Abstract
OBJECTIVES
Evaluate the degree of vascular occlusion, vascular recanalization, and necrosis of the vascular wall caused by polyvinyl alcohol-covered polyvinyl acetate (PVAc) particles compared to trisacryl particles after renal embolization.
METHODS
Seventy-nine female albino New Zealand rabbits underwent arterial catheterization of the right kidney. Thirty-three animals were embolized with trisacryl particles, thirty-one with PVAc particles, and fifteen were kept as controls. Four animals were excluded (three trisacryl and one PVAc) due to early death. Five subgroups of six animals were created. The animals in the different groups were sacrificed either 48 hours, 5 days, 10 days, 30 days, or 90 days after embolization. The control group was divided into subgroups of three animals each and kept for the same periods of time. The kidneys were dyed with hematoxylin-eosin and Masson’s trichrome and then examined using optical microscopy.
RESULTS
There were significant differences in the degree of vascular occlusion caused by the trisacryl and the PVAc particles between the five-day and the ten-day groups. Additional differences were noted between the five-day and 48-hour groups in regard to the amount of necrosis. For both findings, the PVAc group members showed adequate tissue reaction (ischemia and volumetric reduction) and less recanalization than those treated with trisacryl.
CONCLUSION
The use of PVAc as an embolization material exhibited an adequate tissue reaction (ischemia and volumetric reduction), more expressive vascular occlusion and necrosis, and less recanalization than the trisacryl material.
Keywords: Therapeutic embolization, Particles, Microspheres, Interventional radiology, Kidney
INTRODUCTION
Endovascular embolization is a very important strategy in the treatment of tumors and arteriovenous malformations (AVMs).1,3 This technique is based on the injection of a synthetic material after selective catheterization of the blood vessels that irrigate the region being treated. The synthetic material interrupts the supply of nutrients to this particular region, leading to necrosis and a decrease in size of the organ. Thus, embolization favors the recovery of the tissue after a variable amount of time.
Different materials have been proposed to be used for vascular occlusion through embolization. Latchaw and Gold mention the use of metal coils, silicon rubber, carbon microspheres, and polyvinyl alcohol particles as suitable materials for embolization.2 In each case, one should consider the compatibility between the size of the material to be injected, the diameter of the catheter used, the vessels to be treated, and the suitability of the material for sterilization.
Among the above-mentioned materials, vinyl polyalcohol, or polyvinyl alcohol (PVA), deserves special attention because of its many interesting properties. The interesting properties of PVA include elevated compressibility, good elasticity, and good chemical resistance to acids, bases, and detergents.1 In fact, PVA foams have the ability to resume their original shape after compression when they are exposed to blood. This property makes PVA a very appealing material for embolization because the size of the catheter limits the size of the particles. Unfortunately, currently available PVA has some undesirable aspects. First, the flake-like shape and very uneven surfaces of PVA particles4,5 facilitates their agglutination and aggregation, making their flow through a catheter very difficult.6,7 In addition, currently available PVA is prone to degradation over time (biodegradability), increasing the possibility of future recanalization of the treated vessels.
In recent years, several sources have used spherical embolization agents. These spherical agents are easier to use and more effective at promoting vascular occlusion compared to non-spherical agents.8,9 An advantage of spherical particles is their defined size and the ability to calibrate particle size. Previous studies have demonstrated that particles with diameters closely matched to the vessels targeted for occlusion make the embolization procedure safer and more accurate.10 Spherical PVA and trisacryl are among several spherical agents that have already been produced and tested for medical use.10,11
Several trials were undertaken in the Chemical Engineering program of the Group of Research on Engineering (COPPE) of the Federal University of Rio de Janeiro (UFRJ). The aims of these trials were to produce a new spherical particle using alternative initializing mechanisms, such as ionizing ultraviolet light,12 and to suggest new viable routes for synthesizing PVA that can be used as an embolizing agent. Some of the classic polymerization procedures for vinyl acetate (a precursor of polyvinyl alcohol) under heating were considered, followed by hydrolysis through basic solutions and purification steps, as well as the polymerization of vinyl acetate in ultraviolet chambers. The aim of these new techniques was to control of the amount of ramification in the polyvinyl acetate leading to the generation of a spherical particle that consists of a core of polyvinyl acetate coated by PVA produced according to the technique published by Peixoto et al.13
The objective of this study was to evaluate the effects of arterial embolization with trisacryl and PVAc particles in the kidneys of albino New Zealand rabbits. The effect of these particles was measured by the degree of vascular occlusion, the extent of necrosis of the vascular wall, and the presence of recanalization of the vessel lumen.
