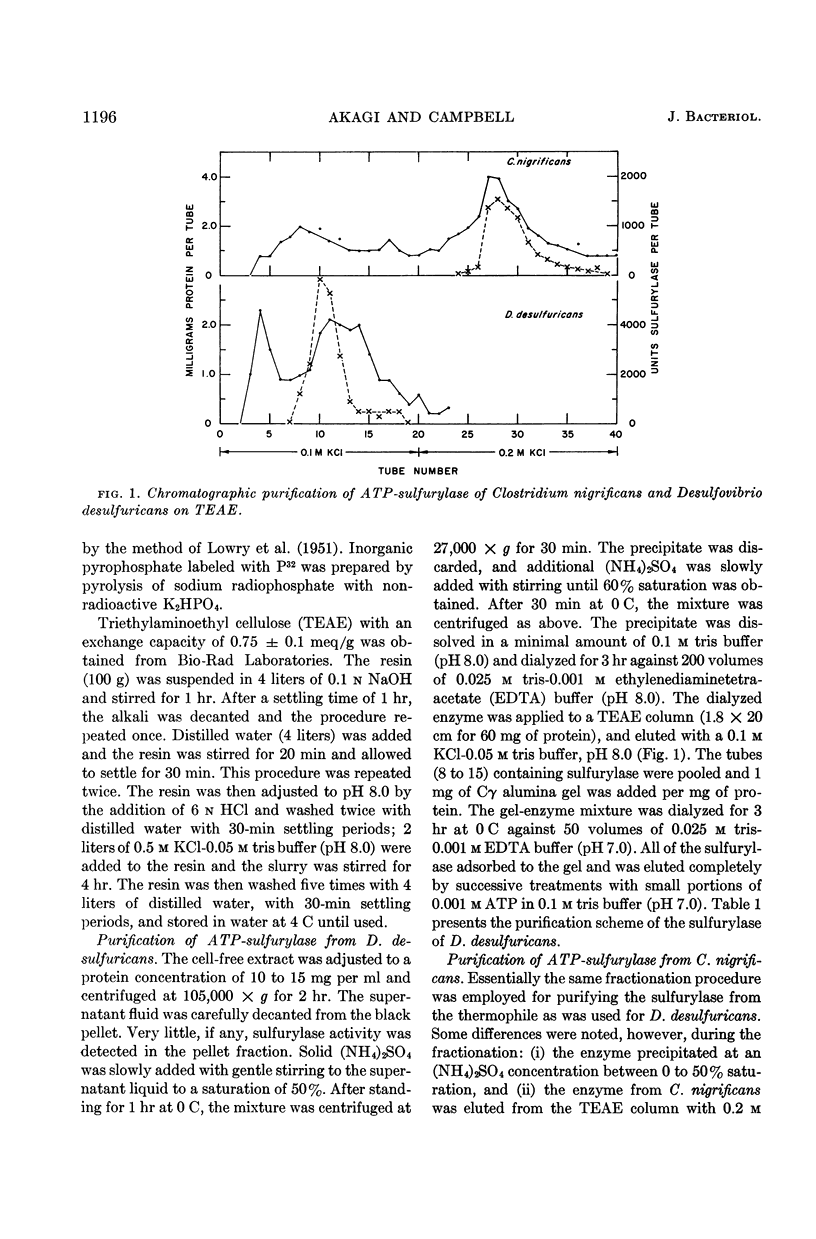
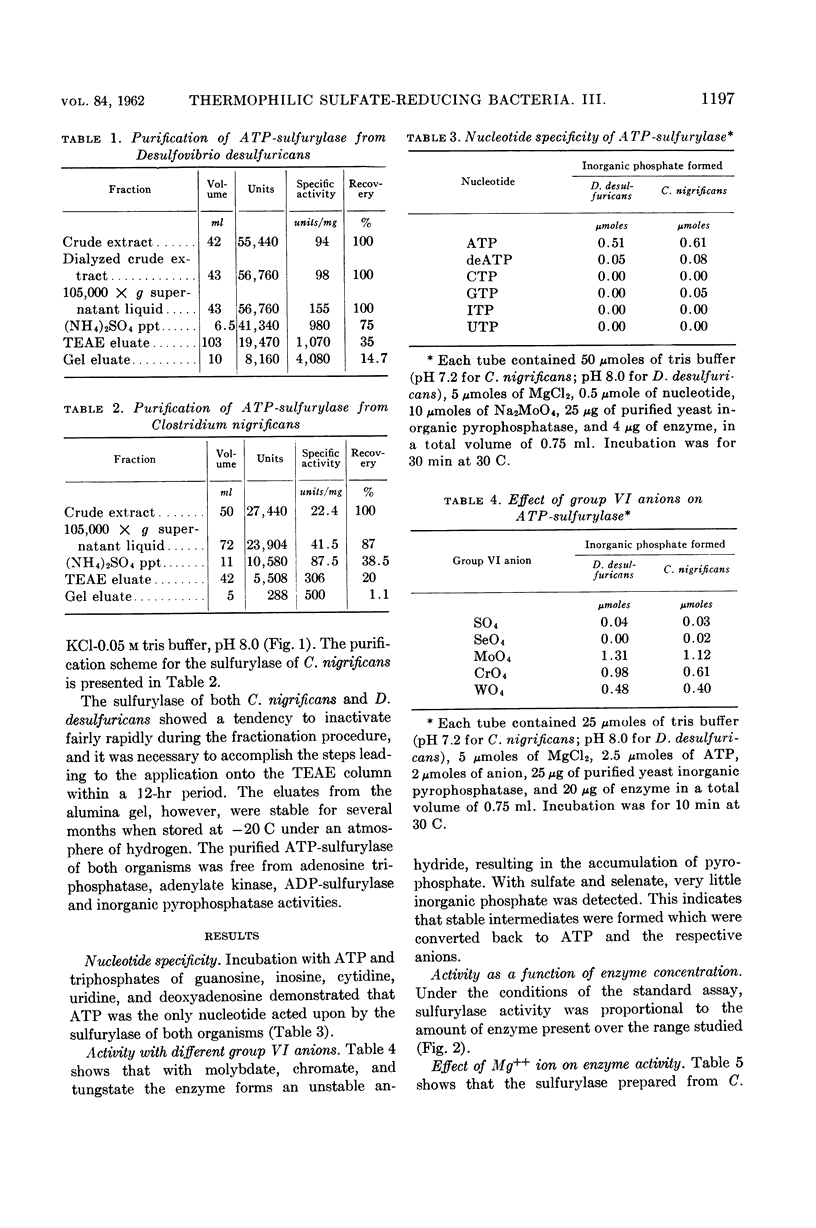
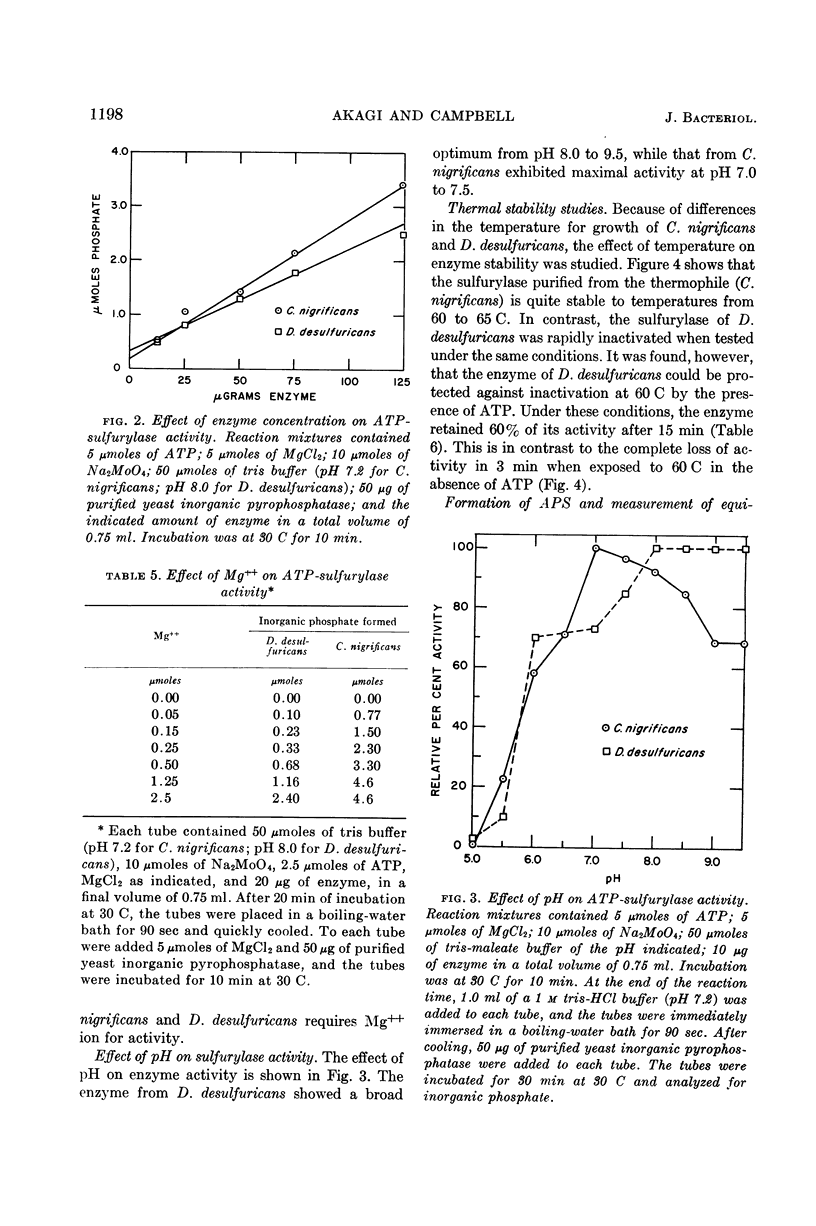
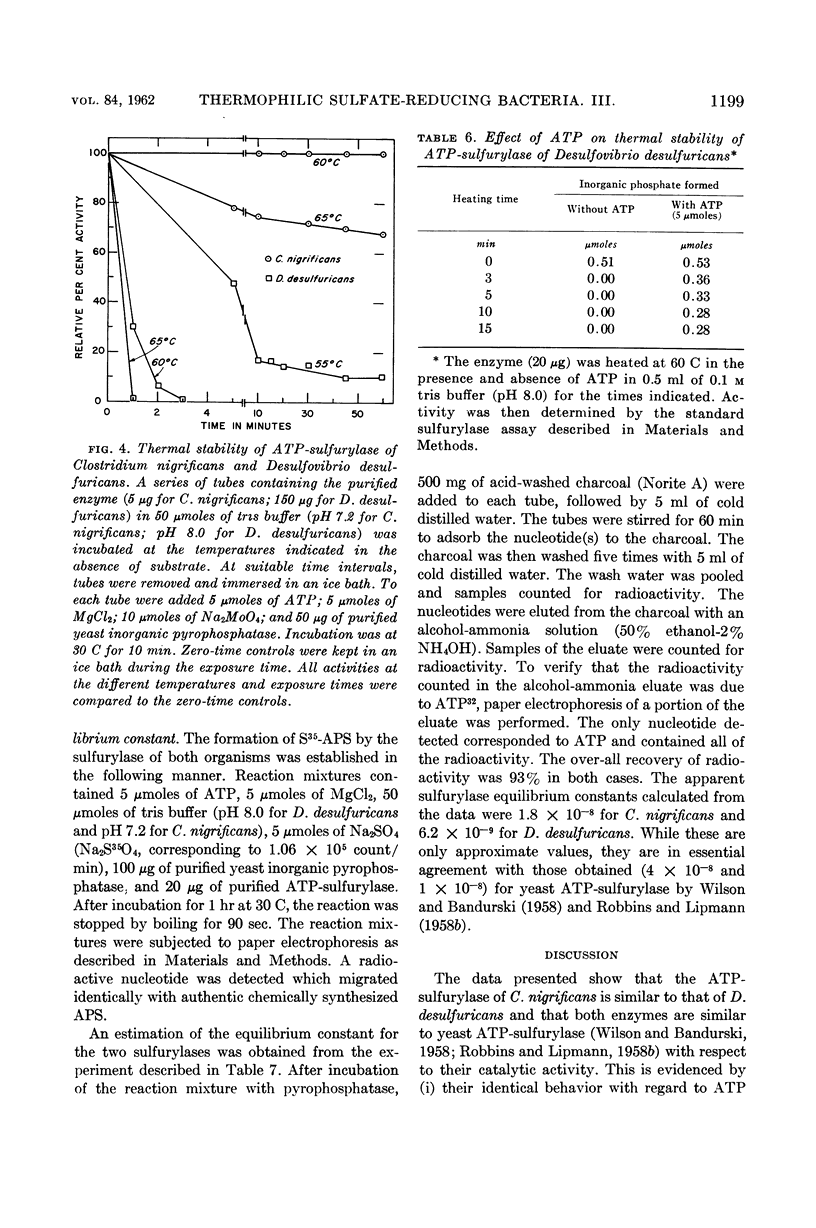
Abstract
Akagi, J. M. (University of Kansas, Lawrence) and L. Leon Campbell. Studies on thermophilic sulfate-reducing bacteria. III. Adenosine triphosphate-sulfurylase of Clostridium nigrificans and Desulfovibrio desulfuricans. J. Bacteriol. 84:1194–1201. 1962.—Adenosine triphosphate (ATP)-sulfurylase, which catalyzes the formation of adenosine-5′-phosphosulfate (APS) from ATP and SO4=, has been purified from crude extracts of Clostridium nigrificans and Desulfovibrio desulfuricans by (NH4)2SO4 fractionation and triethylaminoethyl column chromatography. The enzyme from both sources operates over a broad pH range from 6.0 to 9.5. Below pH 6.0, activity decreases sharply, with no detectable activity at pH 5.0. Of the nucleotides tested (ATP and the triphosphates of deoxyadenosine, uridine, inosine, and guanosine), only ATP was acted upon by the enzyme from either source. The enzyme requires Mg++ for activity. Incubation of the enzyme from both organisms with ATP and S35O4= in the presence of helium resulted in the formation of an S35-labeled nucleotide whose electrophoretic mobility was identical to that of chemically prepared APS. When