 introduced into the background by the iodate solution was subtracted from observed peak areas of peak 8 for solutions that contained the iodate solution to assess changes in phosphate concentration before and after oxidation.
Geothermal Pool #1 contained 0.04 ± 0.01 μM of phosphate before oxidation and 0.16 ± 0.09 μM after oxidation. The resulting change in phosphate was an increase of 0.13 ± 0.10 μM. A potassium permanganate solution (23) was used to oxidize the same Geothermal Pool #1 sample in a second oxidation experiment. The resulting change in phosphate was an increase of 0.51 ± 0.07 μM, which indicates the permanganate solution was more effective at oxidizing reduced phosphorus species than the iodate solution. Because in each oxidation experiment the measured increase in phosphate after oxidation was greater than the 0.06 ± 0.02 μM of phosphite in Geothermal Pool #1, these data suggest that the geothermal water sample contains additional forms of reduced phosphorus oxyanions susceptible to iodate and permanganate solution oxidation. A likely candidate is the compound hypophosphite in the P(I) oxidation state, which has been shown to be oxidized by iodate solutions in laboratory studies (22).
Both laboratory and field based studies have shown hypophosphite and phosphite are often observed in the same samples. Numerous laboratory studies exploring the conversion of calcium phosphate in apatite to reduced phosphorus as a potentially significant process in biogenesis have been conducted (6–9). These studies have repeatedly referred to detectable concentrations of hypophosphite, in support of the results of this work (6,9). In fact, DeGraff and Schwartz (9) consistently measured higher concentrations of hypophosphite than phosphite in water extracts analyzed after exposing apatite submerged in water to simulated lightening. Field-based research conducted by Morton et al. (24) has previously identified hypophosphite and phosphite in process water from thermal phosphorus production. Raw waste water analyzed in these samples consistently contained over three times as much hypophosphite as phosphite, despite the fact that hypophosphite is the less thermodynamically stable compound of the two. Based on these previous investigations, it is likely that hypophosphite is present in geothermal pools at higher concentrations than phosphite, and this compound alone may account for the additional phosphate formed after oxidation of the geothermal pool water. Further studies, looking explicitly for hypophosphite are necessary to determine if the presence of hypophosphite in Hot Creek accounts for the concentration of total reactive phosphorus unaccounted for in the current study (17).
Data obtained from the analysis of Hot Creek stream water before and after the iodate oxidation procedure are shown in Figure 3b. Column aging can contribute to the observed retention time shifts for peak 8 (phosphate) observed in Figure 3 panels (a) and (b). Additional experiments (Supporting Information Figure S2) have confirmed that the new peak (peak 9) eluting at approximately 17.85 minutes in Figure 3 panel (b) is arsenate, a known component in Hot Creek stream originating from the geothermal pools (13–16). Peak 5 was not observed in the stream water before or after oxidation, and consequently changes in phosphite could not be quantified in this sample. These data also show that the areas obtained from peak 8 (corrected for background) are not significantly different from background levels, suggesting that reduced phosphorus oxyanions were not present in the stream samples.
