Abstract
OBJECTIVE
To observe the effects of consuming repeatedly heated soy oil on the aortic tissues of estrogen-deficient rats.
METHODS
Thirty female Sprague Dawley rats (200–250 g) were divided equally into five groups. One group served as the normal control (NC) group. The four treated groups were ovariectomized and were fed as follows: 2% cholesterol diet (OVXC); 2% cholesterol diet + fresh soy oil (FSO); 2% cholesterol diet + once-heated soy oil (1HSO); and 2% cholesterol diet + five-times-heated soy oil (5HSO). After four months, the rats were sacrificed, and the aortic tissues were obtained for histological studies.
RESULTS
After four months of feeding, the NC, FSO and 1HSO groups had a lower body weight gain compared to the OVXC and 5HSO groups. The tunica intima/media ratio in the 5HSO group was significantly thicker (p < 0.05) compared to the NC, OVXC and FSO groups. Electron microscopy showed that endothelial cells were normally shaped in the FSO and NC groups but irregular in the 1HSO and 5HSO groups. A greater number of collagen fibers and vacuoles were observed in the 5HSO group compared to the other treatment groups.
CONCLUSIONS
Fresh soy oil offered protection in the estrogen-deficient state, as these rats had similar features to those of the NC group. The damage to the tunica intima and the increase in the ratio of tunica intima/media thickness showed the deleterious effect of consuming repeatedly heated soy oil in castrated female rats.
Keywords: Soy oil, Heating; Aorta, Estrogen deficiency, Atherosclerosis
INTRODUCTION
According to International Cardiovascular Disease Statistics by the World Health Organization (WHO), cardiovascular diseases are the leading cause of death globally. The WHO estimates that by 2015, almost 20 million people will die from cardiovascular diseases, which is projected to remain the single leading cause of death.1 Atherosclerosis is a cardiovascular disease that leads to morbidity and mortality in developed countries.2 The major risk factors of atherosclerosis are hyperlipidemia, hypertension, cigarette smoking and diabetes. It is also associated with increasing age, male gender, family history and genetic abnormalities.3
One of the best ways to prevent atherosclerosis in the society is to alter dietary habits, including a reduced consumption of saturated animal fats. Vegetable oils, such as olive and soy, are recommended for human consumption due to their high content of monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA).4 Soy oil, which is produced from soybean (Glycine max), is the most widely marketed edible oil in the world.5 Soy oil contains approximately 60% of PUFA, 24% of MUFA and 16% of saturated fatty acids.6 This high level of PUFA dietary intake can improve the blood lipid profile status.7 In addition, with its high content of tocopherols, soy oil is known to exhibit various antioxidant actions against lipid peroxidation.6
Edible oil, however, is often used in deep-frying, which can lead to a series of chemical reactions involving hydrolysis, oxidation and polymerization. These changes may degrade the oil’s quality.8 The hydrolysis produces free fatty acids, which subsequently oxidizes and generates peroxide and hydroperoxide compounds, and secondary lipid oxidation products, such as aldehydes, ketones and alcohols.9,10 The consumption of these oxidation products has various adverse health effects, such as liver dysfunction, hypertension and renal damage.11,12,13
We previously reported the adverse effects of consuming repeatedly heated palm oil by observing the ultrastructure of aortic tissues in estrogen-deficient rats.14 Estrogen-deficient rats were used to simulate an estrogen-deficient state observed postmenopausal women who are more susceptible to developing cardiovascular diseases, such as atherosclerosis.15,16 In the present study, we observed the effects of consuming repeatedly heated soy oil on the aorta of rats following 16 weeks of consumption.
MATERIALS AND METHODS
Preparation of animal diets
Commercial soy oil was purchased (Yee Lee Edible Oils, Malaysia) in three forms: fresh, heated once and heated five times.11,17 A total of 1 kg of ‘keropok lekor’ (fish-flavored chips) was deep fried using 2.5 L soy oil at 180ºC for 10 minutes. The oil was then cooled to room temperature and stored in a tightly sealed container before being mixed with animal diets. For five-times-heated soy oil, the same heating procedure was performed. After each round, the oil was cooled for 5 hours before the heating process was repeated with a fresh batch of keropok lekor. No fresh soy oil was added between batches to replace any lost oil absorbed by the frying material.
