Abstract
Adrenomedullin (AM) is a multifunctional peptide vasodilator that signals through a G-protein-coupled receptor when the receptor, called calcitonin receptor-like receptor (CL), is associated with a receptor activity-modifying protein 2 (RAMP2). We demonstrated previously that haploinsufficieny for each of these genes led to reduced maternal fertility, and that even a modest genetic reduction of AM peptide caused maternal defects in implantation, placentation, and fetal growth. Here, we further demonstrate that Adm+/− female mice displayed reduced pregnancy success rates that were not caused by defects in folliculogenesis, ovulation, or fertilization. The poor fertility of Adm+/− female mice could not be rescued by transfer of wild-type blastocysts, which suggested an underlying defect in uterine receptivity. In fact, we found that Adm, Calcrl, and Ramp2 gene expressions are tightly and spatiotemporally regulated in the luminal epithelial cells of the uterus during the estrus cycle and the peri-implantation period. RAMP3, which also generates an AM receptor when associated with CL, had a diametrically opposite expression pattern than that of Adm, Calcrl, and Ramp2 and was most robustly induced in the stroma of the uterus. Finally, we discovered that Adm+/− female mice have a substantially reduced number of pinopodes on the uterine luminal epithelial surface, which is indicative and possibly causative of the poor uterine receptivity. Taken together, our studies identify a new class of pharmacologically tractable proteins that are involved in establishing uterine receptivity through the regulation of pinopode formation.
Keywords: adrenomedullin, female fertility, female reproductive tract, gene-targeted mouse models, gene targeting, implantation, pinopodes, uterine receptivity, uterus
Genetic reduction of ADM, a peptide that is dynamically and highly expressed in the uterine epithelium, causes reduced uterine receptivity.
INTRODUCTION
It is widely accepted that tightly controlled molecular cues from both the maternal endometrium and the preimplanting blastocyst are required for establishing a normal pregnancy. A precisely orchestrated maternal-fetal dialogue driven by maternal hormones leads to the expression and secretion of growth factors, vasodilators, adhesion molecules, and innate immune response factors that are required for the attachment and invasion of the blastocyst to the uterine epithelium [1]. Although studies in rodent and primate models have uncovered a multitude of gene products implicated in the normal growth and development of the early blastocyst, remarkably fewer genes have been shown to have a causal role in the establishment of a receptive uterus. As a consequence, there are few clinically useful biomarkers for diagnosing or potentially treating a nonreceptive uterus in human patients with infertility [2].
Adrenomedullin (AM) is a 52-amino acid peptide vasodilator that circulates in the plasma and can influence many biological processes [3]. The gene coding for AM peptide, Adm, is widely expressed throughout the body and is most robust in endothelial cells and vascular smooth muscle cells [4]. Consequently, highly vascularized tissues, such as the placenta and uterus, produce high levels of AM peptide [5, 6]. Adrenomedullin transduces its biological activity by binding to a G-protein-coupled receptor, calcitonin receptor-like receptor (CL for protein, Calcrl for gene), when the receptor is associated with a single-transmembrane accessory protein called receptor activity-modifying protein 2 (RAMP2) [7]. Interestingly, pharmacological studies have also demonstrated high affinity for AM binding when CL is associated with RAMP3, but the physiological significance of this interaction remains unknown [8]. Therefore, careful evaluation of the coexpression of Calcrl as well as the Ramp2 and Ramp3 genes is required to define the tissues and cells in which AM can transduce its signal through an autocrine or paracrine mode of action.
The genes that encode for AM and its receptor components are highly expressed in the human and rodent uterus under the direct control of estrogen and progesterone [6, 9–14]. Transcriptional profiling of wild-type and estrogen receptor knockout mice revealed that Ramp3 was one of the most highly estrogen-induced genes in the uterus [15, 16], and other studies using chromatin immunoprecipitation assays have shown a direct association between the estrogen receptor and transcriptional regulatory elements of both the Adm and Ramp3 genes [16]. Taken together, these gene expression studies suggest that hormonal regulation of AM signaling in the uterus during the estrus cycle and pregnancy may play important physiological roles.
Numerous studies in humans and experimental animal models have supported an important role for AM signaling during pregnancy [17, 18]. In humans, maternal plasma levels of AM rise throughout gestation and eventually peak to four to five times over prepregnancy values before returning to basal levels 24 h after delivery [19–23]. It has been widely speculated that during complications of pregnancy, like preeclampsia, gestational diabetes, small for gestational age infants, and spontaneous abortions, altered levels of maternal AM may be indicative of the condition and/or even contribute to poor reproductive outcomes [24–30]. Our previous studies using genetically engineered mice heterozygous for deletion of the Adm gene have supported an essential role for AM during pregnancy. Although Adm−/− mice are embryonic lethal, we found that pregnant Adm+/− mice displayed abnormal implantation, uterine overcrowding, and placental abnormalities, resulting in fetal growth restriction that was largely independent of the genotype of the embryo [31]. Therefore, even a modest reduction in maternal Adm gene expression caused profound defects in the implantation and placentation processes.
During the course of these previous studies, we also noticed that Adm+/− female mice appeared to have significantly lower success rates for establishing a pregnancy following a successful mating. Here, we describe in more detail the apparent cause for poor reproductive outcomes preceding implantation in Adm+/− female mice.
