Abstract
We examined the role of cyclooxygenase-2 (COX-2) in the late phase of ischemic preconditioning (PC). A total of 176 conscious rabbits were used. Ischemic PC (six cycles of 4-min coronary occlusions/4-min reperfusions) resulted in a rapid increase in myocardial COX-2 mRNA levels (+231 ± 64% at 1 h; RNase protection assay) followed 24 h later by an increase in COX-2 protein expression (+216 ± 79%; Western blotting) and in the myocardial content of prostaglandin (PG)E2 and 6-keto-PGF1α (+250 ± 85% and +259 ± 107%, respectively; enzyme immunoassay). Administration of two unrelated COX-2 selective inhibitors (NS-398 and celecoxib) 24 h after ischemic PC abolished the ischemic PC-induced increase in tissue levels of PGE2 and 6-keto-PGF1α. The same doses of NS-398 and celecoxib, given 24 h after ischemic PC, completely blocked the cardioprotective effects of late PC against both myocardial stunning and myocardial infarction, indicating that COX-2 activity is necessary for this phenomenon to occur. Neither NS-398 nor celecoxib lowered PGE2 or 6-keto-PGF1α levels in the nonischemic region of preconditioned rabbits, indicating that constitutive COX-1 activity was unaffected. Taken together, these results demonstrate that, in conscious rabbits, up-regulation of COX-2 plays an essential role in the cardioprotection afforded by the late phase of ischemic PC. Therefore, this study identifies COX-2 as a cardioprotective protein. The analysis of arachidonic acid metabolites strongly points to PGE2 and/or PGI2 as the likely effectors of COX-2-dependent protection. The recognition that COX-2 mediates the antistunning and antiinfarct effects of late PC impels a reassessment of current views regarding this enzyme, which is generally regarded as detrimental.
The late phase of ischemic preconditioning (PC) is an adaptive response of the heart to a mild ischemic stress that confers relative resistance to a subsequent ischemic insult occurring 12–72 h later (1–18). The development of late PC is triggered by the generation of nitric oxide (NO) (3, 13, 17) and reactive oxygen species (15) during the initial ischemic challenge and the subsequent recruitment of protein kinase C (1, 10, 12, 14), protein tyrosine kinases (5, 7, 11), and NF-κB (18), culminating in the synthesis of cardioprotective proteins that are the effectors (mediators) of protection 12–72 h after the PC stimulus (4, 9). Despite intense research over the past decade, the precise identity of the protein(s) responsible for the protective effects of late PC remains unclear. Pharmacological and genetic evidence indicates that up-regulation of inducible NO synthase (iNOS) is essential for late PC (2, 6, 16). However, it is unknown whether iNOS is the only effector of protection or whether other protein(s) is/are also involved.
The induction of iNOS in response to stresses such as cytokines, hypoxia, and ischemia is associated with simultaneous induction of cyclooxygenase-2 (COX-2) in various cell types including cardiac myocytes (19). COX is the rate-limiting enzyme in prostaglandin (PG) synthesis, catalyzing the conversion of arachidonic acid to PGH2 (20). Two distinct COX isoforms have been characterized so far: COX-1, which is present in most cells and is responsible for constitutive PG formation, and COX-2, which is induced in response to stress (20). The signaling elements that control the expression of COX-2 after stress appear to be similar to those that control the induction of iNOS, because they include reactive oxygen species (21), protein kinase C (20), protein tyrosine kinases (20), and NF-κB (22, 23), suggesting that COX-2 may be up-regulated in the heart during late PC. At present, however, no information is available regarding whether brief myocardial ischemia can induce COX-2 expression in vivo, and virtually nothing is known regarding the functional role of COX-2 in myocardial ischemia/reperfusion in general.
The goal of this study was to determine whether COX-2 contributes to the protective effects of the late phase of ischemic PC. Using a well-characterized rabbit model, we first determined whether COX-2 is induced by ischemic PC. The up-regulation of COX-2 was thoroughly analyzed not only in terms of mRNA expression and protein content but also in terms of enzymatic activity by measuring the myocardial content of all of the major metabolites of arachidonic acid (PGD2, PGE2, PGF2α, 6-keto-PGF1α, and TXB2). We then determined whether COX-2 selective inhibitors interfere with the delayed protective effects elicited by ischemic PC. Both late PC against myocardial stunning and late PC against myocardial infarction were examined to provide a comprehensive assessment of the functional role of COX-2 in this adaptive process. All studies were conducted in conscious animals. The results demonstrate that COX-2 is up-regulated in preconditioned myocardium in vivo and that this phenomenon plays an essential role in late PC. Therefore, the present investigation identifies COX-2 as a cardioprotective protein.
Methods
The conscious rabbit model of myocardial ischemia has been described in detail previously (2, 3, 5, 10, 12–14, 16–18). Briefly, New Zealand White male rabbits (2.4 ± 0.1 kg) were instrumented under sterile conditions with a balloon occluder around a major branch of the left coronary artery, a 10-MHz pulsed Doppler ultrasonic crystal in the center of the region to be rendered ischemic, and bipolar ECG leads. Rabbits were allowed to recover for a minimum of 14 days after surgery. The study consisted of three consecutive phases (phases I, II, and III).
