Abstract
Glutamate transporters, also called excitatory amino acid transporters (EAATs), uptake extracellular glutamate and regulate neurotransmission. Activation of protein kinase C (PKC) increases the activity of EAAT type 3 (EAAT3), the major neuronal EAAT. We designed this study to determine which amino acid residue(s) in EAAT3 may be involved in this PKC effect. Selective potential PKC phosphorylation sites were mutated. These EAAT3 mutants were expressed in the Xenopus oocytes. Phorbol 12-myristate 13-acetate, a PKC activator, significantly increased wild-type EAAT3 activity. Mutation of serine 465 to alanine or to aspartic acid, but not the mutation of threonine 5 to alanine, abolished PKC-increased EAAT3 activity. Our results suggest a critical role of serine 465 in the increased EAAT3 activity by PKC activation.
Keywords: Glutamate, glutamate transporter, protein kinase C, site-directed mutagenesis
Introduction
Glutamate, the major excitatory neurotransmitter in the central nervous system (CNS), is neurotoxic when its extracellular concentration is high. Glutamate transporters, also called excitatory amino acid transporters (EAATs), play an important role in removing glutamate from extracellular space into cells (Kanai and Hediger, 1992, Danbolt, 2001). Dysfunction of EAATs causes extracellular glutamate accumulation that results in glutamate excitatoxicity. Five EAATs (EAAT1 - 5) have been characterized; each has about 520–580 amino acid residues (Danbolt, 2001). EAAT1 and EAAT2 are glial EAATs. EAAT3 and EAAT4 are mainly expressed in neurons. The expression of EAAT5 is found in retina. Since EAAT3 is highly expressed in many brain regions and EAAT4 is predominantly expressed in the cerebellum, EAAT3 is the major neuronal EAAT in the CNS (Danbolt, 2001).
Many studies have suggested that EAAT3 activity can be increased by protein kinase C (PKC) (Davis et al., 1998, Do et al., 2002, Gonzalez et al., 2002). We previously showed that isoflurane, a commonly used volatile anesthetic, induced a PKCα-dependent increase of EAAT3 activity (Huang and Zuo, 2005). This increase requires the phosphorylation of serine 465 in EAAT3 (Huang et al., 2006). There are at least 11 PKC isozymes. Direct activation of PKCs by phorbol esters can increase EAAT3 activity (Do et al., 2002, Gonzalez et al., 2002). We hypothesize that phosphorylation of serine 465 is also required for the phorbol esters-induced increase of EAAT3 activity. To test this hypothesis, we mutated selective potential PKC phosphorylation sites and determine their activity in the presence or absence of phorbol ester stimulation.
Materials and methods
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Virginia. The recommendations from the Declaration of Helsinki and the internationally accepted principles in the care and use of experimental animals had been adhered to during the study.
Materials
Mature female Xenopus laevis frogs were purchased from Xenopus I (Ann Arbor, MI, USA). All agents, unless specified below, were obtained from Sigma (St. Louis, MO, USA). Phorbol 12-myristate 13-acetate (PMA) was initially dissolved in 0.1% dimethyl sulfoxide (DMSO) and then diluted into 100 nM. The final concentrations of DMSO in the PMA solution were 0.025% or less. QuikChange site-directed mutagenesis kit was from Stratagene (La Jolla, CA). Isoflurane was purchased from Abbott Laboratories (North Chicago, IL).
Oocyte preparation and injection
As we described before (Do et al., 2002), frogs were anesthetized in 500 ml of 0.2% 3-aminobenzoic acid ethyl ester in water until unresponsive to painful stimuli (toe pinching) and underwent surgery on ice. Oocytes were surgically retrieved and placed immediately in modified Barth’s solution containing 88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 0.41 mM CaCl2, 0.82 mM MgSO4, 0.3 mM Ca(NO3)2, 0.1 mM gentamicin and 15 mM HEPES with pH adjusted to 7.5. The oocytes were defolliculated with gentle shaking for approximately 2 h in calcium-free OR-2 solution containing 82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, 4 mM HEPES and 0.1% collagenase type Ia with pH adjusted to 7.5 and then incubated in modified Barth’s solution that does not contain collagenase at 16° for 1 day before the injection of mRNA of the wild type rat EAAT3 or its mutants (S465A, S465D and T5A).