MATERIALS AND METHODS
Mice
Generation of mice with a targeted deletion of the Adm gene has been described previously [32]. We and others historically and commonly refer to the Adm gene-targeted allele as AM+/−, and so we emphasize that the nomenclature used in the current study does not describe a new allele, but rather conforms to established nomenclature rules. The Adm+/− line is maintained as a heterozygote breeding colony on an isogenic 129S6/SvEv background. Genotyping was performed by a three-primer, PCR-based strategy: primer 1, 5′-CAGTGAGGAATGCTAGCCTC-3′; primer 2, 5′-GCTTCCTCTTGCAAAACCACA-3′; and primer 3, 5′-TCGAGCTTCCAAGGAAGACCAGG-3′. Primers 1 and 3 amplified a 1.8-kb wild-type product, whereas primers 2 and 3 amplified a 1.3-kb targeted product. Unless otherwise stated, experimental and control animals were strain and age matched. For timed pregnancies, Adm+/− or wild-type 129S6/SvEv breedings were established, and the day when the vaginal plug was detected was considered Embryonic Day 0.5 (Day E0.5). Scoring of successful pregnancies following detection of a vaginal plug was evaluated either at Day E9.5 (postimplantation) or Postnatal Day 1. All experiments were approved by the Institutional Animal Care and Use Committee of The University of North Carolina at Chapel Hill.
Ovulation, Superovulation, and Fertilization Assay
Natural matings of one wild-type male with two wild-type females or two Adm+/− females were established. On Day E1.5, females were euthanized and oviducts were flushed with M2 medium (M7167; Sigma). Two-cell-stage embryos and degenerated oocytes were counted. For superovulation, wild-type or Adm+/− female mice were injected intraperitoneally with 5 IU eCG (G4527; Sigma) and 44 h later with 5 IU hCG (C8554; Sigma). Mating pairs were established, and embryos were flushed from the oviducts at Day E1.5 as described above.
Blastocyst Transfer
The 3.5-day-old blastocysts were collected from the uteri of superovulated C57BL/6 donor female mice purchased from Harlan. A total of 14–16 blastocysts were transferred into the uteri of 8- to 12-wk-old Adm+/− females or wild-type recipient females anesthetized with avertin (0.5 mg/g body weight) at Pseudopregnant Day E3.5. In breedings with vasectomized males, the morning of the vaginal plug was designated as Day E0.5. Recipient females were euthanized 5 days later, and the number of implanted blastocysts in the pregnant mice was calculated.
In Situ Hybridization
Wild-type 129S6/SvEv female mice at various stages of the estrus cycle or at Days E0.5, E2.5, or E5.5 were euthanized. Uteri were fixed in 4% paraformaldehyde overnight and 30% sucrose in diethylpyrocarbonate-PBS overnight and serially cryosectioned (20 μm). Sense and antisense RNA probes were synthesized from plasmids containing cDNA sequences of the murine Adm, Calcrl, Ramp2, or Ramp3 genes. Nonradioactive in situ hybridization reagents were used (1745816, 1585762, and 1093274; Roche Diagnostics). Following prehybridization, sections were hybridized to digoxigenin-labeled sense or antisense RNA probes for 12–16 h at 60°C. After washing, the sections were incubated with anti-digoxigenin Fab-AP diluted 1:2000 in blocking buffer at room temperature for 2 h. After washing, the sections were developed in nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate solution in the dark overnight, then rinsed several times in PBS at room temperature and mounted. Sections hybridized with the sense probe served as negative controls and showed little to no positive signal. Photos were taken with Nikon Microphot-FXA microscope.
Scanning Electron Microscopy of Day E4.5 Mouse Uteri
Day E4.5 mouse uteri were dissected, cut open longitudinally, and pinned to a silicone rubber mat to expose the uterine luminal epithelium. The uteri were rinsed gently with PBS to remove surface debris and covered with a fixative containing 2% paraformaldehyde/2.5% glutaraldehyde/0.15 M Karlsson and Schultz sodium phosphate buffer, pH 7.4. Samples were stored at 4°C overnight to several days before processing. After several rinses in buffer, the samples were dehydrated through an increasing series of ethanol (30%, 50%, 75%, 90%, 100%, and 100%) and dried using a Balzers CPD 020 critical point dryer (Balzers Union Ltd.) using liquid carbon dioxide as the transition solvent. The uteri were mounted onto aluminum scanning electron microscopy stubs with colloidal silver paste and were sputter coated with gold:palladium alloy (60:40) to a thickness of 20 nm using a Hummer X Sputter Coater (Anatech Ltd., Alexandria, VA). Specimens were viewed and photographed with a Cambridge Stereoscan S200 scanning electron microscope (LEO Electron Microscopy Inc., Thornwood, NY) using an accelerating voltage of 20 kV.
Statistics
Statistical analyses were performed with JMP software (SAS Institute). One-way ANOVA was used to determine significance at P < 0.05. In all figures, error bars represent SEM.