Phase I: Studies of COX-2 Expression and Activity.
Rabbits were assigned to six groups. Group I (control) did not undergo coronary occlusion. The rabbits were euthanized, and myocardial samples (≈500 mg) were rapidly removed from the anterior and posterior left ventricular (LV) walls, frozen in liquid N2, and stored at −140°C until used. Groups II, III, and IV (PC) underwent a sequence of six 4-min coronary occlusions interspersed with 4 min of reperfusion with no treatment and were euthanized 1 h (group II), 3 h (group III), or 24 h (group IV) after the last reperfusion. Myocardial samples were rapidly removed from the ischemic-reperfused region and from the nonischemic region (posterior LV wall), frozen in liquid N2, and stored at −140°C until used. Groups V (PC + NS-398) and VI (PC + celecoxib) underwent the same sequence of occlusion/reperfusion cycles and then received an i.p. injection of NS-398 (5 mg/kg) or celecoxib (3 mg/kg), respectively, 24 h after the last reperfusion. NS-398 (Cayman Chemicals, Ann Arbor, MI) and celecoxib (Searle) were dissolved in 20% DMSO in saline. Rabbits were euthanized 40 min after the injection of NS-398 or celecoxib (time corresponding to the interval elapsed between treatment and coronary occlusion in phases II and III), and myocardial samples were obtained as described above.
RNase Protection Assay (RPA).
A 294-bp HincII/ScaI fragment of the rabbit COX-2 cDNA [kindly supplied by M. Breyer, Vanderbilt University, Nashville, TN (24)] was subcloned into the pCRII vector (Invitrogen) at the EcoR V site and linearized with EcoRI for use as a template in the RPA. A 359-nt antisense riboprobe was transcribed with T7 RNA polymerase [MAXIscript kit, Ambion (Austin, TX)] by using [α-32P]UTP (DuPont-NEN, specific activity, 800 Ci/mmol). RPAs were carried out by using the RPAIII kit (Ambion). Briefly, gel-purified riboprobes [105 cpm for COX-2 and 3 × 104 cpm for glyceraldehyde-3-phosphate dehydrogenase (GAPDH)] were hybridized with 30 μg total cellular RNA extracted from rabbit hearts or tRNA at 45°C for 18 h followed by RNase A/T1 digestion at 37°C for 30 min. Protected fragments were heat denatured and separated on 5% denaturing polyacrylamide gels. A rabbit GAPDH antisense probe was used as a control. Radioactive signals were recorded and quantitated by using a PhosphorImager. The experiment was performed four times, each of which used a new set of rabbit hearts. Each COX-2 signal was normalized to the GAPDH signal from the same sample, and the normalized values were expressed as a percentage of the signal in the anterior LV wall of group I (control).
Western Immunoblotting Analysis.
Tissue samples were homogenized in buffer A [25 mM Tris⋅HCl (pH 7.4)/0.5 mM EDTA/0.5 mM EGTA/1 mM PMSF/25 μg/ml leupeptin/1 mM DTT/25 mM NaF/1 mM Na3VO4] and centrifuged, and the resulting supernatants were collected as cytosolic fractions (10). The pellets were incubated in a lysis buffer (buffer A + 1% Triton X-100) for 2 h and centrifuged, and the resulting supernatants were collected as membranous fractions (10). The expression of COX-2 was assessed by standard SDS/PAGE Western immunoblotting techniques (10, 14, 18). Gel transfer efficiency was recorded carefully by making photocopies of membranes dyed with reversible Ponceau staining (10, 14); gel retention was determined by Coomassie blue staining (10, 14). Specific monoclonal anti-COX-2 antibodies were purchased from Transduction Laboratories (Lexington, KY). The COX-2 signals and the corresponding records of Ponceau stains of nitrocellulose membranes were quantitated by an image scanning densitometer, and each COX-2 signal was normalized to the corresponding Ponceau stain signal (10, 14). In all samples, the content of COX-2 protein was expressed as a percentage of the COX-2 protein in the anterior LV wall of group I (control group).
PG Enzyme Immunoassay (EIA).
PGs were extracted from tissue samples by using ODS-silica reverse-phase columns (Sep-Pak C18, Waters) as described by Powell (25). By using [3H]PGE2 as an internal standard, percent recovery was estimated to be 81 ± 1 (n = 10). The myocardial content of PGD2, PGE2, PGF2α, 6-keto-PGF1α, and TXB2 was determined by using EIA kits (PGD2, PGE2, and PGF2α EIA kits from Cayman Chemical; 6-keto-PGF1α and TXB2 kits from Amersham Life Science) as described (26, 27) and expressed as picogram/milligram of protein.
Phase II: Studies of Myocardial Stunning.
