Abstract
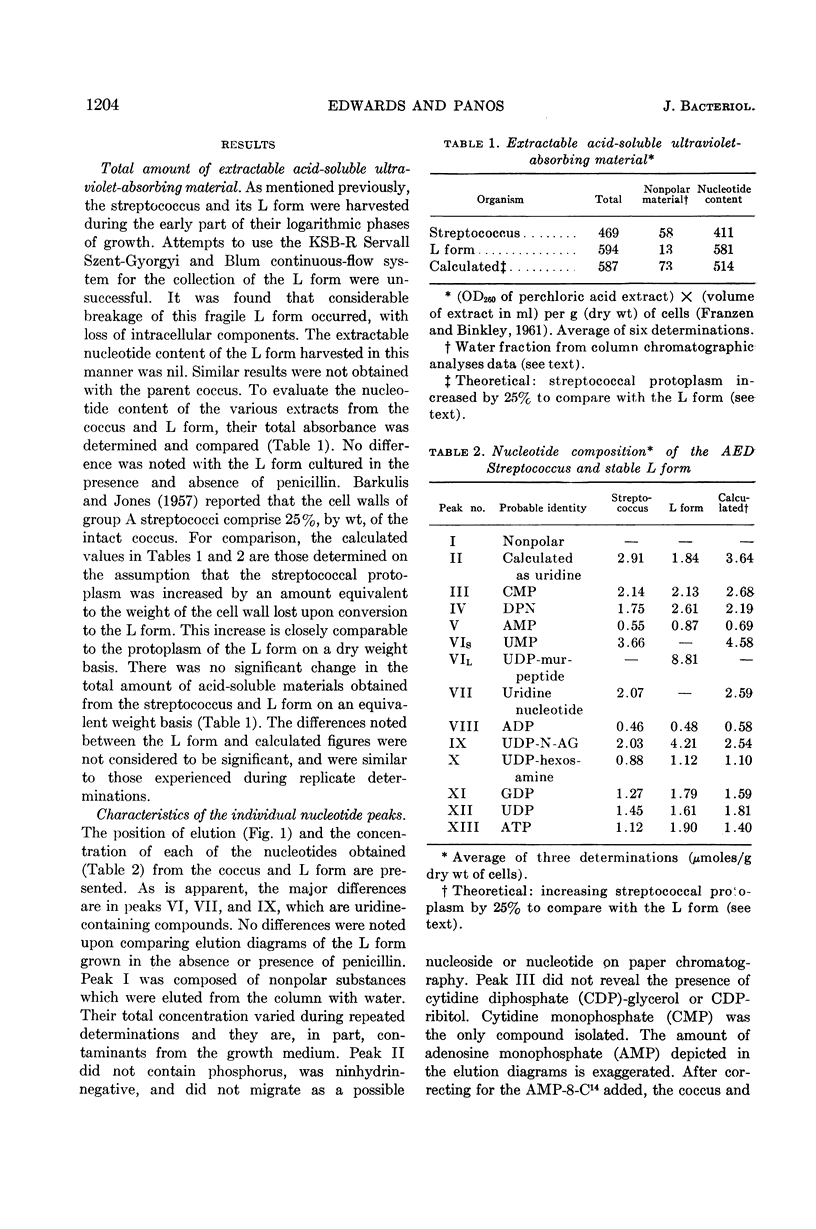
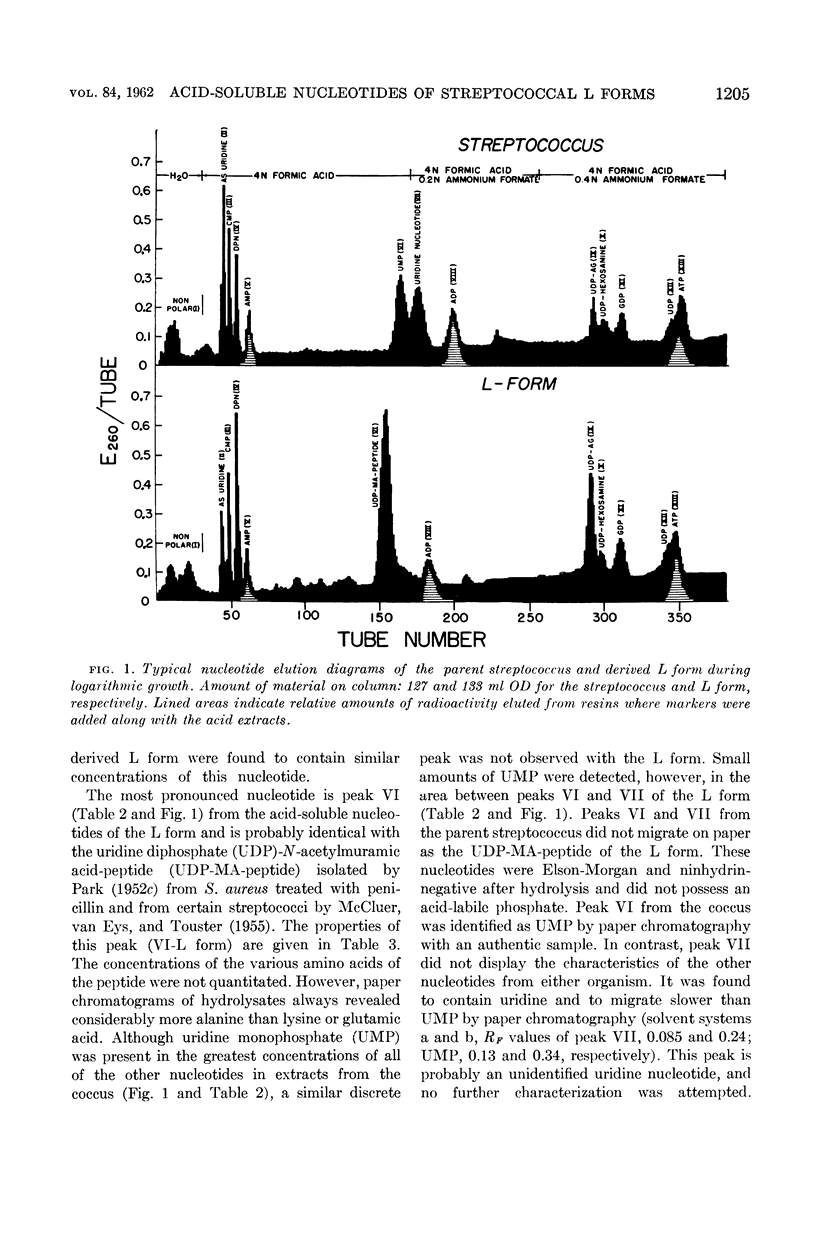
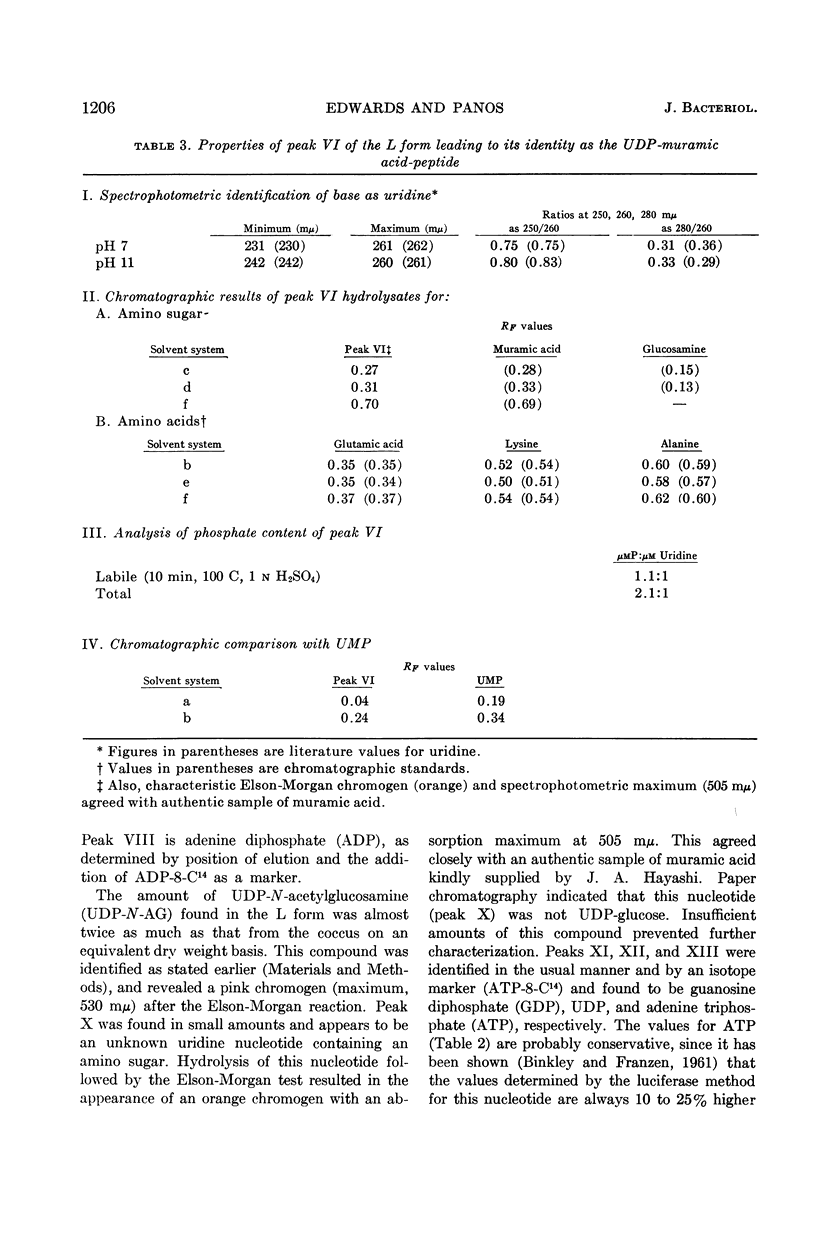
Edwards, John (University of Illinois College of Medicine, Chicago, and Albert Einstein Medical Center, Philadelphia, Pa.) and Charles Panos. Streptococcal L-forms. V. Acid-soluble nucleotides of a group A Streptococcus and derived L form. J. Bacteriol. 84:1202–1208. 1962.—This report deals with a comparison of the acid-soluble nucleotides from a group A, type 12, β-hemolytic streptococcus and a derived stable L form. This is the first report of the presence of a cell-wall precursor (uridine diphosphate-muramic acid-peptide) in a stable L form. Cells of each organism were obtained during their logarithmic phases of growth, harvested by centrifugation, and washed with osmotically protective NaCl solutions. The analytical procedures were essentially those of Franzen and Binkley. Calculated values indicated that these results could not be accounted for by dry-weight differences due to loss of the streptococcal cell wall. It was found that both organisms contained the same amount of total nucleotide material. The L form contained no uridine monophosphate (UMP), a large concentration of uridine diphosphate (UDP)-muramic acid-peptide, and a significant increase of UDP-N-acetylglucosamine. A similar nucleotide containing muramic acid-peptide was not demonstrable in the parent coccus. Instead, UMP and an unidentified uridine nucleotide were resolved in this region. Analyses of extracts from this streptococcal L form indicate the probable presence of two of the three nucleotides originally isolated by Park from penicillin-treated Staphylococcus aureus. The presence of