RESULTS
Genetic Reduction of AM Causes Low Pregnancy Success Rates
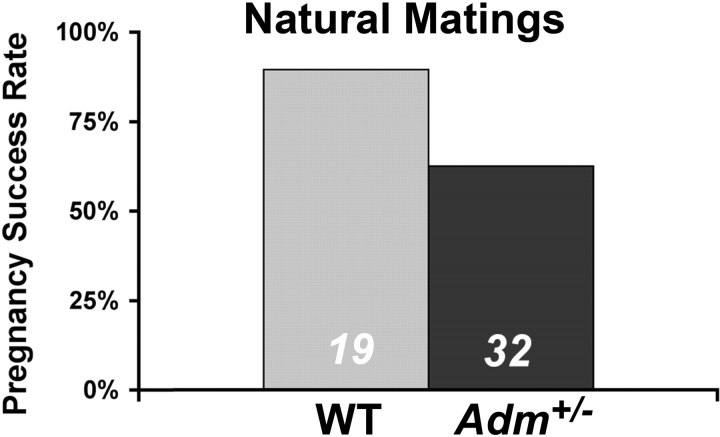
Based on our previous difficulties in maintaining a large and robust colony of Adm+/− mice, we speculated that the rate of successful pregnancies might be much lower for Adm+/− females than their isogenic wild-type control littermates. To directly address this hypothesis, we scored the number of successful pregnancies beyond implantation that resulted from natural matings in which visualization of a vaginal plug was evident the morning after mating. As shown in Figure 1, the pregnancy success rate for wild-type 129S6/SvEv female mice was 89%, whereas only 63% of the Adm+/− females with vaginal plugs resulted in successful pregnancies. Therefore, although our previous studies have shown clearly that maternal genetic reduction of AM signaling leads to abnormal implantation and placentation, these data further demonstrate that genetic dosage of AM is also crucial to establishing a pregnancy before implantation.
FIG. 1.
Reduced pregnancy success rates in Adm+/− female mice. Percentage of female mice that had successful pregnancies beyond implantation following visualization of a vaginal plug. The number at the bottom of the bars denotes the total number of female mice with an observed vaginal plug. WT, Wild-type.
Adm+/− Female Mice Exhibit Normal Ovulation and Fertilization Rates
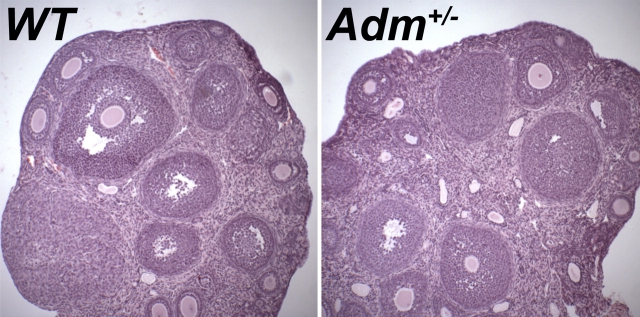
To determine whether the reduced pregnancy success rates of Adm+/− females could be caused by defects in folliculogenesis or corpus luteum formation, we compared the ovaries of Adm+/− females to those of wild-type controls. Histological examination did not reveal any significant differences between the two genotypes (Fig. 2). Specifically, the Adm+/− ovaries appeared normal in size and contained follicles at all stages of development, as well as corpus lutea.
FIG. 2.
Ovarian histology of Adm+/− mice appears normal. Hematoxylin-eosin stains of ovary sections from wild-type (WT) and Adm+/− mice do not reveal any overt defects in folliculogenesis or corpus luteum formation. Original magnification ×40.
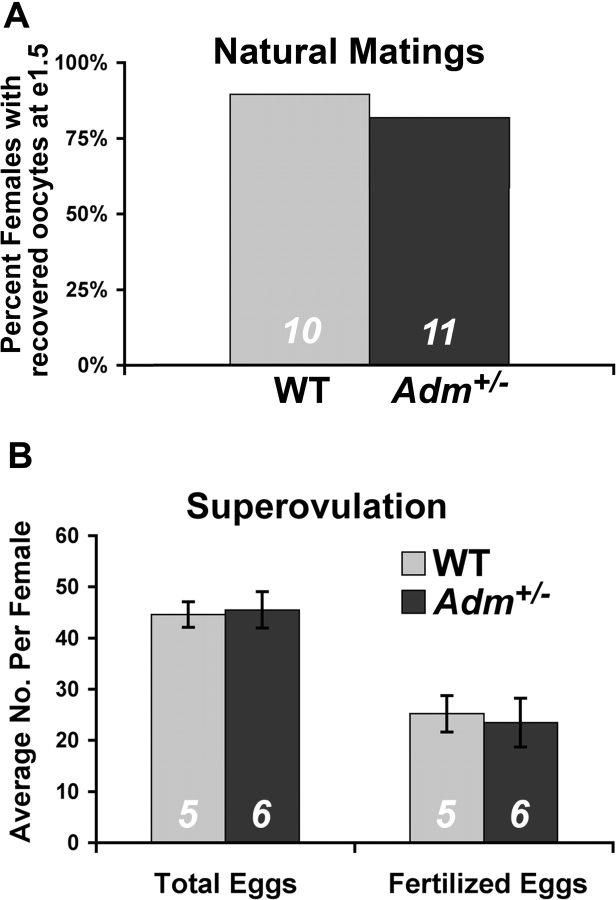
To determine whether Adm+/− female mice had a reduction in ovulation rates, we established natural timed matings with wild-type males and collected oocytes from the oviduct at Day 1.5 of pregnancy. Our ability to recover oocytes from the oviducts of plug-positive females was equivalent in both wild-type (90%) and Adm+/− (82%) females (Fig. 3A). Moreover, the total number of oocytes recovered from the two groups was roughly equivalent (54 oocytes from nine wild-type females compared with 44 oocytes from nine Adm+/− females). Consistent with the normal ovarian histology, these data demonstrate that mating and overall ovulation rates were not significantly affected by genetic reduction of Adm+/−.
FIG. 3.
Ovulation rates and fertilization occur normally in Adm+/− female mice. A) Percentage of female mice from which oocytes were collected in the oviduct 1.5 days after natural mating (e1.5). B) Average number of recovered eggs, total and fertilized, from wild-type and Adm+/− female mice following superovulation. The numbers at the bottom of the bars represent N. WT, Wild-type.