The experimental protocol consisted of 3 consecutive days of coronary artery occlusions (days 1, 2, and 3, respectively). On each day, the rabbits were subjected to a sequence of six 4-min coronary occlusion/4-min reperfusion cycles. Rabbits were assigned to six groups. Group VII (untreated control) received no treatment. In group VIII (DMSO control), rabbits received an i.p. injection of DMSO (0.25 ml/kg) 40 min before the first coronary occlusion on day 2. This dose of DMSO was the same as that given to groups IX, X, XI, and XII. In group IX (NS-398 on day 1), rabbits received an i.p. injection of NS-398 [5 mg/kg (the same dose used in group V in phase I)] 40 min before the first coronary occlusion on day 1. In group X (NS-398 on day 2), rabbits received the same dose of NS-398 40 min before the first coronary occlusion on day 2. In group XI (celecoxib on day 1), rabbits received an i.p. injection of celecoxib [3 mg/kg (the same dose used in group VI in phase I)] 40 min before the first coronary occlusion on day 1. In group XII (celecoxib on day 2), rabbits received the same dose of celecoxib 40 min before the first coronary occlusion on day 2. LV systolic wall thickening (WTh), range gate depth, and the ECG were continuously recorded on a thermal array chart recorder. Regional myocardial function was assessed as systolic thickening fraction by using the pulsed Doppler probe (3). The total deficit of systolic WTh (an integrative assessment of the overall severity of myocardial stunning) was calculated by measuring the area comprised between the systolic WTh-vs.-time line and the baseline (100% line) during the 5-h recovery phase after the sixth reperfusion (2, 3, 5, 12, 14, 17, 18).
Phase III: Studies of Myocardial Infarction.
Rabbits were subjected to a 30-min coronary artery occlusion followed by 3 days of reperfusion. Diazepam was administered 20 min before the onset of ischemia (6 mg/kg i.p.) to relieve the stress caused by the coronary occlusion. Rabbits were assigned to seven groups. Group XIII (untreated control) underwent the 30-min occlusion with no PC protocol or drug pretreatment. Group XIV (PC) was preconditioned with a sequence of six 4-min coronary occlusion/4-min reperfusion cycles 24 h before the 30-min coronary occlusion. Group XV (PC + DMSO) was preconditioned with the sequence of six occlusion/reperfusion cycles 24 h before the 30-min occlusion and was given an i.p. injection of DMSO (0.25 ml/kg) 40 min before the 30-min coronary occlusion (same dose of DMSO that was used in groups XVI, XVII, XVIII, and XIX). Groups XVI (PC + NS-398) and XVIII (PC + celecoxib) were preconditioned with the sequence of six occlusion/reperfusion cycles 24 h before the 30-min occlusion and then received NS-398 (5 mg/kg, i.p.) or celecoxib (3 mg/kg, i.p.) 40 min before the 30-min coronary occlusion (the same doses that were used in groups V, IX, and X for NS-398 and in groups VI, XI, and XII for celecoxib). In groups XVII (NS-398) and XIX (celecoxib), rabbits were given the same doses of NS-398 and celecoxib as in groups XVI and XVIII, respectively, without prior PC.
Postmortem Tissue Analysis.
At the conclusion of the study, the occluded/reperfused vascular bed and the infarct were identified by postmortem perfusion of the heart with Phthalo blue dye and triphenyltetrazolium (13, 16–18). Infarct size was calculated by using computerized videoplanimetry (13, 16–18).
Statistical Analysis.
Data are reported as means ± SEM. Measurements were analyzed with a one-way or two-way repeated-measures ANOVA, as appropriate, followed by paired or unpaired Student's t tests with the Bonferroni correction.
Results
Of the 176 rabbits instrumented for the study, 22 (12.5%) were excluded [the reasons for exclusion are specified in supplemental Table 1 (see www.pnas.org)].
Phase I: Expression of COX-2 mRNA.
A representative example of an RPA is illustrated in Fig. 1A. Low but detectable COX-2 mRNA levels were present in control rabbits (group I). COX-2 mRNA levels in the ischemic-reperfused region were markedly increased (+231 ± 64%) at 1 h after ischemic PC (group II), remained elevated at 3 h (group III), and returned to near control values by 24 h (group IV) (Fig. 1).
Figure 1.
Effect of ischemic PC on COX-2 mRNA levels in rabbit myocardium. Tissue samples were obtained from the anterior and posterior LV wall of control rabbits (group I) and from the ischemic-reperfused and nonischemic regions of rabbits that underwent ischemic PC with six 4-min coronary occlusion/4-min reperfusion cycles and were euthanized 1 h (group II), 3 h (group III), or 24 h (group IV) later. (A) Representative RPA gel; (B) densitometric analysis of COX-2 mRNA signals. Each COX-2 signal was normalized to the GAPDH signal from the same sample to control for RNA loading. The normalized values are expressed as percentage of the signal in the anterior LV wall of control hearts. Data are means ± SEM.
Expression of COX-2 Protein.