The wild type rat EAAT3 complementary DNA (cDNA) construct was provided by Dr. M.A. Hediger (Brigham and Women’s Hospital, Harvard Institutes of Medicine, Boston, MA). The cDNA was subcloned in a commercial vector (BluescriptS Km). As described before (Fang et al., 2002, Huang et al., 2006), mutagenesis was done by using QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) with a pair of 28 base primers containing the desired mutation. The mutations were confirmed by sequencing about 500 bases that included the mutated sites. The plasmid DNA of EAAT3 or its mutants was linearized with a restriction enzyme (NotI) and mRNA was synthesized in vitro with a commercially available kit (Ambion, Austin, TX). The resulting mRNA was quantified spectrophotometrically and diluted in sterile water. Oocytes were injected with 40 ng of cRNA of EAAT3 or its mutants (S465A, S465D and T5A) by using an automated microinjector (Nanoject; Drummond Scientific Co., Broomall, PA). Oocytes were then incubated at 16°C in modified Barth’s solution for 3 days before voltage-clamping experiments.
Electrophysiological recordings
Experiments were performed at room temperature (approximately 21°C – 23°C). A single oocyte was placed in a recording chamber that was < 1 ml in volume and was perfused with 5 ml/min Tyrode’s solution containing 150 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 10 mM dextrose and 10 mM HEPES with pH adjusted to 7.5. Clamping microelectrodes were pulled from capillary glass (10 µl Drummond Microdispenser, Drummond Scientific) on a micropipette puller (model 700C; David Kopf Instruments, Tujunga, CA). Tips were broken at a diameter of approximately 10 µm and filled with 3 M KCl obtaining resistance of 1–3 MΩ. Oocytes were voltage-clamped using a two-electrode voltage clamp amplifier (OC725-A; Warner Corporation, New Haven, CT) that was connected to a data acquisition and analysis system running on a personal computer. The acquisition system consisted of a DAS-8A/D conversion board (Keithley-Metrabyte, Taunton, MA). Analyses were performed with pCLAMP7 software (Axon Instruments, Foster City, CA). All measurements were performed at a holding potential of −70 mV. Oocytes that did not show a stable holding current less than 1 µA were excluded from analysis. L-Glutamate was diluted in Tyrode’s solution and superfused over the oocyte for 25 s (5 ml/min). L-Glutamate-induced inward currents were sampled at 125 Hz for 1 min: 5 s of baseline, 25 s of L-glutamate application and 30 s of washing with Tyrode’s solution. We used 30 µM of L-glutamate in this study because the EC50 of L-glutamate to induce EAAT3 responses was 27 – 30 µM (Do et al., 2002, Kim et al., 2003). Since the transport of one negatively charged glutamate molecule co-transports 2 – 3 Na+ into the cell (Kanai and Hediger, 1992, Danbolt, 2001), we measured the glutamate-induced peak currents to reflect the amount of transported glutamate.
Administration of experimental chemicals
In the control experiments, oocytes were perfused with Tyrode’s solution for 3 min before the application of Tyrode’s solution containing L-glutamate for the electrophysiological recording. The effects of PKC activation on the activity of EAAT3 or its mutants were examined by pre-incubating oocytes with a PKC activator, 100 nM PMA, in Bath’s solution for 10 min before voltage-clamp recordings were taken. The application of isoflurane was performed as we described previously (Do et al., 2002). Oocytes were perfused with Tyrode’s solution containing 0.7 mM isoflurane for 3 min before the voltage-clamp recordings were taken. The isoflurane containing solution was prepared by gassing the Tyrode’s solution with 1.5% isoflurane in air for 10 min and the aqueous concentration of isoflurane was measured by gas chromatography.