To determine whether fertilization could occur normally in Adm+/− females independently of endogenous hormonal regulation, we used gonadotropin stimulation to drive superovulation and compared the total number of released oocytes and fertilized eggs to that of similarly treated wild-type females. As shown in Figure 3B, the total number of released oocytes, as well as fertilized eggs, did not differ between wild-type and Adm+/− females. Taken together, these results demonstrate that follicular development, ovulation, and fertilization were not significantly impaired in the Adm+/− mice, and suggest that abnormal uterine receptivity may underlie the low pregnancy success rates.
Transfer of Wild-Type Blastocysts to Adm+/− Mice Fails to Rescue Low Pregnancy Success Rates
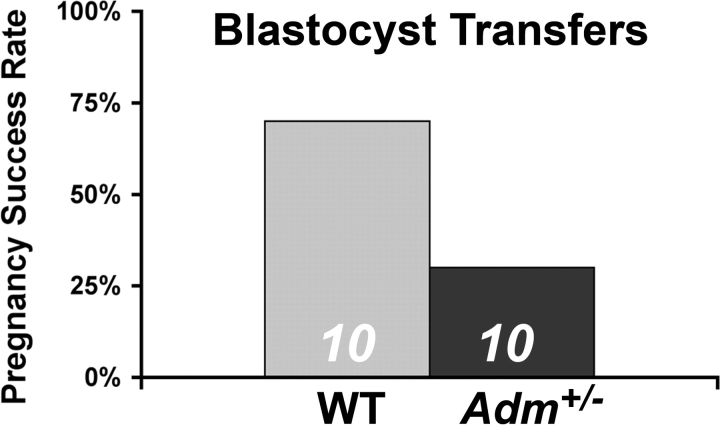
To evaluate whether uterine receptivity was altered in Adm+/− females, we next performed blastocyst transfer experiments using wild-type blastocysts from donor females transferred into either wild-type or Adm+/− females. As shown in Figure 4, 70% of wild-type females receiving wild-type donor blastocytsts resulted in successful pregnancies beyond implantation. However, only 30% of the Adm+/− females were able to maintain a pregnancy beyond implantation with wild-type blastocysts. These results demonstrate that maternal genetic reduction of AM caused defects in uterine receptivity.
FIG. 4.
Reduced uterine receptivity in Adm+/− female mice. Transfer of wild-type blastocysts into wild-type females (WT) resulted in successful pregnancies in 7 of 10 mice. In contrast, only 3 of 10 Adm+/− female mice resulted in successful pregnancies following wild-type blastocyst transfers.
AM and Its Receptor Components Are Dynamically Expressed in the Cycling Uterus
To provide a comprehensive assessment of the localization and level of expression of Adm, Calcrl, Ramp2, and Ramp3 in the mouse uterus during the estrus cycle, we performed in situ hybridization. As shown in Figure 5, there was diffuse staining for Adm in the stroma at proestrus and estrus, but the highest levels of Adm expression were observed in the luminal epithelial cells and somewhat lower in the glandular epithelium. The expression of Adm then declined dramatically throughout the uterus during metestrus and diestrus. Colocalization of Adm expression with its receptor components, Calcrl and Ramp2, was observed in proestrus and estrus. Notably, the expression of Ramp2 was remarkably higher in the luminal epithelial cells of the estrus phase than in any other phase of the estrus cycle. Calcrl and Ramp2 expression persisted in the luminal epithelium at metestrus and then dynamically switched to the glandular epithelium and vasculature at diestrus. Ramp3, which if coexpressed with Calcrl can also generate an AM receptor, had a remarkably contrasting expression pattern to that of Adm, Calcrl, and Ramp2. As shown in the bottom row of Figure 5, Ramp3 was potently induced at estrus but was spatially restricted to the first several cell layers of the underlying stroma and was completely absent in the luminal epithelium, where Adm, Calcrl, and Ramp2 gene expressions were found to be most robust. Taken together, these data indicate that genes that confer AM signaling are dynamically expressed in a spatiotemporally regulated manner in the uterus during the estrus cycle. The highest and most colocalized site of expression was found on the luminal epithelial cells during proestrus and estrus.
FIG. 5.
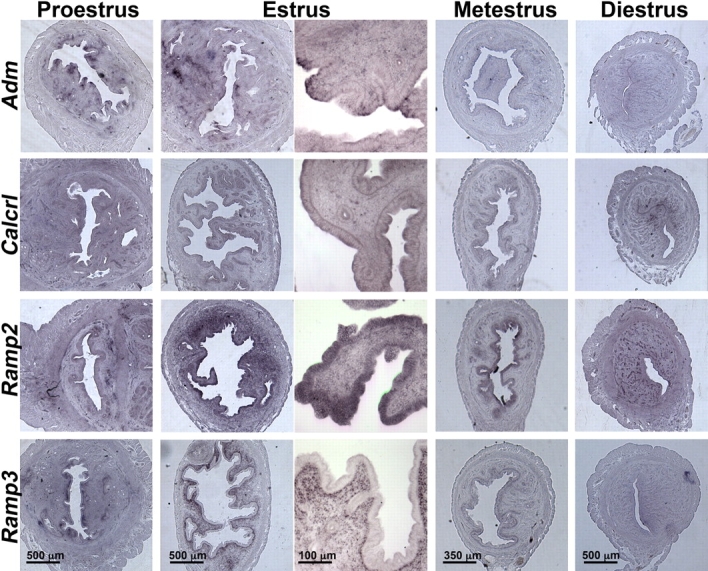
In situ hybridization of Adm and its receptor components during the uterine estrus cycle. In situ hybridization reveals that Adm, Calcrl, and Ramp2 are coordinately expressed in uterine luminal epithelial cells at estrus, whereas Ramp3 expression is restricted to the stroma. Scale bars are applicable to all images within a column.