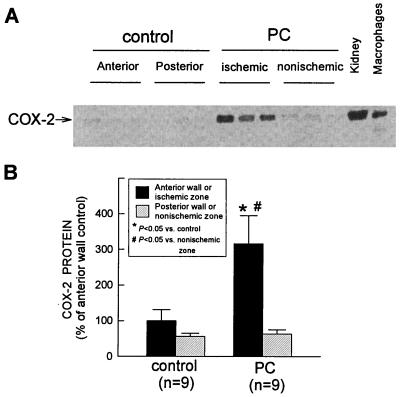
In control rabbits (group I), over 99% of total COX-2 protein was found in the membranous fraction, which is consistent with previous reports (20). A representative Western immunoblotting analysis of COX-2 is illustrated in Fig. 2A. A weak COX-2 signal was detected in control hearts (group I). When rabbits were preconditioned 24 h earlier (group IV), the expression of COX-2 in the ischemic-reperfused region increased markedly (+216 ± 79%) (Fig. 2). No expression of COX-2 protein was detectable in the cytosolic fractions of preconditioned hearts (data not shown).
Figure 2.
Effect of ischemic PC on the expression of COX-2 protein in rabbit myocardium. Tissue samples were obtained as described in the legend to in Fig. 1 from control rabbits (group I) and from rabbits that underwent ischemic PC 24 h earlier (group IV). (A) COX-2 immunoreactivity in the membranous fraction increased markedly in the ischemic-reperfused region 24 h after ischemic PC. Robust expression of COX-2 was observed in rabbit kidney and in murine macrophages stimulated with interferon-γ and lipopolysaccharide (positive controls). (B) Densitometric analysis of COX-2 signals in the membranous fraction. In all samples, the densitometric measurements of COX-2 immunoreactivity were expressed as a percentage of the average value measured in the anterior LV wall of control rabbits. Data are means ± SEM.
Myocardial PG Content.
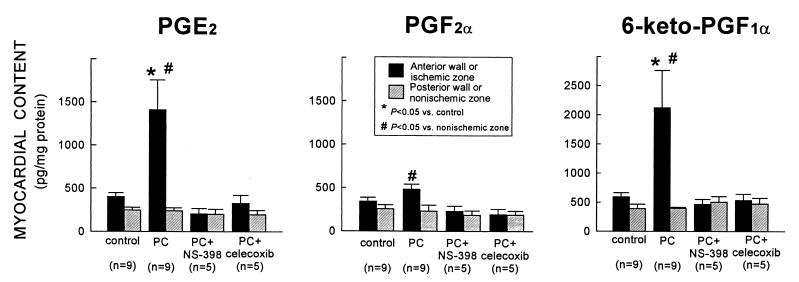
To determine whether the increase in COX-2 protein expression was associated with increased COX-2 enzymatic activity, the myocardial content of the major arachidonic acid metabolites [PGD2, PGE2, PGF2α, 6-keto-PGF1α (stable metabolite of PGI2), and TXB2 (stable metabolite of TXA2)] was measured by using EIA. Ischemic PC resulted in a robust increase in PGE2 and 6-keto-PGF1α levels (+250 ± 85% and + 259 ± 107%, respectively, vs. controls) in the ischemic-reperfused region 24 h later (group IV) (Fig. 3). PGF2α levels were also elevated but to a much lesser extent (Fig. 3). The increase in PGE2, PGF2α, and 6-keto-PGF1α was completely abrogated when rabbits preconditioned 24 h earlier were given the selective COX-2 inhibitors NS-398 (group V) or celecoxib (group VI) 40 min before euthanasia (Fig. 3). Thus, the dosages of NS-398 and celecoxib used in this study were effective in blocking the increase in COX activity associated with ischemic PC. Importantly, neither NS-398 nor celecoxib lowered PG levels in the nonischemic zone below control values (Fig. 3), indicating that constitutive COX activity (COX-1) was not suppressed by these drugs. There was no significant difference in the myocardial content of PGD2 and TXB2 between control and preconditioned hearts [supplemental Table 2 (see www.pnas.org)].
Figure 3.
Effect of ischemic PC on the myocardial content of PGE2, PGF2α, and 6-keto-PGF1α (measured by EIA). In rabbits that underwent ischemic PC 24 h earlier (group IV), the levels of PGE2 and 6-keto-PGF1α in the ischemic-reperfused region increased markedly vs. control rabbits (group I); the levels of PGF2α were higher than in the nonischemic region of the same group (group IV) but did not differ significantly from group I. The increase in PGE2, PGF2α, and 6-keto-PGF1α was completely abrogated when rabbits were given NS-398 (group V) or celecoxib (group VI) 24 h after ischemic PC (40 min before euthanasia). The myocardial content of PGE2 and 6-keto-PGF1α in the nonischemic region was similar in groups V and VI vs. group IV, indicating that the COX-2 inhibitors did not affect constitutive production of these eicosanoids by COX-1. Data are means ± SEM.
Phase II.