Data analysis
Responses are reported as mean ± S.D. Each experimental condition was applied to oocytes from at least three different frogs. Since the expression level of transporter proteins in oocytes of different batches may vary, variability in response among batches of oocytes is common. Thus, responses were normalized to the mean values of the same-day controls for each batch. Statistical analysis was performed by unpaired student’s t-test. A P < 0.05 was accepted as significant.
Results
L-Glutamate (30 µM) induced no current in the oocytes without injection of EAAT3 mRNA (data not shown). Glutamate-induced inward current was observed in voltage-clamped oocytes after injection of EAAT3 mRNA of wild and mutant types (S465A, S465D, and T5A). DMSO (0.025%) used to dissolve PMA did not affect the glutamate-induced current responses in EAAT3-expressing oocytes (data not shown).
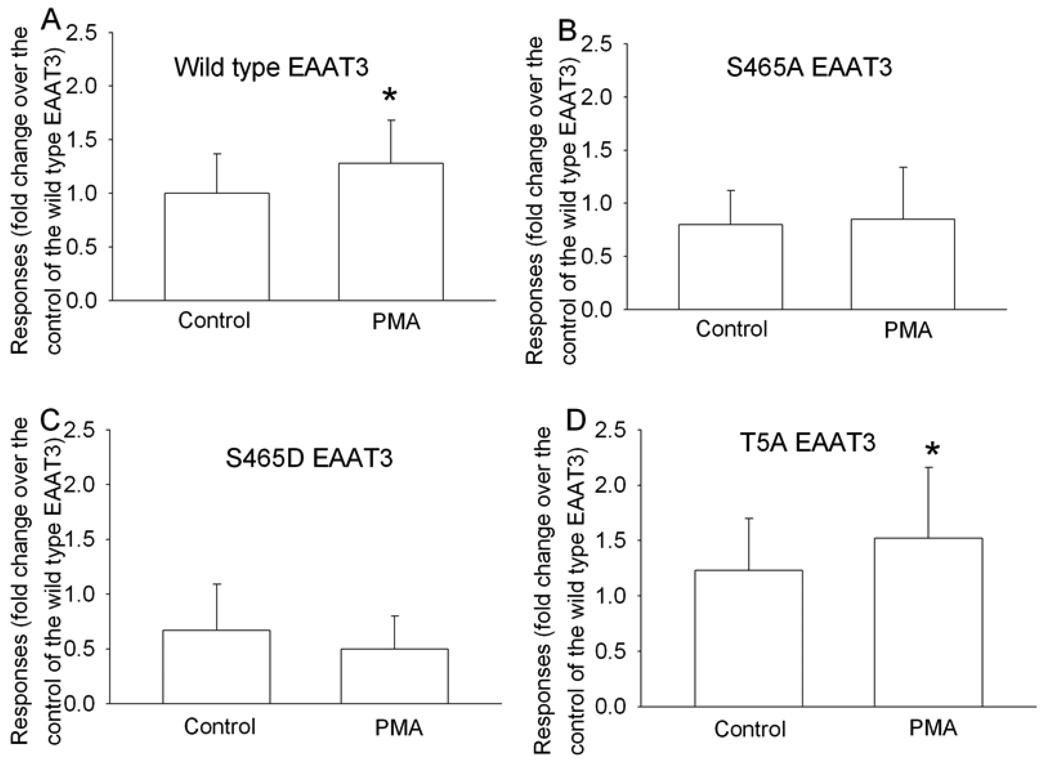
Preincubation of the oocytes with 100 nM PMA, a PKC activator, increased wild type EAAT3 activity (1.28 ± 0.40 fold of control) significantly compared to the control (Fig. 1A). When serine 465 was replaced by alanine (S465A) or by aspartic acid (S465D), preincubation with 100 nM PMA did not significantly change the activity of these mutants (Figs. 1B and 1C). However, when threonine 5 was replaced by alanine (T5A), preincubation with 100 nM PMA increased the activity of this mutant (1.23 ± 0.47 fold of the corresponding control without PMA incubation) (Fig. 1D). Similar to serine 465, threonine 5 may be a potential PKC phosphorylation site based on the EAAT3 sequence (Kanai and Hediger, 1992).