AM and Its Receptor Components Are Robustly Increased Prior to the Uterine Receptive Phase
At Day 0.5 of pregnancy, before the blastocyst has attached to the luminal epithelium of the uterus, we noticed a remarkable induction of Adm gene expression in the luminal epithelial cells of the uterus (Fig. 6). Although substantially weaker than Adm, the expressions of Calcrl and Ramp2 also were evident in the luminal epithelial layer. Adm, Calcrl, and Ramp2 all exhibited moderate expression in the stroma at Day E0.5 of pregnancy. In contrast, Ramp3 expression was highly expressed in the stroma at Day E0.5. In situ hybridization of pregnant uteri at Day E2.5 of pregnancy revealed similar staining patterns as E0.5; however, expression of all genes in the glandular epithelium and stroma was markedly enhanced by Day E2.5. Finally, at Day E5.5 we found that the expression of Adm was highly upregulated in the maternal PDZ domain (the first few underlying layers of stromal tissue surrounding the conceptus). The expressions of Calcrl and Ramp2 were most abundant in endothelial cords of the developing decidua but not in the PDZ domain. Ramp3 also was expressed in endothelial cords of the decidua, but it was robustly expressed in the underlying stroma adjacent to the conceptus. These data support previous studies demonstrating an increase in Adm expression during pregnancy and highlight the dynamic and robust changes in AM signaling that occur locally within the receptive uterus.
FIG. 6.
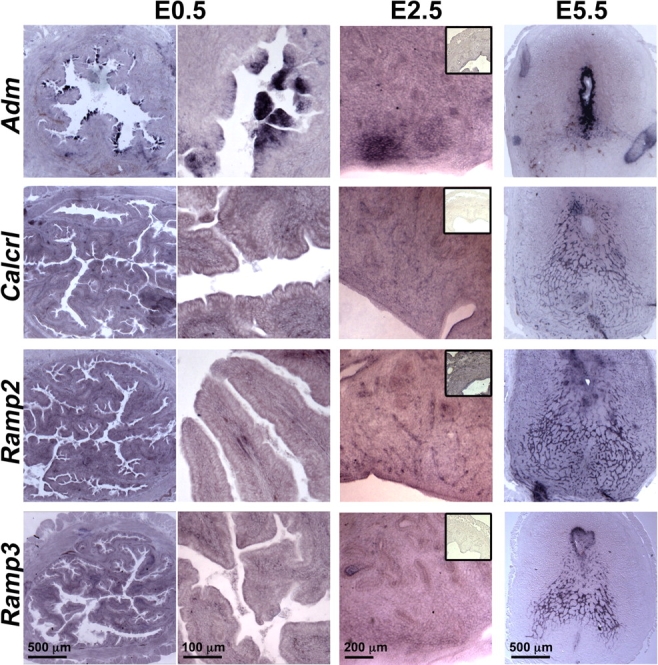
In situ hybridization of Adm and its receptor components during the peri-implantation phase. In situ hybridization of the uterus at Days E0.5, E2.5, and E5.5 of pregnancy reveals that Adm, Calcrl, and Ramp2 are highly upregulated in uterine luminal epithelial cells. By Day E2.5 the expression of all genes becomes apparent in the stroma and glandular epithelium. Inset images at Day E2.5 show sense control probes. At Day E5.5, the expression of Adm is very strong in the first few cell layers surrounding the implanted conceptus, whereas the expression of Calcrl and Ramp2 is mostly localized to endothelial cell cords of the decidua. Scale bars are applicable to all images within a column. Insets were imaged with 2.5× objective.
Uterine Pinopodes Are Remarkably Reduced in Adm+/− Female Mice
Thus far, we demonstrated that genetic reduction of AM caused low pregnancy success rates due to poor uterine receptivity and that genetic components of AM signaling were spatiotemporally regulated in the uterus during the estrus cycle and the peri-implantation period. We next wanted to determine whether biological markers of uterine receptivity were altered in Adm+/− female mice. In rodents, the presence of uterine pinopodes serves as a faithful indication of uterine receptivity. Thus, we used scanning electron microscopy to determine whether Adm+/− females had normal pinopode formation during implantation. As shown in Figure 7, wild-type mice had abundant and large plasma membrane projections of ∼3 microns in diameter along the apical surface of the uterine epithelium. In contrast, we rarely observed pinopodes in Adm+/− females. The presence of a few pinopode-like projections confirmed that the process of pinopode formation was not entirely defective in Adm+/− female mice. However, the unusually small number of pinopodes, which often were surrounded by smaller plasma membrane blebs, demonstrated that appropriate genetic dosage of AM may be required for pinopode formation in the epithelium.
FIG. 7.