The size of the occluded-reperfused vascular bed was similar in groups VII-XII [supplemental Table 3 (see www.pnas.org)]. There were no appreciable differences in heart rate throughout the experimental protocol among the six groups (supplemental Table 4). In addition, there were no differences in thickening fraction among the six groups at baseline (before administration of DMSO, NS-398, or celecoxib) and just before coronary occlusion (supplemental Table 5). Neither NS-398 nor celecoxib produced significant changes in mean arterial pressure before, during, or after the six occlusion/reperfusion cycles (supplemental Table 6).
In all groups, thickening fraction on day 1 remained significantly (P < 0.05) depressed for 4 h after the sixth reperfusion and returned to values not significantly different from preocclusion values by 5 h (supplemental Figs. 6, 7, and 8; see www.pnas.org). Thus, as previously observed in this model (2, 3, 5, 12, 14, 17, 18), the sequence of six 4-min coronary occlusion/4-min reperfusion cycles resulted in severe stunning that lasted, on average, for 4 h. As expected (2, 3, 5, 12, 14, 17, 18), in control rabbits (group VII) the recovery of WTh during the 5-h reperfusion period was improved on days 2 and 3 compared with day 1 (supplemental Fig. 6), resulting in a significant decrease in the total deficit of WTh after the sixth reperfusion on days 2 and 3, respectively, compared with day 1 (P < 0.05 for both) (Fig. 4). This indicates the development of late PC against stunning (2, 3, 5, 12, 14, 17, 18). Similar results were obtained in rabbits given DMSO (the vehicle used for both NS-398 and celecoxib) on day 2 (group VIII) (Fig. 4). When rabbits were given NS-398 (group X) or celecoxib (group XII) on day 2, however, the recovery of WTh during the 5-h reperfusion period was not improved on day 2 compared with day 1 (supplemental Figs. 7 and 8), so that the total deficit of WTh did not differ significantly between day 1 and day 2 (Fig. 4). Thus, the protective effects of late PC against stunning on day 2 were completely abrogated by the administration of either NS-398 or celecoxib. In these two groups (groups X and XII), a full PC effect [induced by the PC ischemia on day 2 (2, 3, 5, 12, 14, 17, 18)] became apparent on day 3, as demonstrated by the fact that the recovery of WTh was markedly improved (supplemental Figs. 7 and 8) and the deficit of WTh markedly reduced (Fig. 4) compared with day 2. Administration of NS-398 (group IX) or celecoxib (group XI) on day 1 had no effect on the recovery of WTh (data not shown) and on the total deficit of WTh (Fig. 4) on the same day, indicating that the drugs did not augment the severity of myocardial stunning in nonpreconditioned myocardium.
Figure 4.
Total deficit of WTh after the sixth reperfusion (a measure of the severity of myocardial stunning) on days 1, 2, and 3 in groups VII (control, n = 10), VIII (DMSO on day 2, n = 7), IX (NS-398 on day 1, n = 9), X (NS-398 on day 2, n = 10), XI (celecoxib on day 1, n = 9) and XII (celecoxib on day 2, n = 10). The total deficit of WTh was measured in arbitrary units, as described in the text. Data are means ± SEM.
Phase III.
There were no significant differences in heart rate throughout the experimental protocol among groups XIII-XIX (supplemental Table 7; see www.pnas.org). In addition, the size of the region at risk did not differ among the seven groups (supplemental Table 3). In rabbits preconditioned 24 h earlier (group XIV), infarct size was significantly (P < 0.05) smaller than in controls (group XIII), indicating a late PC effect against myocardial infarction (Fig. 5). Administration of vehicle (DMSO) on day 2 (group XV) did not interfere with this effect (Fig. 5). In contrast, in rabbits treated with either NS-398 (group XVI) or celecoxib (group XVIII) on day 2, infarct size was similar to that measured in controls (Fig. 5), indicating that both drugs abrogated the late PC effect against infarction. In rabbits given NS-398 (group XVII) or celecoxib (group XIX) without ischemic PC, infarct size did not differ from that observed in controls (Fig. 5), indicating that these drugs did not affect the extent of cell death in nonpreconditioned myocardium. Thus, the abrogation of the infarct-sparing effect of late PC observed in groups XVII and XIX cannot be ascribed to deleterious actions of NS-398 or celecoxib on infarct size independent of PC. ANCOVA confirmed that for any given size of the region at risk, the resulting infarction was greater in preconditioned rabbits treated with either NS-398 or celecoxib than in untreated preconditioned rabbits (supplemental Fig. 9; see www.pnas.org).
Figure 5.
Myocardial infarct size in groups XIII (control, n = 10), XIV (PC, n = 9), XV (PC + DMSO, n = 10), XVI (PC + NS-398, n = 8), XVII (NS-398, n = 9), XVIII (PC + celecoxib, n = 8), and XIX (celecoxib, n = 9). Infarct size is expressed as a percentage of the region at risk of infarction. Solid circles represent means ± SEM.