The normal rat chow was comprised of 20% crude protein, 5% crude fiber, 2.5% crude fat, 13% moisture, 7% ash, 0.7–1.4% calcium, 0.6–1.2% total phosphorus and 51% of nitrogen-free extract. Other additives, such as vitamins A, D3, E, C, K and B12, thiamine, riboflavin, pantothenic acid, niacin, pyridoxine, folic acid, choline, antioxidants and trace minerals, were also present in the normal rat chow.
The experimental soy oil contained 14% saturated fatty acids, 26% MUFA and 60% PUFA. The cholesterol diet (2%) was purchased from MP Biomedicals, Inc. (Seven Hills, Australia). This cholesterol diet had the same composition as the normal rat chow with an addition of 2% cholesterol. The cholesterol diet was ground and mixed with the respective soy oil at a ratio of 85:15. The mixture was then formed into pellets and dried in an oven overnight at 80ºC.
Study design
Thirty 2- to 4-month-old female Sprague Dawley rats weighing 200 to 250 g were used. Prior ethical approval was obtained from the animal unit of Universiti Kebangsaan Malaysia (UKMAEC: FAR/2003/Kamsiah/25 Jan/090).
The rats were randomly divided into five groups: normal control (NC), ovariectomized control (OVXC), fresh soy oil (FSO), once-heated soy oil (1HSO), and five-times-heated soy oil (5HSO). All rats, except the NC group, were ovariectomized after 1 week of acclimatization. After recovery, each group was assigned with their respective diets, i.e., the NC group was fed with normal rat chow, the OVXC group was fed with the 2% cholesterol diet, the FSO group was fed with a 2% cholesterol diet fortified with fresh soy oil, the 1HSO group was fed with a 2% cholesterol diet fortified with once-heated soy oil and the 5HSO group was fed with a 2% cholesterol diet fortified with five-times-heated soy oil. The rationale for the number of heating was based on an earlier documented study in our laboratory.14 The rats were fed for 4 months before being sacrificed, after which their aortic tissues were taken for histological analyses. Their body weight and food intake were recorded every week during the study period.
Histomorphometric study
The proximal portion of the aortic tissues were removed and preserved in 10% formalin. They underwent standard tissue processing techniques, and aortic cross sections were stained with Verhoeff van Gieson for easy identification of the elastic fibers. The structure of the section was observed under light microscope (Eclipse 80i, Nikon Corporation, Tokyo, Japan), and images were taken with a camera (Qimaging MicroPublisher 5.0, Surrey, Canada). The images were analyzed using Image-Pro Plus version 5.0.2.9 (Media Cybernetics, Inc., Bethesda, USA), and the thickness of tunica intima (TI) and tunica media (TM) of the aorta were measured at 0º, 90º, 180º and 270º in each of the cross sections. Their average readings were taken for statistical analysis.
Electron microscopic study
The arch of the aortic tissues was cut into 0.5 mm rings, which were fixed with glutaraldehyde at 4ºC for 12–16 hours. They were then washed with 0.1 M phosphate buffer, post-fixed with 1% buffered osmium tetroxide for 1–2 hours, and washed with distilled water. The samples were then bulk-stained with uranyl acetate for 30 minutes before being dehydrated in graded concentrations of ethanol, infiltrated in propylene oxide and embedded in resin at 60ºC for 24 hours. They were cut into 50 nm slices using an ultramicrotome. The ultrathin sections were stained with 3% uranyl acetate and Reynold’s lead citrate. The ultrastructure of the TI was viewed under a transmission electron microscope (Philips HMG 400, Philips, Eindhoven, The Netherlands).