Ion Chromatography with Conductivity and Mass Spectrometer Detection
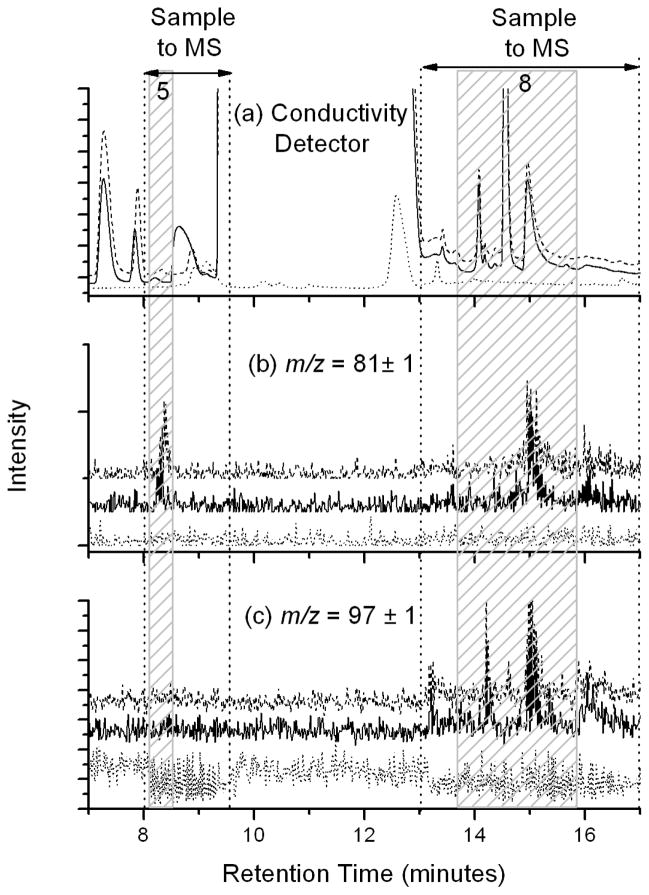
Additional confirmation of the peak assignments were made by using a mass spectrometer connected in series to the ion chromatograph. Figure 4 is a representative ion chromatogram of geothermal pool water analyzed in tandem with suppressed conductivity (CD) and electrospray mass spectrometry (MS) detectors (18). Each panel presents the unperturbed geothermal pool sample (solid lines) along with an aliquot of the same sample spiked with sodium phosphite dibasic pentahydrate and sodium phosphate dibasic dihydrate each at concentrations of 0.05 μM (dashed lines), and an 18.2 MΩ water blank (dotted line). The conductivity data is presented in Figure 4a. Single ion monitoring experiments were conducted using the mass spectrometer detector at m/z = 81± 1 (Figure 4b) and m/z = 97 ± 1 (Figure 4c), which correspond to the parent masses of monovalent phosphite (H2PO3−) and phosphate (H2PO4−), respectively. Divalent anions could not be detected in this study, because their mass-to-charge ratios fall below the m/z = 50 low-end cut off for the mass spectrometer. The spiked samples were used to determine that phosphite eludes at 8.4 minutes and phosphate eludes at 15.0 minutes in the IC/CD/MS experiments. Consequently, the data presented in Figure 4 have been truncated to highlight the 7 – 17 minute retention time range where phosphite and phosphate elude.
FIGURE 4.
Representative chromatographs of a spiked geothermal pool (dashed line), geothermal pool (solid line), and 18.2 MΩ water (dotted line) samples. Each were acquired using conductivity detection in series with mass spectrometer (MS) detection. The three panels present (a) conductivity detector data, (b) single ion monitoring data at m/z = 81 ± 1, and (c) single ion monitoring data at m/z = 97 ± 1, respectively. Shaded areas represent the retention times of (peak 5) phosphite and (peak 8) phosphate, respectively. IC effluent was directed to the MS only during time intervals bracketed between the dotted lines. Data are offset for clarity.
Peaks are present at the retention time of 8.4 minutes in both the conductivity detector and in the mass spectrometer at m/z = 81 ± 1 in the geothermal water sample, that is not present in the 18.2 MΩ water blank. The addition of 0.05 μM of phosphite to the geothermal water results in an increase in the peak area of the 8.4 minute peak, which supports the conclusion that phosphite is present in geothermal water in agreement with the conductivity detector data presented in Figures 1 – 3.
The conductivity detector data presented in Figure 4a show three distinct peaks in the 13.7 to 15.8 minute retention time window used to identify phosphate compounds in this experiment. However, only the peak at 15.0 minutes produces a signal in the conductivity detector, at m/z = 81 ± 1, and at m/z = 97 ± 1. It is likely that the m/z = 81 ± 1 signal is a fragment of the m/z = 97 ± 1 parent created by the loss of one oxygen atom (16 amu) from the phosphate parent during ionization. Although electrospray ionization is an inherently soft ionization method that favors the formation of parent ions, high-energy ionization is known to create in-source collisionally-induced-dissociation (CID) of analytes (25) resulting in MS spectra containing both parent and daughter fragment ions. An unusually high needle voltage of 8 kV was used to promote desolvation of the high surface tension, low volatility water droplets. Consequently, the observed ion signal at m/z = 81 for the phosphate peak is likely a daughter fragment created through the loss of an oxygen atom (m/z = 16) from the monovalent negatively-charged phosphate ion (H2PO4−) observed at m/z = 97.