the UDP-muramic acid-peptide cell-wall precursor in the L form cultured in the continual absence of penicillin points to an inability of this form to resynthesize the rigid cell wall and indicates that this synthetic mechanism has been permanently impaired.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARKULIS S. S. Biochemical properties of a virulent and an avirulent strain of group A hemolytic Streptococcus. Ann N Y Acad Sci. 1960 Nov 21;88:1034–1053. doi: 10.1111/j.1749-6632.1960.tb20095.x. [DOI] [PubMed] [Google Scholar]
- BARKULIS S. S., JONES M. F. Studies of streptococcal cell walls. I. Isolation, chemical composition, and preparation of M protein. J Bacteriol. 1957 Aug;74(2):207–216. doi: 10.1128/jb.74.2.207-216.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- DIENES L., WEINBERGER H. J. The L forms of bacteria. Bacteriol Rev. 1951 Dec;15(4):245–288. doi: 10.1128/br.15.4.245-288.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANZEN J. S., BINKLEY S. B. Comparison of the acid-soluble nucleotides in Escherichia coli at different growth rates. J Biol Chem. 1961 Feb;236:515–519. [PubMed] [Google Scholar]
- HURLBERT R. B., SCHMITZ H., BRUMM A. F., POTTER V. R. Nucleotide metabolism. II. Chromatographic separation of acid-soluble nucleotides. J Biol Chem. 1954 Jul;209(1):23–39. [PubMed] [Google Scholar]
- LEDERBERG J. Mechanism of action of penicillin. J Bacteriol. 1957 Jan;73(1):144–144. doi: 10.1128/jb.73.1.144-144.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANOS C., BARKULIS S. S., HAYASHI J. A. Streptococcal L forms. II. Chemical composition. J Bacteriol. 1959 Dec;78:863–867. doi: 10.1128/jb.78.6.863-867.