MATERIALS AND METHODS
Animals
Seventy-nine albino New Zealand female rabbits with weights ranging from 2300 to 3200 g were used. The animals were kept in dedicated laboratories of the School of Medicine of the University of São Paulo (FMUSP) in cages constructed solely for the purpose of this study.
Of the 79 animals, 33 underwent intra-arterial embolization with trisacryl (Embosphere®; BioSphere® Medical), 31 underwent intra-arterial embolization with PVAc, and the remaining 15 were kept as controls. Each experimental group was divided into five subgroups of six rabbits each (n=6). The subgroups were kept captive for post-operative periods of either 48 hours, 5 days, 10 days, 30 days, or 90 days. Four animals were excluded (three trisacryl and one PVAc) due to early deaths. The control group (Sham) was divided into five subgroups of three animals each (n=3) for the same periods of time. Within each animal, the non-manipulated contralateral kidney was also analyzed as a part of the control group.
Anesthesia
Ketamine chloridrate (Vetanarcol®, König) at a concentration of 20 mg/kg and xylazine chloridrate (Rompum®, Bayer) at a concentration of 2 mg/kg were both used intramuscularly. A solution of 1% lidocaine chloridrate (Xylocaine®, AstraZeneca) was used at the inguinal incision site.
Surgical Procedure
A peripheral intravenous catheter (Jelco®, Smiths Medical) was used for the inguinal surgical approach, necessitating the identification and selection of the right femoral artery. After the dissection, the artery was catheterized with a Jelco® 22G, through which a 0.018″ guidewire, and then a pediatric sheath introducer 4F (Cordis®, Johnson & Johnson) was placed. The animal was then infused with 20 mL of saline.
An angiographic catheter 4F (Cordis ®, Johnson & Johnson) with a hydrophilic 0.035” guidewire (Terumo®, Terumo Medical Corporation) was progressed through a catheter to the right renal artery. One milliliter of low-osmolarity, non-ionic contrast (Optiray®, Mallinckrodt) was injected under fluoroscopy and an angiographic study was then obtained, evaluating the arterial, parenchymatous, and venous phases.
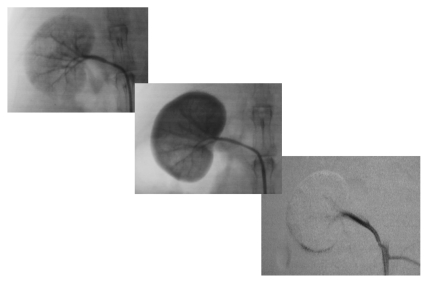
The intra-arterial embolization with a diameter of 100–300 μm was carried out with a 20 mL suspension of 0.9% saline with either trisacryl or PVAc. Injection of the particles was performed slowly and under fluoroscopic control (to avoid possible counter-flow to the aorta) until complete renal artery occlusion was obtained. Devascularization of the kidney was confirmed with a post-embolization angiography, which did not show renal parenchyma opacification (Fig. 1). The angiographic catheter was taken out, hemostasia performed, and the skin was then closed with 4–0 monofilament Nylon (Mononylon®, Ethicon-Johnson & Johnson) in a running suture. Intramuscular dipirone (Novalgina®, Aventis Pharma) and penicillin (Penicilina®, Pfizer) were given and the animals were sent back to the original laboratory using ground transportation. The surgical procedures took place in the Interventionist Vascular Radiology department of the Hospital das Clínicas (General Hospital) of the School of Medicine of the University of São Paulo (FMUSP).