In conclusion, these results show that an active geothermal pool in Hot Creek Gorge near Mammoth Lakes, California is a natural source of the bioactive, water soluble, reduced phosphorus oxyanion phosphite. It was measured at a concentration of 0.06 ± 0.02 μM approximately six days after the sample was removed from the pool and because phosphite is thermodynamically unstable, it is likely that the actual concentration is somewhat higher. These results elucidate the reasons why exotic microorganisms in the modern-world have the ability to utilize phosphite as an energy source for metabolism. The presence of phosphite in such a system supports the idea that calcium phosphate can lead to phosphite formation and hence the support of biological activity. The use of on-site analytical techniques for monitoring reduced phosphorus oxyanions, especially hypophosphite and phosphite is imperative to the future of these investigations. We are currently exploring the development of such techniques.
Supplementary Material
Acknowledgments
The authors are grateful to the National Science Foundation CREST program (HDR_9805529) for funding this research project. K.L.F. and G.H. also thank the National Institute of Health RIMI program (1P20MD001824-01) for making this research possible. K.L.F. and T.M.S. thank the National Institute of Health SCORE program (5S06 08101 and 5SC3GM083682-02) for their support. Finally, we are eternally grateful to M. Ivey, L. Ke, R. Barco, M. Orozco, M.M. McDowell, and the other numerous research assistants who participated in the method development and field campaigns that supported this research.
Footnotes
Brief: The reduced phosphorus oxyanion phosphite has been quantified in a pristine geothermal pool supporting the theory that this compound may have played a significant role in biogenesis.
Supporting Information Available
Supporting information for this article includes one table and three figures. These results include: a summary of phosphite and phosphate measurements in geothermal pool and stream samples collected over three days and two seasons; representative chromatographs presenting standard additions of phosphite and phosphate to geothermal pool water; and two chromatographs presenting phosphite, phosphate and arsenate standards used for peak identification. This information is available free of charge via the internet at http://pubs.acs.org.
Literature Cited
- 1.Hallberg RO. Sediments: Their Interaction with Biogeochemical Cycles through Formation and Diagenesis, in Global Biogeochemical Cycles. In: Butcher SS, Charlson RJ, Orians GH, Wolfe GV, editors. Global Biogeochemical Cycles. Academic Press; New York, NY: 1992. [Google Scholar]
- 2.Jahnke RA. The Phosphorus Cycle. In: Butcher SS, Charlson RJ, Orians GH, Wolfe GV, editors. Global Biogeochemical Cycles. Academic Press; New York, NY: 1992. [Google Scholar]
- 3.Schlesigner WH. Biogeochemistry: An Analysis of Global Change. Academic Press; London: 1991. [Google Scholar]
- 4.Stevenson FJ. Cycles of Soil: Carbon, Nitrogen, Phosphorus, Sulfur, Micronutrients. John Wiley & Sons, Inc; New York, NY: 1986. [Google Scholar]
- 5.Pasek MA. Rethinking early earth phosphorous geochemistry. Proc Natl Acad Sci USA. 2008;105:853–858. doi: 10.1073/pnas.0708205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glindemann D, DeGraaf RM, Schwartz AW. Chemical reduction of phosphate on the primative earth. Origins Life Evol Biosphere. 1999;29:555–561. doi: 10.1023/a:1006622900660. [DOI] [PubMed] [Google Scholar]
- 7.Glindemann D, Edwards M, Morgenstern P. Phosphine from rocks: Mechanically driven phosphate reduction. Environ Sci Technol. 2005;39:8295–8299. doi: 10.1021/es050682w. [DOI] [PubMed] [Google Scholar]