Statistical analysis
All analyses were conducted using Statistical Product and Service Solutions (SPSS) (Chicago, IL, USA). Data are presented as the mean ± standard error of mean (SEM). Kolmogorov-Smirnov test was used to test the distribution of all data. Normally distributed data were analyzed using analysis of variance (ANOVA) followed by Tukey’s HSD post-hoc test. Data that were not normally distributed were analyzed using Kruskall-Wallis test. A p-value less than 0.05 was considered statistically significant.
RESULTS
Average food intake and body weight gain (Table 1)
Table 1.
Average food intake and body weight gain of rats after 4 months of feeding
| Group | Average food intake (g/week) | Body weight gain after 4 months (g) |
|---|---|---|
| NC | 116 ± 2 | 79 ± 35 |
| OVXC | 110 ± 4 | 114 ± 15 a |
| FSO | 78 ± 1 ab | 93 ± 42 b |
| 1HSO | 79 ± 2 ab | 91 ± 7 b |
| 5HSO | 86 ± 3 ab | 122 ± 32 acd |
Results are mean ± SEM (n = 6).
= significant compared to NC (p < 0.05).
= significant compared to OVXC (p < 0.05).
= significant compared to FSO (p < 0.05).
= significant compared to 1HSO (p < 0.05).
NC, normal control; OVXC, ovariectomized control; FSO, fresh soy oil-treated group; 1HSO, once-heated soy oil-treated group; 5HSO, five-times-heated soy oil-treated group.
Rats treated with soy oil (i.e., the FSO, IHSO and 5HSO groups) showed a reduction of average food intake compared to the NC and OVXC groups. Interestingly, the food intake was the highest in the NC group. After 16 weeks of feeding, the NC, FSO and 1HSO groups had lower body weight gain compared to the OVXC and 5HSO groups (p < 0.05).
Histomorphometric study
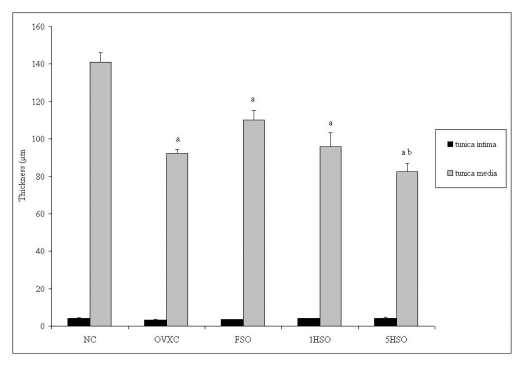
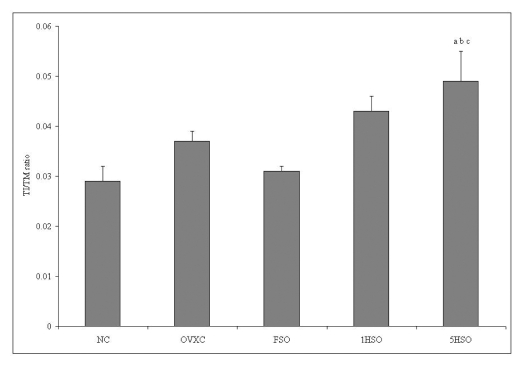
Figure 1 shows the average intimal thickness and medial thickness of each group. There was no significant difference in the intimal thickness among the groups. Conversely, all ovariectomized groups showed significant differences in the medial thickness compared to the NC group. The 5HSO group also showed a significantly smaller medial thickness compared to the FSO group. Additionally, the 5HSO group showed the highest TI/TM ratio compared to the NC, OVXC and FSO groups (Figure 2).
Figure 1.
Intimal and medial thickness of rats according to treatment group. Results are mean ± SEM (n = 6). a = significant compared to the NC group (p < 0.05). b = significant compared to the FSO group (p < 0.05). NC, normal control; OVXC, ovariectomized control; FSO, fresh soy oil-treated group; 1HSO, once-heated soy oil-treated group; 5HSO, five-times-heated soy oil-treated group.
Figure 2.