Figure 1.
Renal angiography - Arterial stage and parenchymal before and after embolization
The right kidney was chosen solely to maintain sample uniformity while the left kidney was preserved to maintain the vital functions of the animal until the time of sacrifice.
Sample Harvesting
The animals were sacrificed with an intravenous injection of sodium thiopental (Pentothal®, Abbott Laboratories) and potassium chloride in the marginal vein of the ear. The kidneys were excised (both the embolized and the contralateral) through a median laparotomy and were sectioned into two halves on the longitudinal (coronal) plane, photographed, and immediately immersed in 10% formaldehyde.
Histopathological analysis
Histopathological studies were undertaken in the Renal Physiopathology Research Laboratory (LIM-16) at the University of São Paulo with conventional optical microscopy. Fragments of both kidneys (averaging 1.0 x 1.0 cm) were taken and processed in 10% formol, dehydrated using increasing concentrations of ethylic alcohol, sheared in xylol, and immersed in paraffin blocks. They were then sliced into four-micron-thick slices and dyed by the hematoxylin-eosin (HE) and Masson’s trichromic methods.
The degree of vascular occlusion was evaluated as follows: Grade I – few particles, scarcely occluding the vascular lumen; Grade II – more numerous particles, aggregated among themselves and to the vascular wall, without total lumen occlusion; and Grade III – numerous particles, aggregated among themselves and to the vascular wall, with total lumen occlusion.
Necrosis of the vascular wall was classified as Grade I through Grade III. Grade I was assigned to lesions of up to 25% of the vessel wall, Grade II was a lesion of up to 50% of the vessel wall, and Grade III included injury to more than 50% of the vessel wall.
The degree of vascular recanalization was also classified as Grade I through Grade III. The recanalization was deemed Grade I if there were a few discrete lacunas of small caliber. Grade II represented moderate recanalization with more numerous lacunas and/or of an increased caliber. Grade III encompassed intense recanalization with numerous lacunas of a larger caliber and blood cells in the vascular lumen.
The ischemic necrosis of the parenchyma was evaluated in percentages related to the normal parenchyma and correlated with the number of days elapsed since embolization with trisacryl or PVAc.
Statistical Analysis
The non-parametric Kruskal-Wallis test was used through the SPSS software (Statistical Package for the Social Sciences, Version 8.0, Chicago, Illinois, USA, 1977).
This study was approved by the Ethics Committee for the analysis of research projects of the CAPPesq of the Faculty of Medicine, University of São Paulo. The guidelines to protect the animals in the vivarium of the School of Medicine, University of São Paulo, were followed, as were the national laws for the use of animals in laboratories.
RESULTS
Both the trisacryl and the PVAc particles were injected with the same ease and none of the catheters clogged in either group.
Macroscopy
In the animals sacrificed after 48 hours of embolization, macroscopic study of the embolized kidneys showed a subcapsular cortical surface paleness and, after cutting, discrete blurring of the cortico-medullar limits associated with slight hyperemia of the medullar zone. Such alterations were proportionally enhanced in the five-day and ten-day groups, with paleness in the medullar zone and a complete loss of cortico-medullar limits when compared to the control contralateral kidneys.
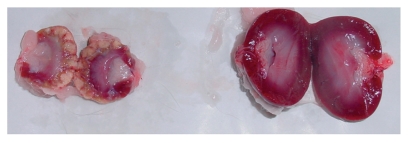
After 30 and 90 days of embolization, the kidneys showed a decrease in longitudinal diameter of over 1.0 cm (Fig. 2). The specimens embolized with trisacryl had external cortical surfaces and section surfaces similar to those observed after 10 days of embolization; however, the kidneys embolized with PVAc had several depressed scars on the cortical surface, as well as a thickened and adherent renal capsule. After sectioning, homogeneous areas with a cartilaginous appearance, as well as other areas that were yellowish and serpiginous, were noted.
Figure 2.