Discussion
Although COX-2 is known to be rapidly induced in response to various stresses, its functional role in myocardial ischemia/reperfusion is essentially unknown. From a broader perspective, the biological significance of COX-2 induction in the cardiovascular system remains poorly understood (28). The present study, performed in a conscious animal model, reveals a novel facet of the pathophysiology of this enzyme. The results of phase I demonstrate that brief episodes of myocardial ischemia/reperfusion result in a rapid increase in COX-2 mRNA levels followed by a robust induction of COX-2 protein 24 h later. The enhanced expression of COX-2 is associated with a marked increase in the myocardial content of PGE2 and 6-keto-PGF1α, indicating that these PGs are the major enzymatic products of the newly synthesized protein. The results of phases II and III demonstrate that two unrelated COX-2 selective inhibitors (NS-398 and celecoxib), given at doses that block the increase in PGE2 and 6-keto-PGF1α levels associated with late PC, completely block the cardioprotective effects of late PC against both mild reversible ischemic injury (myocardial stunning) and severe irreversible injury (myocardial infarction). Taken together, these data demonstrate that COX-2 plays an essential role in the cardioprotection afforded by the late phase of ischemic PC and therefore identify a heretofore unrecognized molecular effector of this phenomenon.
All the studies reported herein were performed in conscious animals in an effort to rigorously test the role of COX-2 under conditions that are as physiological as possible. Open-chest animal preparations are associated with a number of potentially confounding factors that may interfere with the induction of COX-2 (29), with the production of arachidonic acid metabolites (30), and with myocardial stunning (31), myocardial infarction (32), and/or ischemic PC (33). Moreover, because the focus of the present study was to examine the role of COX-2, which has been implicated as a mediator of inflammation (20, 29), it seemed important to eliminate experimental conditions, such as surgical trauma, that lead to inflammatory reactions. Hence, all rabbits were allowed to recover for a minimum of 14 days after surgery.
To evaluate the physiological significance of COX-2 induction, we used two structurally unrelated agents, so as to minimize the possibility that the abrogation of late PC could be caused by nonspecific actions of either drug. NS-398 and celecoxib have been reported to be 168 times and 375 times selective, respectively, for COX-2 vs. COX-1 (IC50 for COX-1 and COX-2: 16.8 and 0.1 μM, respectively, for NS-398, and 15.0 and 0.04 μM for celecoxib) (29). Our measurements of myocardial PGE2, PGF2α, and 6-keto-PGF1α levels directly demonstrate that both NS-398 and celecoxib, at the doses used in the present study, selectively blocked the increase in COX-2 activity without lowering PG concentration below control levels, which would have implied inhibition of constitutive COX-1 activity (Fig. 3). The absence of any discernible hemodynamic effects of NS-398 and celecoxib (supplemental Tables 4–7; see www.pnas.org) further supports the conclusion that the doses used herein were selective for COX-2.
One important aspect of this study is that it defines the specific products of COX-2 activity in adult myocardium. No information is currently available regarding this issue. COX-2 generates PGH2, which can be a precursor of many different eicosanoids, including PGD2, PGE2, PGF2α, PGI2, and TXA2 (20). The precise range of PGs generated by COX-2 depends on the cell types and their inherent prostanoid synthetic pathways (20, 29). Because both beneficial and detrimental effects of eicosanoids have been described (28, 30), we reasoned that it was important to precisely define the profile of the products of COX-2 activity during late PC to elucidate the molecular basis whereby this enzyme contributes to cardioprotection. Our data demonstrate that the two main products of COX-2 activity in preconditioned myocardium are PGE2 and PGI2 (Fig. 3); PGF2α increased only marginally (Fig. 3), and PGD2 and TXB2 did not change (supplemental Table 2; see www.pnas.org). It appears, therefore, that the cardioprotective effects of COX-2 in late PC are related to the biological actions of PGE2 and/or PGI2. Both of these PGs have been reported to exert salutary actions during myocardial ischemia/reperfusion (30), resulting in attenuation of stunning (34, 35) and reduction in infarct size (36–38). The cytoprotective actions of these PGs have been attributed to antagonism of adenylyl cyclase (39), activation of ATP-sensitive potassium channels (36), inhibition of Ca2+ influx (39), and attenuation of neutrophil infiltration (37, 38).
The conclusion of the present study that COX-2 mediates the beneficial effects of late PC may appear to be in contrast with the extensive literature documenting a detrimental role of COX-2 activity in various pathophysiological conditions (28, 29). However, there is also evidence suggesting salutary actions of COX-2 in other situations (21, 40). We propose that the pathophysiological role of COX-2 is much more complex than heretofore appreciated, and that this enzyme may exert either beneficial or deleterious effects depending on the intensity of its induction, the pathophysiological setting, and the ability of specific cells to metabolize PGH2 produced by COX-2 into cytoprotective PGs.
Previous investigations have shown that iNOS mediates late PC (2, 6, 16). Because nitric oxide has been reported to directly activate COX-2 (41), it is possible to speculate that iNOS might be upstream of COX-2 in the protective pathway of late PC. Further investigation will be necessary to determine whether these two enzymes act in series or serve as independent effectors of cardioprotection.