Intima/media (TI/TM) ratio of rats according to treatment group. Results are mean ± SEM (n = 6). a = significant compared to the NC group (p < 0.05). b = significant compared to the OVXC group (p < 0.05). c = significant compared to the FSO group (p < 0.05). NC, normal control; OVXC, ovariectomized control; FSO, fresh soy oil-treated group; 1HSO, once-heated soy oil-treated group; 5HSO, five-times-heated soy oil-treated group.
Electron microscopic findings
Rat aorta from the NC group (Fig. 3A)
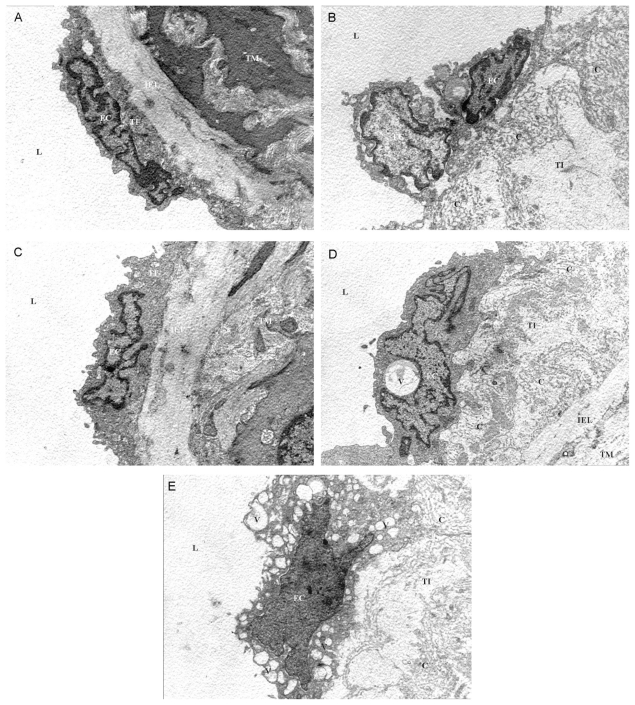
Figure 3.
A) A normal control rat aorta. B) An ovariectomized control rat aorta. C) The aorta of a rat fed with fresh soy oil. D) The aorta of a rat fed with once-heated soy oil. E) The aorta of a rat fed with five-times-heated soy oil. Note the collagen fibrils (c) in the subendothelial layer (in B) and vacuolization (v) of the endothelial cells (in D and E). L, lumen; TI, tunica intima; IEL, internal elastic lamina; TM, tunica media; EC, endothelial cell (7,700x)
This tissue showed the normal endothelial cell shape. Endothelial cells were located near the internal elastic lamina.
Rat aorta from the OVXC group (Fig. 3B)
This tissue contained endothelial cells whose shapes were irregular. The endothelial cells were distant from the internal elastic lamina.
Rat aorta from the FSO group (Fig. 3C)
The characteristic features of the endothelial cells were very similar to the NC group.
Rat aorta from the 1HSO group (Fig. 3D)
Endothelial cells were irregularly shaped. The subendothelial layer was thicker. Abundant collagen fibers were observed in the subendothelial layer, and some endothelial cells contained vacuoles.
Rat aorta from the 5HSO group (Fig. 3E)
Endothelial cells were irregularly in shape. The subendothelial layer was thicker. Abundant collagen fibers were observed in the subendothelial layer. More vacuoles were observed in the endothelial cells, and the endothelial cells were further away from the internal elastic lamina compared to the 1HSO group.