incubated with ATP and the group VI anions (CrO4, MoO4, WO4), the enzyme from both organisms formed an unstable intermediate, resulting in the accumulation of pyrophosphate. Thermal stability studies revealed that the ATP-sulfurylase of C. nigrificans was stable at higher temperatures than the enzyme obtained from D. desulfuricans. Exposure of the enzyme from C. nigrificans to 65 C for 2 hr gave virtually no decrease in activity. In contrast, the enzyme from D. desulfuricans was completely inactivated after 30 min at 55 C, after 3 min at 60 C, or after 1 min at 65 C.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKAGI J. M., CAMPBELL L. L. Studies on thermophilic sulfate-reducing bacteria. II. Hydrogenase activity of Clostridium nigrificans. J Bacteriol. 1961 Dec;82:927–932. doi: 10.1128/jb.82.6.927-932.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER F. D., PAPISKA H. R., CAMPBELL L. L. Choline fermentation by Desulfovibrio desulfuricans. J Bacteriol. 1962 Nov;84:973–978. doi: 10.1128/jb.84.5.973-978.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL L. L., Jr, FRANK H. A., HALL E. R. Studies on thermophilic sulfate reducing bacteria. I. Identification of Sporovibrio desulfuricans as Clostridium nigrificans. J Bacteriol. 1957 Apr;73(4):516–521. doi: 10.1128/jb.73.4.516-521.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEPPEL L. A., HILMOE R. J. Purification of yeast inorganic pyrophosphatase. J Biol Chem. 1951 Sep;192(1):87–94. [PubMed] [Google Scholar]