Macroscopic photographs of the right (embolized) kidney and the contralateral (control) kidney immediately after sacrificing the animal (these kidneys are from an animal in the 90-day post-embolization PVAc group)
Histopathological Study
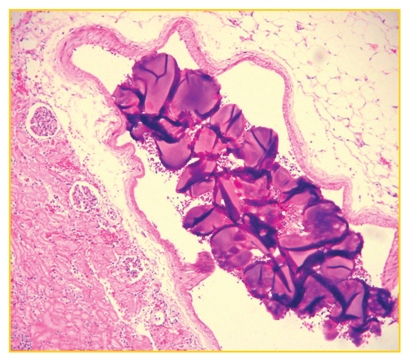
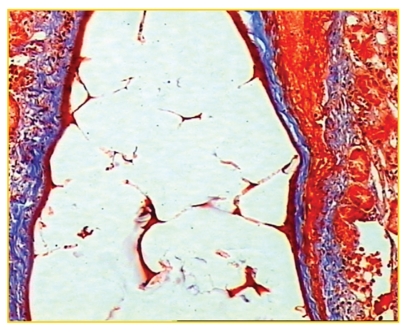
The histological study of the group of rabbits after 48 hours of embolization showed that the trisacryl group (T) particles appeared to be flexible, often demonstrating a deformed aspect (Fig. 3). In contrast, the PVAc group (P) had a more stable spherical shape (Fig. 4).
Figure 3.
Deformed trisacryl particles and spaces left by them in the vessel lumen
Figure 4.
PVA particles retain their original shape and occupy all of the space within the vessel
In both groups, the particles were concentrated mainly in the renal hilum arteries and arcuate arteries. Some particles could be observed in smaller arteries (interlobular arteries), mainly in the T group.
There was less aggregation between the group T particles, which were generally found isolated in the vascular lumen. The P particles were found to be more aggregated, resulting in more vascular occlusion (p=0.1179). This observation was also noted in the subsequent time span groups. A progressive increase in the occlusion rate was noted in the five-day group (p=0.0068) and the ten-day group (p=0.0037; Table 1).
Table 1.
Vascular lumen occlusion related to the time after embolization with either trisacryl (T) or PVAc (P)
| Vascular Lumen Occlusion | ||||||||
|---|---|---|---|---|---|---|---|---|
| 48 h | 5 D | 10 D | 30 D | |||||
| T | P | T | P | T | P | T | P | |
| (n=6) | (n=6) | (n=6) | (n=6) | (n=6) | (n=6) | (n=6) | (n=5) | |
| Grade I | 2 | 1 | 3 | 0 | 4 | 0 | 0 | 0 |
| Grade II | 4 | 2 | 2 | 0 | 2 | 1 | 6 | 0 |
| Grade III | 0 | 3 | 1 | 6 | 0 | 5 | 0 | 5 |
| P-values (Kruskal-Wallis | p=0.1179 | p=0.0068 | p=0.0037 | Does not apply | ||||
In both the trisacryl and the PVAc groups, there were necrotizing lesions in the walls of the obstructed vessels. These lesions were observed 48 hours after embolization (p=0.027) and were more extensive in the group of rabbits embolized with the PVAc particles. After 10 days, only the kidneys treated with PVAc particles still had vascular wall necrosis (Table 2).
Table 2.
Vascular wall necrosis related to the number of days after embolization with trisacryl (T) or PVAc (P)
| Vascular Wall Necrosis | ||||||
|---|---|---|---|---|---|---|
| 48 h | 5 D | 10 D | ||||
| T | P | T | P | T | P | |
| (n=6) | (n=6) | (n=6) | (n=6) | (n=6) | (n=6) | |
| Grade I | 5 | 1 | 0 | 0 | 0 | 3 |
| Grade II | 1 | 5 | 4 | 2 | 0 | 2 |
| Grade III | 0 | 0 | 2 | 4 | 0 | 1 |
| P-values (Kruskal-Wallis) | p=0.027 | p=0.2689 | Does not apply | |||
Ten days after embolization, all group T kidneys presented moderate or severe recanalization of the occluded vessels. The group P kidneys, analyzed at the same time, showed no recanalization in more than half of the specimens studied (p=0.32). The recanalization was mild in both of the group P kidneys in which it was observed. After 30 days, predominant vascular recanalization was still observed in the trisacryl-treated rabbits (p=0.4073). Ninety days after embolization, recanalization could be observed in the renal vessels of both groups (p=0.2352), with no significant differences between them (Table 3).