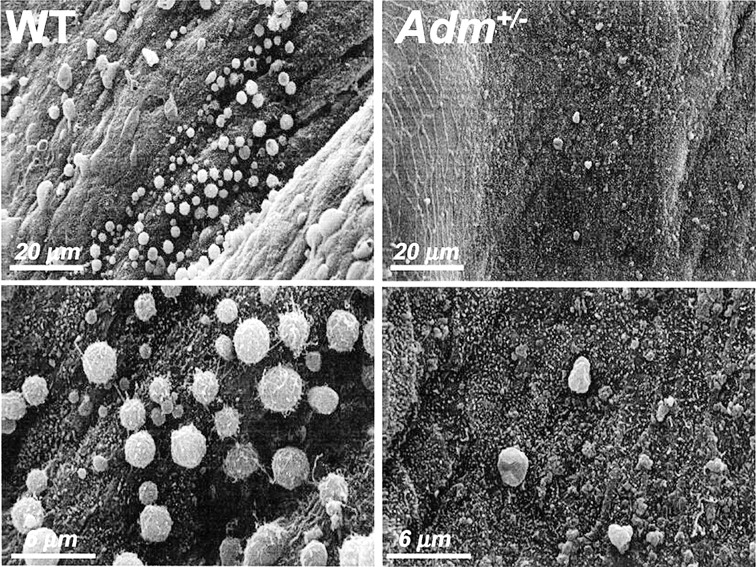
Adm+/− female mice have reduced pinopode formation. Scanning electron micrographs of the luminal surface of the uterus at Day E4.5 of pregnancy. Wild-type (WT) mice show robust and evenly distributed coverage of the luminal epithelium with ∼3-μm membrane projections called pinopodes. Adm+/− female mice had very few to no pinopodes present throughout the uterus. Much smaller (<1 μm) membrane blebs were sometimes found protruding from the epithelial surface, but rarely reached the size or density of pinopodes seen in wild-type mice.
DISCUSSION
The major finding of our current study was that female Adm+/− mice with a modest genetic reduction of AM peptide had reduced pregnancy success rates due to poor uterine receptivity. The poor uterine receptivity in Adm+/− female mice was most remarkably illustrated by reduction in uterine pinopodes during the preimplantation stage. The actual function of uterine pinopodes (also referred to as uterodomes in humans [33]) remains unclear [34], but some researchers suggest that they allow for reorganization of the epithelial cell surface, which in turn reduces the nonadherent glycocalyx coating of the uterus [35]. Others have suggested that pinopodes, as their name implies, may serve as cellular projections that engulf relatively large volumes of fluid from the uterine lumen [36]. We do not yet know whether there exists a direct causal link between haploinsufficieny for AM peptide and reduced pinopode formation in the uterine luminal epithelial cells of Adm+/− female mice. However, it is intriguing to speculate that AM peptide, which can potently regulate the permeability of endothelial cells through reorganization of the cellular cytoskeleton [37], may have also acquired important roles in mediating epithelial cellular fluid balance by influencing pinopode formation.
A few other studies using gene knockout mice with reduced fertility or abnormal uterine receptivity have shown reduced pinopode formation. For example, female mice that are homozygous null for either Lif or Hoxa10 display maternally dependant failure of implantation or decidualization, respectively, which are apparently associated with reduced pinopode formation [38, 39], although these findings have been contested recently by others [40]. Therefore, to our knowledge this is the first report that haploinsufficiency of a gene product can lead to reduction in pinopode formation. Hence, our studies emphasize the critical importance of tightly regulating the expression and dosage of AM signaling during pregnancy.
Our study also demonstrates that the expression of AM receptor components is equivalently dynamically regulated in uterine tissue during the estrus cycle, receptive phase, and implantation. Due to the lack of robust antibodies for the murine proteins, our study was limited to addressing the spatiotemporal expression of the genes that encode for AM, CL, RAMP2, and RAMP3. Although our findings await confirmation of protein distribution, we convincingly found that Calcrl and Ramp2 were spatiotemporally colocalized with AM peptide in most phases of the uterus. We also observed a remarkable dichotomy between the expression patterns of Ramp2 and Ramp3. Consistent with our findings, several groups have shown previously that Ramp3, which contains functional estrogen receptor DNA-binding sites in its promoter, is one of the most potently estrogen-induced genes in the uterus [15, 16]. However, the spatial localization of Ramp3 to the stroma, where Calcrl and Adm were consistently low, might suggest that the biological function of RAMP3 in the uterus involves other, as yet unidentified G-protein-coupled receptors. In support of this hypothesis is our finding that Calcrl+/− and Ramp2+/− female mice exhibit reduced fertility similarly to Adm+/− mice, but female mice that are homozygous null for Ramp3 appear to have normal litter sizes [41]. Nevertheless, the identification and functional validation of a conserved signaling paradigm involving AM, CL, and RAMP2 in uterine epithelial cells and stroma offers a unique and pharmacologically tractable system for potentially modulating uterine receptivity.
Pinpointing the precise biological function of AM in the uterus remains complex. The hallmark function of AM peptide is to induce potent relaxation of vascular smooth muscle cells, and so one obvious role for AM in the peri-implantation period might be to induce vasodilation in order to increase blood flow to the implantation site. However, in conflict with this notion is our current finding that the most robust induction of AM and its receptors during the estrus cycle and the peri-implantation phase occurred in uterine luminal epithelial cells, rather than vascular smooth muscle cells of the blood vasculature. Adrenomedullin peptide also acts as an angiogenic factor, and our recent studies in knockout mice have demonstrated that AM signaling is required for the development of the lymphatic vascular system [42]. Therefore, increased levels of AM signaling at the implantation site may help drive placental angiogenesis. Finally, it should be noted that the AM-binding protein (AMBP-1) is also known as complement factor H, a negative regulator of the complement activation cascade and innate immune response [43]. So, the production and secretion of AM during the peri-implantation period, in connection to its interaction with an innate immune regulator, may be involved in downregulating the maternal innate immune response to fetal implantation. Of course, the actual role of AM in the uterus may incorporate all of these functions, and future studies using in vivo replacement strategies or genetically engineered mouse models should continue to elucidate these roles.