- 8.Glindemann D, Edwards M, Schrems O. Phosphine and methylphosphine production by simulated lightening - a study for the volatile phosphorus cycle and cloud formation in the earth atmosphere. Atmos Environ. 2004;38:6867–6874. [Google Scholar]
- 9.DeGraaf RM, Schwartz AW. Reduction and activation of phosphate on the primative earth. Origins Life Evol Biosphere. 2000;30:405–410. doi: 10.1023/a:1006700512902. [DOI] [PubMed] [Google Scholar]
- 10.Schink B, Friedrich M. Phosphite oxidation by sulphate reduction. Nature. 2000;406:37. doi: 10.1038/35017644. [DOI] [PubMed] [Google Scholar]
- 11.Schink B, Thiemann V, Laue H, Friedrich MW. Desulfotignum phosphitoxidans sp nov., a new marine sulfate reducer that oxidizes phosphite to phosphate. Arch Microbiol. 2002;177:381–391. doi: 10.1007/s00203-002-0402-x. [DOI] [PubMed] [Google Scholar]
- 12.California Department of Water Resources. Investigation of Geothermal Waters in the Long Valley Area. Mono County: 1967. [Google Scholar]
- 13.Eccles L. Sources of arsenic in streams tributary to Lake Crowley, CA. Water Resour Invest. 1976;76-36 [Google Scholar]
- 14.Mariner RH, Willey LM. Geochemistry of thermal waters in Long Valley, Mono County, California. J Geophys Res. 1976;81:792–800. [Google Scholar]
- 15.Wilkie JA, Hering JG. Rapid oxidation of geothermal arsenic(III) in streamwaters of the eastern Sierra Nevada. Environ Sci Technol. 1998;32:657–662. [Google Scholar]
- 16.Wilkie JA. Doctor of Philosophy. University of California; Los Angeles: 1997. Processes controlling arsenic mobility in natural and engineered systems. [Google Scholar]
- 17.Hanrahan G, Salmassi TM, Khachikian CS, Foster KL. Reduced inorganic phosphorus in the natural environment: Significance, speciation and determination. Talanta. 2005;66:435–444. doi: 10.1016/j.talanta.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Ivey MM, Foster KL. Detection of phosphorus oxyanions in synthetic geothermal water using ion chromatography – mass spectrometry techniques. J Chromatogr, A. 2005;1098:95–103. doi: 10.1016/j.chroma.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 19.McDowell MM, Ivey MM, Lee ME, Firpo VVVD, Salmassi TM, Khachikian CS, Foster KL. Detection of hypophosphite, phosphite, and orthophosphate in natural geothermal water by ion chromatography. J Chromatogr, A. 2004;1039:105–111. doi: 10.1016/j.chroma.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 20.Sorey ML. Evolution and present state of the hydrothermal system in Long Valley Caldera. J Geophys Res. 1985;90(11):219–211. [Google Scholar]
- 21.Robards K, McKelvie ID, Benson RL, Worsfold PJ, Blundell NJ, Casey H. Determination of carbon, phosphorus, nitrogen and silicon species in waters. Anal Chim Acta. 1994;287:147–190. [Google Scholar]
- 22.Barco RA, Patil DG, Xu W, Ke L, Khachikian CS, Hanrahan G, Salmassi T. The development of iodide-based methods for batch and on-line determinations of phosphite in aqueous samples. Talanta. 2006;69:1292–1299. doi: 10.1016/j.talanta.2006.02.060. [DOI] [PubMed] [Google Scholar]
- 23.Morton SC, Glindermann D, Wang X, Niu X, Edwards M. Analysis of reduced phosphorus in samples of environmental interest. Environ Sci Technol. 2005;39:4369–4376. doi: 10.1021/es0401038. [DOI] [PubMed] [Google Scholar]
- 24.Morton SC, Glindemann D, Edwards MA. Phosphate, phosphites, and phosphides in environmental samples. Environ Sci Technol. 2003;37:1169–1174. doi: 10.1021/es020738b. [DOI] [PubMed] [Google Scholar]
- 25.Bristow AWT, Nichols WF, Webb KS, Conway B. Evaluation of protocols for reproducible electrospray in-source collisionally induced dissociation on various liquid chromatography/mass spectrometry instruments and the development of spectral libraries. Rapid Commun Mass Spectrom. 2002;16:2374–2386. doi: 10.1002/rcm.843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.