Table 3.
Recanalization of the vascular lumen related to the number of days after embolization with either trisacryl (T) or PVAc (P)
| Recanalization of Vascular Lumen | ||||||
|---|---|---|---|---|---|---|
| 10 D | 30 D | 90 D | ||||
| T | P | T | P | T | P | |
| (n=6) | (n=6) | (n=6) | (n=5) | (n=5) | (n=6) | |
| 0 | 0 | 4 | 0 | 1 | 0 | 0 |
| Grade I | 0 | 2 | 0 | 3 | 0 | 2 |
| Grade II | 3 | 0 | 2 | 1 | 3 | 2 |
| Grade III | 3 | 0 | 4 | 0 | 2 | 2 |
| P-values (Kruskal-Wallis) | p=0.32 | p=0.4073 | p=0.2352 | |||
Regarding the renal parenchyma, ischemic lesions and infarction were noted in nearly the entire extent of the treated kidneys in both groups (T and P) 48 hours after embolization. In the animals that were sacrificed at later time points, the ischemic lesions and infarctions were more prominent in the group P rabbits, specifically 30 days after embolization (Table 4).
Table 4.
Average percentage of parenchymal necrosis related to the time elapsed since embolization with either trisacryl (T) or PVAc (P)
| Parenchymal necrosis (%) | ||
|---|---|---|
| T | P | |
| 48H | 100 | 100 |
| 5D | 91.7 | 97 |
| 10D | 76.7 | 93.3 |
| 30D | 57.5 | 89.2 |
| 90D | 84.2 | 70.8 |
After 90 days, the kidneys of both the trisacryl and the PVAc groups had similar appearances. There was generalized fibrosis and foci of dystrophic calcification.
DISCUSSION
This study created an experimental small animal model (rabbit) with a long survival time to allow for an arterial approach to vascular system research. A microsurgery-trained technician and adaptation of surgical tools were required. The mechanisms of action of the available embolic agents and the development of new agents have drawn the attention of researchers worldwide.14 Among the various embolic agents, non-spherical PVA particles are the most commonly used, with well documented biocompatibility, efficacy, and relative stay.1,15 Non-spherical PVA particles are inert and show good compatibility, elasticity, and chemical resistance to acids, bases, and detergents.1,16 Non-spherical PVA particles are susceptible to degradation over time (biodegradability), facilitating future recanalization of the treated vessels.4
In order to overcome the undesirable characteristics inherent to non-spherical PVA particles, different methods of manufacture have been attempted and other substances have been created and tested.7,8,11,14 The uneven shape of the non-spherical PVA particles available on the market appears to be the main reason for their aggregation and agglutination. Due to this problem, spherical agents have been developed. Among these spherical agents, trisacryl is the most widely utilized and best known in the literature. Spherical agents may not be associated with the undesirable characteristics of floccular particles.11,17–19
Derdeyn et al., in an experimental study comparing non-spherical PVA particles and collagen-coated acrylic microspheres (trisacryl, Embosphere®) of the same size, observed a significant difference in the degree of penetration of the particles.15 While the non-spherical PVA particles would often aggregate in the ascendant pharyngeal artery of pigs, the microspherical particles reached and flowed through the microcirculation. These authors suggest that this difference was due to the characteristics of the microspherical particles, including the even hydrophilic surface that was deformable and did not adhere to the other particles. The authors also stated that these characteristics made their injection easier and central accumulation and occlusion of the catheter were avoided.15 The degree of penetration of such non-spherical PVA particles into the vascular system can be affected by several factors.7 The labeled size of a non-spherical PVA particle refers primarily to its intermediate axis. The size of the intermediate axis allows the particle to pass through the square orifices of the strainer used to separate the sizes. When the vascular diameter is smaller than the intermediate diameter of the particle, the particle deforms and adapts itself to the vessel size.20
The PVAc particles did not obstruct any of the catheters used during the experiments performed in the present study. The PVAc particles did not present any subjective difference in the way they were injected compared to the trisacryl particle group.