In summary, the present study identifies a second protein (besides iNOS) that is responsible for the beneficial effects of the late phase of ischemic PC. Our biochemical analyses strongly point to PGE2 and/or PGI2 as the likely effectors of COX-2-dependent cardioprotection. These previously unrecognized cardioprotective actions of COX-2 challenge current paradigms regarding the function of this enzyme, which is generally regarded as detrimental. From a practical standpoint, the recognition that COX-2 is an indispensable co-mediator of protection against ischemia/reperfusion injury during late PC has implications for the design and clinical use of COX-2 selective as well as nonselective COX inhibitors. The concept that the arachidonic acid metabolites, PGE2 and/or PGI2, are necessary for late PC also provides a basis for novel therapeutic strategies aimed at enhancing the biosynthesis of these eicosanoids in ischemic myocardium.
Supplementary Material
Acknowledgments
We thank Dr. Matthew D. Breyer for supplying us with the rabbit COX-2 cDNA. This study was supported in part by National Institutes of Health grants R01 HL-43151 and HL-55757 (R.B.) and HL-59378 (A.B.), by the American Heart Association, Ohio Valley Affiliate grants 9951533V (X.-L.T.), and 9920593V (K.S.), and by the Jewish Hospital Research Foundation, Louisville, KY.
Abbreviations
- COX-2
cyclooxygenase-2
- iNOS
inducible nitric oxide synthase
- LV
left ventricle or left ventricular
- PC
preconditioning
- PG
prostaglandin
- WTh
wall thickening
- RPA
RNase protection assay
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- EIA
enzyme immunoassay
References
- 1.Baxter G F, Goma F M, Yellon D M. Br J Pharmacol. 1995;115:222–224. doi: 10.1111/j.1476-5381.1995.tb15866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolli R, Manchikalapudi S, Tang X L, Takano H, Qiu Y, Guo Y, Zhang Q, Jadoon A K. Circ Res. 1997;81:1094–1107. doi: 10.1161/01.res.81.6.1094. [DOI] [PubMed] [Google Scholar]
- 3.Bolli R, Bhatti Z A, Tang X L, Qiu Y, Zhang Q, Guo Y, Jadoon A K. Circ Res. 1997;81:42–52. doi: 10.1161/01.res.81.1.42. [DOI] [PubMed] [Google Scholar]
- 4.Bolli R, Dawn B, Tang X L, Qiu Y, Ping P, Xuan Y T, Jones W K, Takano H, Guo Y, Zhang J. Basic Res Cardiol. 1998;93:325–338. doi: 10.1007/s003950050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawn B, Xuan Y, Qiu Y, Takano H, Tang X, Ping P, Banergee S, Hill M, Bolli R. Circ Res. 1999;85:1154–1163. doi: 10.1161/01.res.85.12.1154. [DOI] [PubMed] [Google Scholar]
- 6.Guo Y, Jones W K, Xuan Y T, Tang X L, Bao W, Wu W J, Han H, Laubach V E, Ping P, Yang Z, et al. Proc Natl Acad Sci USA. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imagawa J, Baxter G F, Yellon D M. J Mol Cell Cardiol. 1997;29:1885–1893. doi: 10.1006/jmcc.1997.0428. [DOI] [PubMed] [Google Scholar]
- 8.Marber M S, Latchman D S, Walker J M, Yellon D M. Circulation. 1993;88:1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- 9.Marber M S, Yellon D M. Ann NY Acad Sci. 1996;793:123–141. doi: 10.1111/j.1749-6632.1996.tb33510.x. [DOI] [PubMed] [Google Scholar]
- 10.Ping P, Zhang J, Qiu Y, Tang X L, Manchikalapudi S, Cao X, Bolli R. Circ Res. 1997;81:404–414. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- 11.Ping P, Zhang J, Zheng Y T, Li R C, Dawn B, Tang X L, Takano H, Balafanova Z, Bolli R. Circ Res. 1999;85:542–550. doi: 10.1161/01.res.85.6.542. [DOI] [PubMed] [Google Scholar]
- 12.Ping P, Takano H, Zhang J, Tang X L, Qiu Y, Li R C, Banerjee S, Dawn B, Balafonova Z, Bolli R. Circ Res. 1999;84:587–604. doi: 10.1161/01.res.84.5.587. [DOI] [PubMed] [Google Scholar]
- 13.Qiu Y, Rizvi A, Tang X L, Manchikalapudi S, Takano H, Jadoon A K, Wu W J, Bolli R. Am J Physiol. 1997;273:H2931–H2936. doi: 10.1152/ajpheart.1997.273.6.H2931. [DOI] [PubMed] [Google Scholar]