DISCUSSION
Here we observed ultrastructural changes in the aorta of estrogen-deficient rats after the consumption of heated soy oil. We have previously shown that ingestion of repeatedly heated edible oils resulted in adverse effects on serum lipid peroxidation and lipid profiles.18,19 These changes may be due to the degradation products that are produced after the oils are heated at high temperatures. Staprans et al.20 showed that oxidized lipids in the diet were absorbed in the gastrointestinal tract and were transported into circulation. The ingestion of oxidized lipids contributes to the development of atherosclerosis, as oxidized lipoproteins promotes the formation of foam cells, which are the predominant cells in the initial lesion of atherosclerosis.21
The addition of soy oil, either in fresh or heated forms, reduced the average food intake in experimental rats compared to the control rats. This reduction shows that the rats preferred normal rat chow or the 2% cholesterol diet without soy oil. We assume that the addition of oil, which modified the taste and smell of the diet, may have contributed to the reduction of their food intake. The heating process, however, did not have any significant effect on their food intake. All soy oil-treated groups showed a similar food intake, regardless of the number of times the oil was heated. The OVXC group showed a significant body weight gain after 4 months of feeding compared to the NC group. This increase may be due to the 2% cholesterol diet, which is known to contribute to significant weight gain. Despite their low food intake, the 5HSO group exhibited the highest weight gain compared to the NC, FSO and 1HSO groups. We assume that the weight gain in the 5HSO group is caused by water retention. An earlier study reported that water retention may be due to multiple causes, such as renal failure or hypertension, which affected weight gain.22,23 In the present study, however, we did not look into the renal status or hypertension.
The histomorphometric study showed that feeding with soy oil did not result in any significant differences in TI thickness among the soy oil-treated groups. All the ovariectomized rats, however, showed significant medial thinning compared to the NC group. The reason for this change is not clear. Aging and estrogen deficiency may contribute to such changes.24,25 Because ovariectomized rats simulate a postmenopausal condition, it is likely that the observed medial thinning was due to muscular and connective tissue loss secondary to the estrogen deficiency.26,27 Feeding with repeatedly heated soy oil resulted in a significant reduction of medial thickness compared to the FSO group, suggesting that fresh soy oil may have a protective effect in the muscle growth, but such protective effects are lost when the oil is repeatedly heated. Although the TI layer did not show any significant difference, the reduction of the TM layer in ovariectomized rats contributed to the significantly higher TI/TM ratio in the 5HSO group compared to the NC, OVXC and FSO groups. This higher TI/TM ratio indicated an early development in the process of atherosclerosis.24,25
The aortic arch was chosen for the electron microscopic study because this site is most susceptible to atherosclerosis due to any hemodynamic changes that occurred in the vessel.28 The NC group showed normal endothelial cells that were elongated or elliptical in shape.29 Conversely, the OVXC group showed an increase of subendothelial space containing collagen fibrils because the endothelial cell had migrated towards the lumen. The endothelial cell in this group was desquamated from the internal elastic lamina. The observed changes may result in a decrease in vasodilator response and, at the same time, may expose smooth muscle cells to vasoconstrictors, causing artery constriction.30–32 We postulate that these changes may be due to the deficiency of estrogen in the ovariectomized rats. Rats treated with fresh soy oil showed no obvious ultrastructural changes. The endothelial cell was similar to those observed in the NC group and shared tight junctions with the internal elastic lamina, suggesting that feeding fresh soy oil to estrogen-deficient rats had protective benefits against any damage caused by estrogen deficiency coupled with a high cholesterol diet. The high level of antioxidants, primarily tocopherols,33,34 in soy oil may be responsible for the protective effects of soy oil on the aortic ultrastructure observed in this study.
The 1HSO and 5HSO groups showed some abnormal ultrastructural changes compared to normal rats. An increase in the subendothelial layer could be observed in both groups with the presence of abundant collagen fibrils in the area. A large vacuole was observed in the endothelial cell of the 1HSO group because the cell had moved towards the lumen. The endothelial cell of the 5HSO group had also moved further from the internal elastic lamina, and many vacuoles were observed in the endothelial cells. The vacuolization and condensation of cytoplasm in the endothelial cells indicated an early stage of apoptosis.32 Necrosis or apoptosis is known to occur due to an excess of oxidized low-density lipoprotein in macrophages during the progression of atherosclerosis.35 Based on these findings, soy oil, which showed beneficial effects in the FSO group, had lost its potential when heated. The adverse effects of the heated soy oil became more apparent after the oil was heated repeatedly.