Beaujeux et al. noted that microspherical particles should be used in larger sizes than predicted in order to achieve total occlusion of a given vessel during an embolization procedure.10 This recommendation was founded on the fact that the initial choice of a given particle size was based on the medical team’s experience with non-spherical PVA particles.14,20
There is a direct correlation between the size of the trisacryl particles used in embolization and the diameter of the vessel to be occluded.21 The correlation between particle size and vessel diameter confirms the possibility that vessels can be occluded precisely depending on need. Thus, the use of a calibrated particle size with an exact form is necessary. The embolization of distal arteries has a high rate of necrosis. This high rate of necrosis reduces the possibility of revascularization in the occluded vascular territory, a desirable property for tumor and AVM therapies.22
The success of the PVA particles, associated with the benefits of their spherical configuration, has led to the development of spherical embolization agents consisting of PVA (spherical PVA).
Studies performed at the COPPE/UFRJ have led to the manufacture of an inert embolic agent composed of a thin PVA surface over a PVAc core with a spherical shape. These particles are also non-soluble and more resistant to biodegradation. These particles can be made into several convenient sizes and kept with a uniform, even surface and spherical shape in all of the studied samples of different diameters.13 PVAc is currently in clinical testing. Due to its ease of production, PVAc will come to the market at lower prices than trisacryl, leading to a cost decrease of endovascular procedures.
The higher degree of penetration found with the spherical PVAc particles (group P) can be beneficial for achieving a more effective trans-arterial embolization of many lesions. The higher penetration rate seen with the PVAc particles may be due to their uniformity and their evenly spherical, smooth surface.
In this study, both groups (T and P) were shown to be effective at achieving ischemia in the embolized kidneys with the development of fibrosis of the organ and a reduction of their longitudinal diameter. The trisacryl particles seemed to be more flexible and often showed a deformed aspect, whereas the PVAc particles were more stable in regards to the preservation of their initial shape. Renal embolization with PVAc caused a larger vascular occlusion, which was observed in all groups (with a significant difference in the 5-day and 10-day post-embolization groups). This led to necrotizing lesions in the vessel walls, which were found even in the early phases (a significant difference was found after 48 hours) and were more extensive in the PVAc group than in the trisacryl group.
Recanalization of the occluded vessels embolized with trisacryl was also noted in a fairly extensive number of specimens from the 10-day post-embolization group; however, the PVAc analog group did not show any degree of recanalization in more than half of the studied animals. Even after 30 days, there was still predominant recanalization in the trisacryl group. These data suggest that the predominant recanalization in the trisacryl group was due to the larger percentage of preserved renal parenchyma, even though there was no statistical difference. We believe that recanalization in situations where necrosis is already established occurs due to the presence of areas of preserved parenchyma. Therefore, recanalization may affect the long-term effectiveness of embolization and encourage the recrudescence of the lesion.
Trisacryl particles were also found in all of the contralateral kidneys, a phenomenon that did not occur with the PVAc group. The slippery surface of the trisacryl particle is beneficial to the embolization procedure and makes them flow more easily through the catheter; however, this characteristic may make them more dangerous for practical applications. Therefore, we believe that PVAc is easier to control in segmental embolizations, allowing the preservation of some regions of the kidney. As a result, it will be necessary to know the outcome of the clinical phase of research in other organs for a better assessment of embolization with PVAc.
In both groups, the agents (trisacryl and PVAc) were effective, as determined by the great deal of ischemia to the kidneys with subsequent acute and chronic inflammatory phases leading to the development of further organ fibrosis. Neither of the particles induced an inflammatory response when failing to cause a vascular occlusion. In other words, the particles were inert.
The histological study showed that the PVAc particles fit better to the vessel walls and left behind fewer spaces to be filled by blood. As a result, fewer amounts of PVAc particles did not favor vascular recanalization.