- 14.Qiu Y, Ping P, Tang X L, Manchikalapudi S, Rizvi A, Zhang J, Takano H, Wu W J, Teschner S, Bolli R. J Clin Invest. 1998;101:2182–2198. doi: 10.1172/JCI1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun J Z, Tang X L, Park S W, Qiu Y, Turrens J F, Bolli R. J Clin Invest. 1996;97:562–576. doi: 10.1172/JCI118449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takano H, Manchikalapudi S, Tang X L, Qiu Y, Rizvi A, Jadoon A K, Zhang Q, Bolli R. Circulation. 1998;98:441–449. doi: 10.1161/01.cir.98.5.441. [DOI] [PubMed] [Google Scholar]
- 17.Takano H, Tang X L, Qiu Y, Guo Y, French B A, Bolli R. Circ Res. 1998;83:73–84. doi: 10.1161/01.res.83.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xuan Y T, Tang X L, Banerjee S, Takano H, Li R C, Han H, Qiu Y, Li J J, Bolli R. Circ Res. 1999;84:1095–1109. doi: 10.1161/01.res.84.9.1095. [DOI] [PubMed] [Google Scholar]
- 19.LaPointe M C, Isenovic E. Hypertension. 1999;33:276–282. doi: 10.1161/01.hyp.33.1.276. [DOI] [PubMed] [Google Scholar]
- 20.Smith W L, Garavito R M, DeWitt D L. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 21.Adderley S R, Fitzgerald D J. J Biol Chem. 1999;274:5038–5046. doi: 10.1074/jbc.274.8.5038. [DOI] [PubMed] [Google Scholar]
- 22.Schmedtje J F, Jr, Ji Y S, Liu W L, DuBois R N, Runge M S. J Biol Chem. 1997;272:601–608. doi: 10.1074/jbc.272.1.601. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto K, Arakawa T, Ueda N, Yamamoto S. J Biol Chem. 1995;270:31315–31320. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- 24.Guan Y, Chang M, Cho W, Zhang Y, Redha R, Davis L, Chang S, DuBois R N, Hao C M, Breyer M. Am J Physiol. 1997;273:F18–F26. doi: 10.1152/ajprenal.1997.273.1.F18. [DOI] [PubMed] [Google Scholar]
- 25.Powell W. In: Methods Enzymol. Lands W E M, Smith W L, editors. Orlando: Academic; 1982. pp. 466–477. [Google Scholar]
- 26.Nogawa S, Forster C, Zhang F, Nagayama M, Ross M E, Iadecola C. Proc Natl Acad Sci USA. 1998;95:10966–10971. doi: 10.1073/pnas.95.18.10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pradelles P, Grassi J, Maclouf J. Anal Chem. 1985;57:1170–1173. doi: 10.1021/ac00284a003. [DOI] [PubMed] [Google Scholar]
- 28.Wu K K. Circulation. 1998;98:95–96. doi: 10.1161/01.cir.98.2.95. [DOI] [PubMed] [Google Scholar]
- 29.Vane J R, Bakhle Y S, Botting R M. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 30.Pitt B, Shea M J, Romson J L, Lucchesi B R. Ann Intern Med. 1983;99:83–92. doi: 10.7326/0003-4819-99-1-83. [DOI] [PubMed] [Google Scholar]
- 31.Li X Y, McCay P B, Zughaib M, Jeroudi M O, Triana J F, Bolli R. J Clin Invest. 1993;92:1025–1041. doi: 10.1172/JCI116608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duncker D J, Klassen C L, Ishibashi Y, Herrlinger S H, Pavek T J, Bache R J. Am J Physiol. 1996;270:H1189–H1199. doi: 10.1152/ajpheart.1996.270.4.H1189. [DOI] [PubMed] [Google Scholar]
- 33.Haessler R, Kuzume K, Chien G L, Wolff R A, Davis R F, Van Winkle D M. Cardiovasc Res. 1994;28:1574–1580. doi: 10.1093/cvr/28.10.1574. [DOI] [PubMed] [Google Scholar]
- 34.Farber N E, Pieper G M, Thomas J P, Gross G J. Circ Res. 1988;62:204–215. doi: 10.1161/01.res.62.2.204. [DOI] [PubMed] [Google Scholar]
- 35.Farber N E, Gross G J. Am Heart J. 1989;118:17–24. doi: 10.1016/0002-8703(89)90066-5. [DOI] [PubMed] [Google Scholar]
- 36.Hide E J, Ney P, Piper J, Thiemermann C, Vane J R. Br J Pharmacol. 1995;116:2435–2440. doi: 10.1111/j.1476-5381.1995.tb15092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simpson P J, Mickelson J, Fantone J C, Gallagher K P, Lucchesi B R. Circ Res. 1987;60:666–673. doi: 10.1161/01.res.60.5.666. [DOI] [PubMed] [Google Scholar]
- 38.Smalling R W, Feld S, Ramanna N, Amirian J, Felli P, Vaughn W K, Swenson C, Janoff A. Circulation. 1995;92:935–943. doi: 10.1161/01.cir.92.4.935. [DOI] [PubMed] [Google Scholar]
- 39.Narumiya S, Sugimoto Y, Ushikubi F. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 40.McAdam B F, Catella-Lawson F, Mardini I A, Kapoor S, Lawson J A, FitzGerald G A. Proc Natl Acad Sci USA. 1999;96:272–277. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salvemini D, Misko T P, Masferrer J L, Seibert K, Currie M G, Needleman P. Proc Natl Acad Sci USA. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.