We postulate that the loss of the beneficial effects of soy oil was likely due to the significant reduction in tocopherols because we previously reported that more than 80% of α-tocopherol is lost after of the oil was heated five times.36 Repeated heating not only reduces antioxidant contents but also generates free radicals. Soy oil, which contains a high amount of PUFA, is unstable at high temperatures and susceptible to lipid peroxidation. Lipid peroxidation produces volatile and non-volatile compounds. The non-volatile compounds are more important because they remain in the oil and are absorbed by the food. These compounds affect the flavor stability and texture of the fried foods that are later consumed.8,12 Heating the same oil repeatedly generates more degradation products, which may promote progression of numerous diseases, such as atherosclerosis, when this oil is consumed. These statements have been already shown in an earlier study.37
CONCLUSION
Vegetable oil has some protective effects against lipid peroxidation; however, repeated heating may cause deterioration in its quality and formation of new chemical components that are harmful. The practice of using the same oil repeatedly for cooking to save on the cost should therefore be avoided. We conclude that the consumption of fresh soy oil offers protection against vascular changes in an estrogen-deficient state; however, the protective effect is lost when the oil is heated repeatedly. The consumption of repeatedly heated soy oil resulted in alterations in endothelial cells, an increased in the width of the subendothelial layer, and changes in the TI/TM ratio. These findings are indicators of early atherosclerosis. Hence, we do not advise estrogen-deficient subjects to consume repeatedly heated edible oil.
ACKNOWLEDGEMENTS
This study was supported by a grant received from Ministry of Science, Technology and Innovation, Malaysia and UKM Faculty Research Grant. We would like to thank Puan Sinar Suriya Muhamad (Department of Pharmacology), Encik Megat Radzi Abdul Rani (Department of Pathology) and Encik Rafiuz Zaman Haroun (Institute of Bioscience, Universiti Putra Malaysia) for their valuable technical assistances.
REFERENCES
- 1.WHO. International Cardiovascular Disease Statistics. Geneva: World Health Organization; 2008. [Google Scholar]
- 2.Chan-Park MB, Shen JY, Cao Y, Xiong Y, Liu Y, Rayatpisheh S, et al. Biomimetic control of vascular smooth muscle cell morphology and phenotype for functional tissue-engineered small-diameter blood vessels. J Biomed Mater Res A. 2009;88:1104–21. doi: 10.1002/jbm.a.32318. [DOI] [PubMed] [Google Scholar]
- 3.Schoen FJ. Blood vessels. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran pathologic basis of disease. 7th ed. Pennsylvania: Elsevier Saunders; 2005. pp. 511–54. [Google Scholar]
- 4.Chahoud G, Aude YW, Mehta JL. Dietary recommendations in the prevention and treatment of coronary heart disease: do we have the ideal diet yet? Am J Cardiol. 2004;94:1260–7. doi: 10.1016/j.amjcard.2004.07.109. [DOI] [PubMed] [Google Scholar]
- 5.Hayes KC, Khosla P. The complex interplay of palm oil fatty acids on blood lipids. Eur J Lipid Sci Tech. 2007;109:453–64. [Google Scholar]
- 6.Warner K. Effects on the flavor and oxidative stability of stripped soybean and sunflower oils with added pure tocopherols. J Agric Food Chem. 2005;53:9906–10. doi: 10.1021/jf0517593. [DOI] [PubMed] [Google Scholar]
- 7.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–55. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 8.Choe E, Min DB. Chemistry of deep-fat frying oils. J Food Sci. 2007;72:R77–86. doi: 10.1111/j.1750-3841.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- 9.Danowska-Oziewicz M, Karpíska-Tymoszczyk M. Quality changes in selected frying fats during heating in a model system. J Food Lipids. 2005;12:159–68. [Google Scholar]