Fig. 1. The effects of protein kinase C (PKC) activation on the activity of the wild type EAAT3 and its mutants.
Data are means ± S.D. (n = 25 to 36 in each data point). Statistical analysis was performed by unpaired t-test. * P < 0.05 compared to the corresponding control without PMA incubation.
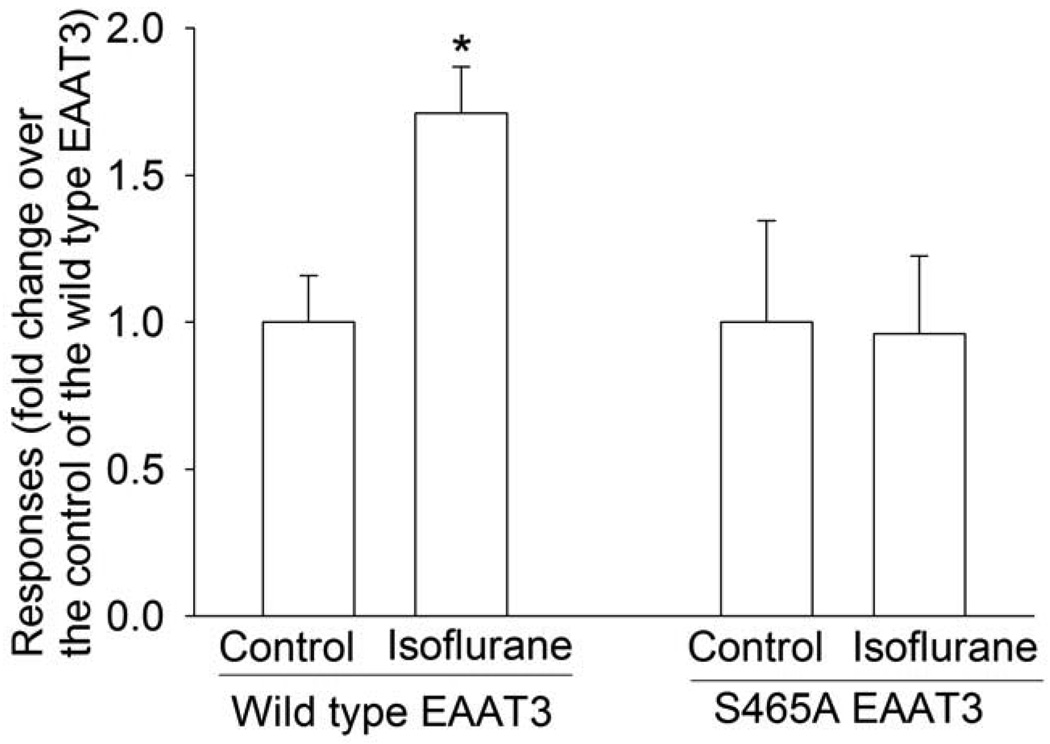
Isoflurane significantly increased the activity of wild type EAAT3. However, isoflurane did not change the activity of S465A EAAT3 activity (Fig. 2).
Fig. 2. The effects of isoflurane (0.7 mM) on the activity of the wild type EAAT3 and its mutant.
Data are means ± S.D. (n = 7 in each data point). Statistical analysis was performed by unpaired t-test. * P < 0.05 compared to the corresponding control without isoflurane exposure.