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANOS C., BARKULIS S. S. Streptococcal L forms. I. Effect of osmotic change on viability. J Bacteriol. 1959 Aug;78:247–252. doi: 10.1128/jb.78.2.247-252.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., STROMINGER J. L. Mode of action of penicillin. Science. 1957 Jan 18;125(3238):99–101. doi: 10.1126/science.125.3238.99. [DOI] [PubMed] [Google Scholar]
- PARK J. T. Uridine-5'-pyrophosphate derivatives. II. A structure common to three derivatives. J Biol Chem. 1952 Feb;194(2):885–895. [PubMed] [Google Scholar]
- PARK J. T. Uridine-5'-pyrophosphate derivatives. II. Isolation from Staphylococcus aureus. J Biol Chem. 1952 Feb;194(2):877–884. [PubMed] [Google Scholar]
- PARK J. T. Uridine-5'-pyrophosphate derivatives. III. Amino acid-containing derivatives. J Biol Chem. 1952 Feb;194(2):897–904. [PubMed] [Google Scholar]
- Panos C. STREPTOCOCCAL L-FORMS IV. : Comparison of the Metabolic Rates of a Streptococcus and Derived L-Form. J Bacteriol. 1962 Nov;84(5):921–928. doi: 10.1128/jb.84.5.921-928.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge S. M. Filter-paper partition chromatography of sugars: 1. General description and application to the qualitative analysis of sugars in apple juice, egg white and foetal blood of sheep. with a note by R. G. Westall. Biochem J. 1948;42(2):238–250. doi: 10.1042/bj0420238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARP J. T. L Colonies from hemolytic streptocci: new technic in the study of L forms of bacteria. Proc Soc Exp Biol Med. 1954 Oct;87(1):94–97. doi: 10.3181/00379727-87-21297. [DOI] [PubMed] [Google Scholar]
- STROMINGER J. L. Biosynthesis of bacterial cell walls. Fed Proc. 1962 Jan-Feb;21:134–143. [PubMed] [Google Scholar]