The inflammatory reaction caused by the PVAc vascular occlusion was more distal and more intense than that caused by trisacryl particles. The inflammatory reaction in the PVAc-treated group promoted cellular growth into the vessel walls and consequent fibrosis. Thus, PVAc treatment rendered a recanalization event more difficult.
In conclusion, PVAc particles showed a higher degree of vascular penetration with a more intense distal occlusion of vessels compared to the trisacryl particles. The group embolized with PVAc particles showed a lower recanalization rate than the group embolized with trisacryl, despite the lack of a significant statistical difference. Both materials (trisacryl and PVAc) were individually effective in promoting necrotizing lesions in the walls of the occluded vessels and ischemia of the embolized kidneys, leading to fibrosis and reduced longitudinal diameter of the kidney.
The data obtained from this study suggest that PVAc is an effective embolic agent. PVAc particles are easy to use (do not clog catheters), possess spherical features that prevent particle agglutination, and are able to reach distal arterial segments. Ultimately, PVAc particles are able to promote vascular occlusion, leading to more intense ischemia and fibrosis and a significant reduction in the volume of the embolized organ.
REFERENCES
- 1.Tadavarthy SM, Moller JH, Amplatz K. Polyvinyl alcohol (Ivalon) - a new embolic material. Am J Roentgenol Radium Ther Nucl Med. 1975;125:609–16. doi: 10.2214/ajr.125.3.609. [DOI] [PubMed] [Google Scholar]
- 2.Latchaw RE, Gold LH. Polyvinyl foam embolization of vascular and neoplastic lesions of head, neck and spine. Radiology. 1979;131:669–79. doi: 10.1148/131.3.669. [DOI] [PubMed] [Google Scholar]
- 3.Silva KR, Costa R, Abi Rached R, Martinelli Filho M, Caldas JG, Carnevale FC, et al. Warfarin prevents venous obstruction after cardiac devices implantation in high-risk patients: partial analysis. Rev Bras Cir Cardiovasc. 2008;23:542–9. doi: 10.1590/s0102-76382008000400015. [DOI] [PubMed] [Google Scholar]
- 4.Jack CR, Jr, Forbes G, Dewanjee MK, Brown ML, Earnest F. Polyvinyl alcohol sponge for embolotherapy: Particle size and morphology. AJNR Am. J Neuroradiol. 1985;6:595–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Repa I, Moradian GP, Dehner LP, Tadarvarthy SM, Hunter DW, Castañeda-Zúñiga WR, et al. Mortalities associated with use of a commercial suspension of polyvinyl alcohol. Radiology. 1989;170:395–9. doi: 10.1148/radiology.170.2.2911663. [DOI] [PubMed] [Google Scholar]
- 6.Kerber CW, Bank WO, Horton JA. Polyvinyl alcohol foam: prepackaged emboli for therapeutic embolization. AJR Am J Roentgenol. 1978;130:1193–4. doi: 10.2214/ajr.130.6.1193. [DOI] [PubMed] [Google Scholar]
- 7.Derdeyn CP, Moran CJ, Cross DT, Dietrich HH, Dacey RG., Jr Polyvinyl alcohol particle size and suspension characteristics. AJNR Am J Neuroradiol. 1995;16:1335–43. [PMC free article] [PubMed] [Google Scholar]
- 8.Dion JE, Rankin RN, Viñuela F, Fox AJ, Wallace AC, Mervart M. Dextran microspheres embolization: experimental and clinical experience with radiologic-pathologic correlation. Radiology. 1986;160:717–21. doi: 10.1148/radiology.160.3.2426727. [DOI] [PubMed] [Google Scholar]
- 9.Wright KC, Anderson JH, Gianturco C, Wallace S, Chuang VP. Partial splenic embolization using polyvinyl alcohol foam, dextran, polystyrene, or silicone. Radiology. 1982;142:351–4. doi: 10.1148/radiology.142.2.6172809. [DOI] [PubMed] [Google Scholar]