Discussion
Sodium-dependent glutamate uptake via EAATs is a major mechanism to clear up extracellular glutamate released during synaptic transmission (Danbolt, 2001). By this function, EAATs regulate neurotransmission and prevent glutamate excitatoxicity (Danbolt, 2001). It has been shown that inhibition of neuronal EAATs induced epilepsy in rats and decreased the inhibitory neurotransmitter GABA-mediated inhibitory postsynaptic current in rat hippocampus via a reduced synthesis of GABA because glutamate taken up by neuronal EAATs is a substrate for GABA synthesis (Sepkuty et al., 2002, Matthews and Diamond, 2003). These results suggest that EAAT3, via its glutamate uptake function, can regulate both the excitatory glutamate neurotransmission and the inhibitory GABA neurotransmission. Studies have shown that long term potentiation, especially the early phase of long term potentiation, and contextural fear conditioning increased EAAT3 activity and redistribution to the plasma membrane in rat hippocampus (Levenson et al., 2002, Pita-Almenar et al., 2006). These results indicate that EAAT3 is involved in the synaptic plasticity and learning and memory functions. A recent study showed that EAAT3 knockout mice had reduced neuronal glutathione levels and, with aging, developed brain atrophy and decreased learning and memory functions (Aoyama et al., 2006). The hippocampi of these mice also had increased oxidant levels and enhanced susceptibility to oxidant injury (Aoyama et al., 2006). These data reveal a critical role of EAAT3 in maintaining neuronal glutathione levels to reduce oxidant-induced neuronal injury and death. Thus, EAAT3 has broad functions, including regulating inhibitory and excitatory neurotransmission, synaptic plasticity and neuroprotection, in the brain.
The activity of EAAT3 can be regulated by PKC. It has been shown that isoflurane induces a PKCα-mediated increase of EAAT3 activity in C6 cells and neurons (Huang and Zuo, 2005). Direct activation of PKC by PMA also increases EAAT3 activity in oocytes, C6 cells and neurons (Do et al., 2002, Gonzalez et al., 2002, Gonzalez et al., 2003). Consistent with these previous studies, we showed here that isoflurane and PMA increased the activity of EAAT3 expressed in oocytes. Two mechanisms have been proposed for the increased activity of EAAT3: increased EAAT3 redistribution to the plasma membrane to increase the number of transporters available for transporting glutamate, which can be mediated by PKCα, and increased catalytic rate of transporter activity, which may be mediated by PKCε (Davis et al., 1998, Gonzalez et al., 2002). Although PMA can activate PKCα and PKCε, occytes express abundant amount of PKCα but do not express detectable PKCε (Johnson and Capco, 1997, Stith et al., 1997). Thus, the PMA-induced increase of EAAT3 activity in this current study may be mediated by PKCα.
Multiple potential PKC phosphorylation sites in EAAT3 have been suggested (Kanai and Hediger, 1992, Huang et al., 2006). We have shown the critical role of S465, a potential PKC phosphorylation site, in isoflurane-induced EAAT3 redistribution to the plasma membrane and increase of EAAT3 activity (Huang et al., 2006). Consistent with our previous findings, we showed that isoflurane increased the wild type EAAT3 activity but did not change the activity of S465A EAAT3 mutant. S465 also plays a critical role in the PMA-increased EAAT3 activity because PMA increased the wild type EAAT3 activity but did not change the activity of S465A and S465D EAAT3 mutants. Alanine can not be phosphorylated by PKC and aspartic acid mimics the acidic charge of a phosphate. Thus, mutation of a potential PKC phosphorylation site to alanine or aspartic acid should eliminate the PKC effect on this site. However, the S465D EAAT3 activity under control condition would have been expected to be increased compared with the control activity of the wild type EAAT3. This was not observed in our study possibly due to the different expression levels of wild type and S465D EAAT3 in oocytes.
In summary, we have shown that the mutation of serine 465, but not of threonine 5, in rat EAAT3 abolished the increased activity after PKC activation. These results suggest a critical role of S465 in EAAT3 activity increase after direct activation of PKC by PMA.
Acknowledgements
This study was supported by National Institutes of Health Grants RO1 GM065211 and RO1 NS045983 (to Z. Zuo).
Footnotes
This work was carried out in the Department of Anesthesiology, University of Virginia.