- KOFFLER H. Protoplasmic differences between mesophiles and thermophiles. Bacteriol Rev. 1957 Dec;21(4):227–240. doi: 10.1128/br.21.4.227-240.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIPMANN F. Biological sulfate activation and transfer. Science. 1958 Sep 12;128(3324):575–580. doi: 10.1126/science.128.3324.575. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PECK H. D., Jr Enzymatic basis for assimilatory and dissimilatory sulfate reduction. J Bacteriol. 1961 Dec;82:933–939. doi: 10.1128/jb.82.6.933-939.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PECK H. D., Jr Evidence for the reversibility of the reaction catalyzed by adenosine 5'-phosphosulfate reductase. Biochim Biophys Acta. 1961 May 27;49:621–624. doi: 10.1016/0006-3002(61)90273-6. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr Symposium on metabolism of inorganic compounds. V. Comparative metabolism of inorganic sulfur compounds in microorganisms. Bacteriol Rev. 1962 Mar;26:67–94. doi: 10.1128/br.26.1.67-94.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PECK H. D., Jr The role of adenosine-5'-phosphosulfate in the reduction of sulfate to sulfite by Desulfovibrio desulfuricans. J Biol Chem. 1962 Jan;237:198–203. [PubMed] [Google Scholar]
- POSTGATE J. R. On the nutrition of Desulphovibrio desulphuricans. J Gen Microbiol. 1951 Oct;5(4):714–724. doi: 10.1099/00221287-5-4-714. [DOI] [PubMed] [Google Scholar]
- Peck H. D. THE ATP-DEPENDENT REDUCTION OF SULFATE WITH HYDROGEN IN EXTRACTS OF DESULFOVIBRIO DESULFURICANS. Proc Natl Acad Sci U S A. 1959 May;45(5):701–708. doi: 10.1073/pnas.45.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Enzymatic synthesis of adenosine-5'-phosphosulfate. J Biol Chem. 1958 Sep;233(3):686–690. [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Isolation and identification of active sulfate. J Biol Chem. 1957 Dec;229(2):837–851. [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Separation of the two enzymatic phases in active sulfate synthesis. J Biol Chem. 1958 Sep;233(3):681–685. [PubMed] [Google Scholar]
- WILSON L. G., BANDURSKI R. S. An enzymatic reaction involving adenosine triphosphate and selenate. Arch Biochem Biophys. 1956 Jun;62(2):503–506. doi: 10.1016/0003-9861(56)90151-5. [DOI] [PubMed] [Google Scholar]
- WILSON L. G., BANDURSKI R. S. Enzymatic reactions involving sulfate, sulfite, selenate, and molybdate. J Biol Chem. 1958 Oct;233(4):975–981. [PubMed] [Google Scholar]