Acknowledgments
The authors gratefully acknowledge Dr. James Cross for expert advice and helpful discussions. We also thank Kimberly Fritz-Six, Victoria Madden, and the UNC-CH Neuroscience Center Expression Localization Core Facility (National Institute of Health grant NS031768) for helpful advice and technical assistance.
Footnotes
1Supported by the Burroughs Wellcome Fund and National Institute of Health grant HD046970 to K.M.C.
REFERENCES
- Wang H, Dey SK.Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet 2006; 7: 185–199.. [DOI] [PubMed] [Google Scholar]
- Sharkey AM, Smith SK.The endometrium as a cause of implantation failure. Best Pract Res Clin Obstet Gynaecol 2003; 17: 289–307.. [DOI] [PubMed] [Google Scholar]
- Gibbons C, Dackor R, Dunworth W, Fritz-Six K, Caron KM.Receptor activity-modifying proteins: RAMPing up adrenomedullin signaling. Mol Endocrinol 2007; 21: 783–796.. [DOI] [PubMed] [Google Scholar]
- Brain SD, Grant AD.Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev 2004; 84: 903–934.. [DOI] [PubMed] [Google Scholar]
- Marinoni E, Casciani V, Marianetti V, Di Rocco A, Moscarini M, Di Iorio R.Localization and distribution of adrenomedullin receptor in the human placenta: changes with gestational age. J Reprod Med 2007; 52: 831–838.. [PubMed] [Google Scholar]
- Cameron VA, Autelitano DJ, Evans JJ, Ellmers LJ, Espiner EA, Nicholls MG, Richards AM.Adrenomedullin expression in rat uterus is correlated with plasma estradiol. Am J Physiol Endocrinol Metab 2002; 282: E139–E146.. [DOI] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM.RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 1998; 393: 333–339.. [DOI] [PubMed] [Google Scholar]
- Hay DL, Poyner DR, Sexton PM.GPCR modulation by RAMPs. Pharmacol Ther 2006; 109: 173–197.. [DOI] [PubMed] [Google Scholar]
- Jerat S, Kaufman S.Effect of pregnancy and steroid hormones on plasma adrenomedullin levels in the rat. Can J Physiol Pharmacol 1998; 76: 463–466.. [DOI] [PubMed] [Google Scholar]
- Nikitenko LL, MacKenzie IZ, Rees MC, Bicknell R.Adrenomedullin is an autocrine regulator of endothelial growth in human endometrium. Mol Hum Reprod 2000; 6: 811–819.. [DOI] [PubMed] [Google Scholar]
- Laoag-Fernandez JB, Otani T, Maruo T.Adrenomedullin expression in the human endometrium. Endocrine 2000; 12: 15–19.. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Arao Y, Otsuka H, Kikuchi A, Kayama F.Estrogen and phytoestrogen regulate the mRNA expression of adrenomedullin and adrenomedullin receptor components in the rat uterus. Mol Cell Endocrinol 2004; 223: 27–34.. [DOI] [PubMed] [Google Scholar]
- Thota C, Yallampalli C.Progesterone upregulates calcitonin gene-related peptide and adrenomedullin receptor components and cyclic adenosine 3′5′-monophosphate generation in Eker rat uterine smooth muscle cell line. Biol Reprod 2005; 72: 416–422.. [DOI] [PubMed] [Google Scholar]
- Xu Q, Ohara N, Chen W, Liu J, Sasaki H, Morikawa A, Sitruk-Ware R, Johansson ED, Maruo T.Progesterone receptor modulator CDB-2914 down-regulates vascular endothelial growth factor, adrenomedullin and their receptors and modulates progesterone receptor content in cultured human uterine leiomyoma cells. Hum Reprod 2006; 21: 2408–2416.. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Collins J, Grissom S, Deroo B, Korach KS.Global uterine genomics in vivo: microarray evaluation of the estrogen receptor alpha-growth factor cross-talk mechanism. Mol Endocrinol 2005; 19: 657–668.. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Takahashi E, Kobayashi M, Goto M, Krust A, Chambon P, Iguchi T.The estrogen-responsive adrenomedullin and receptor-modifying protein 3 gene identified by DNA microarray analysis are directly regulated by estrogen receptor. J Mol Endocrinol 2006; 36: 81–89.. [DOI] [PubMed] [Google Scholar]
- Wilson C, Nikitenko LL, Sargent IL, Rees MC.Adrenomedullin: multiple functions in human pregnancy. Angiogenesis 2004; 7: 203–212.. [DOI] [PubMed] [Google Scholar]
- Di Iorio R, Marinoni E, Letizia C, Cosmi EV.Adrenomedullin in perinatal medicine. Regul Pept 2003; 112: 103–113.. [DOI] [PubMed] [Google Scholar]
- Di Iorio R, Marinoni E, Letizia C, Villaccio B, Alberini A, Cosmi EV.Adrenomedullin production is increased in normal human pregnancy. Eur J Endocrinol 1999; 140: 201–206.. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Kubota T, Aso T, Hirata Y, Imai T, Marumo F.Immunoreactive adrenomedullin (AM) concentration in maternal plasma during human pregnancy and AM expression in placenta. Eur J Endocrinol 2000; 142: 683–687.. [DOI] [PubMed] [Google Scholar]