References
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Davis KE, Straff DJ, Weinstein EA, Bannerman PG, Correale DM, Rothstein JD, Robinson MB. Multiple signaling pathways regulate cell surface expression and activity of the excitatory amino acid carrier 1 subtype of Glu transporter in C6 glioma. J Neurosci. 1998;18:2475–2485. doi: 10.1523/JNEUROSCI.18-07-02475.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do S-H, Kamatchi GL, Washington JM, Zuo Z. Effects of volatile anesthetics on glutamate transporter, excitatory amino acid transporter type 3 Anesthesiology. 2002;96:1492–1497. doi: 10.1097/00000542-200206000-00032. [DOI] [PubMed] [Google Scholar]
- Fang H, Huang Y, Zuo Z. The different responses of rat glutamate transporter type 2 and its mutant (tyrosine 403 to histidine) activity to volatile anesthetics and activation of protein kinase C. Brain Res. 2002;953:255–264. doi: 10.1016/s0006-8993(02)03299-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Bannerman P, Robinson M. Phorbol myristate acetate-dependent interaction of protein kinase C alpha and the neuronal glutamate transporter EAAC1. J Neurosci. 2003;23:5589–5593. doi: 10.1523/JNEUROSCI.23-13-05589.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MI, Kazanietz MG, Robinson MB. Regulation of the neuronal glutamate transporter excitatory amino acid carrier-1 (EAAC1) by different protein kinase C subtypes. Mol Pharmacol. 2002;62:901–910. doi: 10.1124/mol.62.4.901. [DOI] [PubMed] [Google Scholar]
- Huang Y, Feng X, Sando JJ, Zuo Z. Critical Role of Serine 465 in Isoflurane-induced Increase of Cell-surface Redistribution and Activity of Glutamate Transporter Type 3. J Biol Chem. 2006;281:38133–38138. doi: 10.1074/jbc.M603885200. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zuo Z. Isoflurane induces a protein kinase C alpha-dependent increase in cell surface protein level and activity of glutamate transporter type 3. Mol Pharmacol. 2005;67:1522–1533. doi: 10.1124/mol.104.007443. [DOI] [PubMed] [Google Scholar]
- Johnson J, Capco DG. Progesterone acts through protein kinase C to remodel the cytoplasm as the amphibian oocyte becomes the fertilization-competent egg. Mech Dev. 1997;67:215–226. doi: 10.1016/s0925-4773(97)00122-6. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter [see comments] Nature. 1992;360:467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lim YJ, Ro YJ, Min SW, Kim CS, Do SH, Kim YL, Zuo Z. Effects of ethanol on the rat glutamate excitatory amino acid transporter type 3 expressed in Xenopus oocytes: role of protein kinase C and phosphatidylinositol 3-kinase. Alcohol Clin Exp Res. 2003;27:1548–1553. doi: 10.1097/01.ALC.0000092061.92393.79. [DOI] [PubMed] [Google Scholar]
- Levenson J, Weeber E, Selcher JC, Kategaya LS, Sweatt JD, Eskin A. Long-term potentiation and contextual fear conditioning increase neuronal glutamate uptake. Nat Neurosci. 2002;5:155–161. doi: 10.1038/nn791. [DOI] [PubMed] [Google Scholar]
- Matthews G, Diamond JS. Neuronal glutamate uptake contributes to GABA synthesis and inhibitory synaptic strength. The Journal of Neuroscience. 2003;23:2040–2048. doi: 10.1523/JNEUROSCI.23-06-02040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pita-Almenar JD, Sol Collado M, Colbert CM, Eskin A. Different mechanisms exist for the plasticity of glutamate reuptake during early long-term potentiation (LTP) and late LTP. J Neurosci. 2006;26:10461–10471. doi: 10.1523/JNEUROSCI.2579-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepkuty JP, Cohen AS, Eccles C, Rafiq A, Behar K, Ganel R, Coulter DA, Rothstein JD. A neuronal glutamate transporter contributes to neurotransmitter GABA synthesis and epilepsy. The Journal of Neuroscience. 2002;22:6372–6379. doi: 10.1523/JNEUROSCI.22-15-06372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stith BJ, Woronoff K, Espinoza R, Smart T. sn-1,2-diacylglycerol and choline increase after fertilization in Xenopus laevis. Mol Biol Cell. 1997;8:755–765. doi: 10.1091/mbc.8.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]