- 10.Beaujeux R, Laurent A, Wassef M, Casasco A, Gobin YP, Aymard A, et al. Trisacryl gelatin microspheres for therapeutic embolization, II: Preliminary clinical evaluation in tumors and arteriovenous malformations. AJNR Am. J Neuroradiol. 1996;17:541–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Laurent A, Beaujeux R, Wassef M, Rüfenacht D, Boschetti E, Merland JJ. Trisacryl gelatin microspheres for therapeutic embolization, I: development and in-vitro evaluation. AJNR Am J Neuroradiol. 1996;17:533–40. [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto T, Seki S, Fukae R, Sangen O, Kamachi M. High molecular weight poly(vinyl alcohol) through photo-emulsion polymerizations of vinyl acetate. Polymer J. 1990;22:567–71. [Google Scholar]
- 13.Peixoto LS, Silva FM, Niemeyer MAL, Espinosa G, Melo PA, Nele M, et al. Synthesis of Poly(Vinyl Alcohol) and/or Poly(Vinyl Acetate) Particles with Spherical Morphology and Core-Shell Structure and its Use in Vascular Embolization. Macromolecular Symposia. 2006;243:190–9. [Google Scholar]
- 14.Siskin GP, Dowling K, Virmani R, Jones R, Todd D. Pathologic evaluation of a spherical polyvinyl alcohol embolic agent in a porcine renal model. J Vasc Interv Radiol. 2003;14:89–98. doi: 10.1097/01.rvi.0000052296.26939.4c. [DOI] [PubMed] [Google Scholar]
- 15.Derdeyn CP, Graves VB, Salamat MS, Rappe A. Collagen-coated acrylic microspheres for embolotherapy: in vivo and in vitro characteristics. AJNR Am. J Neuroradiol. 1997;18:647–53. [PMC free article] [PubMed] [Google Scholar]
- 16.Castaneda-Zuniga WR, Sanchez R, Amplatz K. Experimental observations on short and long term effects of arterial occlusion with Ivalon. Radiology. 1978;126:783–5. doi: 10.1148/126.3.783. [DOI] [PubMed] [Google Scholar]
- 17.Pelage JP, Laurent A, Wassef M, et al. Uterine fibroid embolization: choice of an embolic particle [abstract] J Vasc Interv Radiol 200011suppl18910716388 [Google Scholar]
- 18.Bendszus M, Klein R, Burger R, Warmuth-Metz M, Hofmann E, Solymosi L. Efficacy of trisacryl gelatin microspheres versus polyvinyl alcohol particles in the preoperative embolization of meningiomas. AJNR Am J Neuroradiol. 2000;21:255–61. [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto A, Imai S, Kobatake M, Yamashita T, Tamada T, Umetani K. Evaluation of Tri-acryl gelatin microsphere embolization with monochromatic X-rays: Comparison with polyvivyl alcohol particles. J Vasc Interv Radiol. 2006;17:1797–802. doi: 10.1097/01.RVI.0000243614.87529.b0. [DOI] [PubMed] [Google Scholar]
- 20.Pelage JP, Laurente A, Wassef M, Bonneau M, Germain D, Rymer R, et al. Uterine artery embolization in sheep: comparison of acute effects with polyvinyl alcohol particles and calibrated microspheres. Radiology. 2002;224:436–45. doi: 10.1148/radiol.2242010847. [DOI] [PubMed] [Google Scholar]
- 21.Laurent A, Wassef M, Chapot R, Houdart E, Merland JJ. Location of vessel occlusion of calibrated tris-acryl gelatin microspheres for tumor and arteriovenous malformation embolization. J Vasc Interv Radiol. 2004;15:491–6. doi: 10.1097/01.rvi.0000124952.24134.8b. [DOI] [PubMed] [Google Scholar]
- 22.Choe DH, Han MH, Kang GH, Yeon KM, Han MC. An experimental study of embolic effect acoording to infusion rate and concentration of suspension in transarterial particulate embolization. Invest Radiol. 1997;32:260–7. doi: 10.1097/00004424-199705000-00002. [DOI] [PubMed] [Google Scholar]