- Kanenishi K, Kuwabara H, Ueno M, Sato C, Sakamoto H, Hata T.Change of adrenomedullin concentrations in plasma and amniotic fluid, and human placental adrenomedullin expression with advancing gestation. Placenta 2001; 22: 244–250.. [DOI] [PubMed] [Google Scholar]
- Hoshimoto K, Hayashi M, Ohkura T.Mature adrenomedullin concentrations in plasma during pregnancy. J Matern Fetal Neonatal Med 2002; 11: 126–129.. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Ueyama H, Mashimo T, Kangawa K, Minamino N.Circulating mature adrenomedullin is related to blood volume in full-term pregnancy. Anesth Analg 2005; 101: 1816–1820.. [DOI] [PubMed] [Google Scholar]
- Lauria MR, Standley CA, Sorokin Y, Yelian FD, Cotton DB.Adrenomedullin levels in normal and preeclamptic pregnancy at term. J Soc Gynecol Investig 1999; 6: 318–321.. [DOI] [PubMed] [Google Scholar]
- Di Iorio R, Marinoni E, Letizia C, Gazzolo D, Lucchini C, Cosmi EV.Adrenomedullin is increased in the fetoplacental circulation in intrauterine growth restriction with abnormal umbilical artery waveforms. Am J Obstet Gynecol 2000; 182: 650–654.. [DOI] [PubMed] [Google Scholar]
- Di Iorio R, Marinoni E, Urban G, Costantini A, Cosmi EV, Letizia C.Fetomaternal adrenomedullin levels in diabetic pregnancy. Horm Metab Res 2001; 33: 486–490.. [DOI] [PubMed] [Google Scholar]
- Yamashiro C, Hayashi K, Yanagihara T, Hata T.Plasma adrenomedullin levels in pregnancies with appropriate for gestational age and small for gestational age infants. J Perinat Med 2001; 29: 513–518.. [DOI] [PubMed] [Google Scholar]
- Nakatsuka M, Habara T, Noguchi S, Konishi H, Kudo T.Increased plasma adrenomedullin in women with recurrent pregnancy loss. Obstet Gynecol 2003; 102: 319–324.. [DOI] [PubMed] [Google Scholar]
- Gratton RJ, Gluszynski M, Mazzuca DM, Nygard K, Han VK.Adrenomedullin messenger ribonucleic acid expression in the placentae of normal and preeclamptic pregnancies. J Clin Endocrinol Metab 2003; 88: 6048–6055.. [DOI] [PubMed] [Google Scholar]
- Marinoni E, Di Iorio R, Lucchini C, Di Netta T, Letizia C, Cosmi EV.Adrenomedullin and nitric oxide synthase at the maternal-decidual interface in early spontaneous abortion. J Reprod Med 2004; 49: 153–161.. [PubMed] [Google Scholar]
- Li M, Yee D, Magnuson TR, Smithies O, Caron KM.Reduced maternal expression of adrenomedullin disrupts fertility, placentation, and fetal growth in mice. J Clin Invest 2006; 116: 2653–2662.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron KM, Smithies O.Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional Adrenomedullin gene. Proc Natl Acad Sci U S A 2001; 98: 615–619.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CR.Understanding the apical surface markers of uterine receptivity: pinopods-or uterodomes? Hum Reprod 2000; 15: 2451–2454.. [DOI] [PubMed] [Google Scholar]
- Lopata A, Bentin-Ley U, Enders A."Pinopodes” and implantation. Rev Endocr Metab Disord 2002; 3: 77–86.. [DOI] [PubMed] [Google Scholar]
- Murphy CR.Uterine receptivity and the plasma membrane transformation. Cell Res 2004; 14: 259–267.. [DOI] [PubMed] [Google Scholar]
- Enders AC, Nelson DM.Pinocytotic activity of the uterus of the rat. Am J Anat 1973; 138: 277–299.. [DOI] [PubMed] [Google Scholar]
- Temmesfeld-Wollbruck B, Hocke AC, Suttorp N, Hippenstiel S.Adrenomedullin and endothelial barrier function. Thromb Haemost 2007; 98: 944–951.. [DOI] [PubMed] [Google Scholar]
- Bagot CN, Kliman HJ, Taylor HS.Maternal Hoxa10 is required for pinopod formation in the development of mouse uterine receptivity to embryo implantation. Dev Dyn 2001; 222: 538–544.. [DOI] [PubMed] [Google Scholar]
- Fouladi-Nashta AA, Jones CJ, Nijjar N, Mohamet L, Smith A, Chambers I, Kimber SJ.Characterization of the uterine phenotype during the peri-implantation period for LIF-null, MF1 strain mice. Dev Biol 2005; 281: 1–21.. [DOI] [PubMed] [Google Scholar]
- Quinn CE, Detmar J, Casper RF.Pinopodes are present in Lif null and Hoxa10 null mice. Fertil Steril 2007; 88: 1021–1028.. [DOI] [PubMed] [Google Scholar]
- Dackor R, Fritz-Six K, Smithies O, Caron K.Receptor activity-modifying proteins 2 and 3 have distinct physiological functions from embryogenesis to old age. J Biol Chem 2007; 282: 18094–18099.. [DOI] [PubMed] [Google Scholar]
- Fritz-Six KL, Dunworth WP, Li M, Caron KM.Adrenomedullin signaling is necessary for murine lymphatic vascular development. J Clin Invest 2008; 118: 40–50.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pio R, Martinez A, Unsworth EJ, Kowalak JA, Bengoechea JA, Zipfel PF, Elsasser TH, Cuttitta F.Complement factor H is a serum-binding protein for adrenomedullin, and the resulting complex modulates the bioactivities of both partners. J Biol Chem 2001; 276: 12292–12300.. [DOI] [PubMed] [Google Scholar]