- 10.Choe E, Min DB. Mechanisms and factors for edible oil oxidation. Compr Food Sci Food Saf Rev. 2006;5:169–86. [Google Scholar]
- 11.Owu DU, Osim EE, Ebong PE. Serum liver enzymes profile of Wistar rats following chronic consumption of fresh or oxidized palm oil diets. Acta Trop. 1998;69:65–73. doi: 10.1016/s0001-706x(97)00115-0. [DOI] [PubMed] [Google Scholar]
- 12.Soriguer F, Rojo-Martínez G, Dobarganes MC, Almeida JMG, Esteva I, Beltrán M, et al. Hypertension is related to the degradation of dietary frying oils. Am J Clin Nutr. 2003;78:1092–7. doi: 10.1093/ajcn/78.6.1092. [DOI] [PubMed] [Google Scholar]
- 13.Totani N, Ojiri Y. Mild ingestion of used frying oil damages hepatic and renal cells in Wistar rats. J Oleo Sci. 2007;56:261–7. doi: 10.5650/jos.56.261. [DOI] [PubMed] [Google Scholar]
- 14.Adam SK, Das S, Jaarin K. A detailed microscopic study of the changes in the aorta of experimental model of postmenopausal rats fed with repeatedly heated palm oil. Int J Exp Path. 2009;90:321–7. doi: 10.1111/j.1365-2613.2009.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker L, Meldrum KK, Wang M, Sankula R, Vanam R, Raiesdana A, et al. The role of estrogen in cardiovascular disease. J Surg Res. 2003;115:325–44. doi: 10.1016/s0022-4804(03)00215-4. [DOI] [PubMed] [Google Scholar]
- 16.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–70. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isong EU, Essein EU, Eka OU, Umoh IB. Sex- and organ-specific toxicity in normal and malnourished rats fed thermoxidized palm oil. Food Chem Toxicol. 2000;38:997–1004. doi: 10.1016/s0278-6915(00)00102-2. [DOI] [PubMed] [Google Scholar]
- 18.Adam SK, Das S, Soelaiman IN, Umar NA, Jaarin K. Consumption of repeatedly heated soy oil increases the serum parameters related to atherosclerosis in ovariectomized rats. Tohoku J Exp Med. 2008;215:219–26. doi: 10.1620/tjem.215.219. [DOI] [PubMed] [Google Scholar]
- 19.Adam SK, Soelaiman IN, Umar NA, Mokhtar N, Mohamed N, Jaarin K. Effects of repeatedly heated palm oil on serum lipid profile, lipid peroxidation and homocysteine levels in a postmenopausal rat model. McGill J Med. 2008;11:145–51. [PMC free article] [PubMed] [Google Scholar]
- 20.Staprans I, Rapp JH, Pan XM, Feingold KR. The effect of oxidized lipids in the diet on serum lipoprotein peroxides in control and diabetic rats. J Clin Invest. 1993;92:638–43. doi: 10.1172/JCI116632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osterud B, Bjorklid E. Role of monocytes in atherogenesis. Physiol Rev. 2003;83:1069–112. doi: 10.1152/physrev.00005.2003. [DOI] [PubMed] [Google Scholar]
- 22.Davy KP, Hall JE. Obesity and hypertension: two epidemics or one? Am J Physiol Regul Integr Comp Physiol. 2004;286:R803–13. doi: 10.1152/ajpregu.00707.2003. [DOI] [PubMed] [Google Scholar]
- 23.Cho S, Atwood JE. Peripheral edema. Am J Med. 2002;113:580–6. doi: 10.1016/s0002-9343(02)01322-0. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Macias KA, Lind L, Naessen T. Thicker carotid intima layer and thinner media layer in subjects with cardiovascular diseases. An investigation using noninvasive high-frequency ultrasound. Atherosclerosis. 2006;189:393–400. doi: 10.1016/j.atherosclerosis.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 25.Naessen T, Rodriguez-Macias K. Menopausal estrogen therapy counteracts normal aging effects on intima thickness, media thickness and intima/media ratio in carotid and femoral arteries. An investigation using noninvasive high-frequency ultrasound. Atherosclerosis. 2006;189:387–92. doi: 10.1016/j.atherosclerosis.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 26.Kamanga-Sollo E, White ME, Chung KY, Johnson BJ, Dayton WR. Potential role of G-protein-coupled receptor 30 (GPR30) in estradiol-17beta-stimulated IGF-I mRNA expression in bovine satellite cell cultures. Domest Anim Endocrinol. 2008;35:254–62. doi: 10.1016/j.domaniend.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Xing D, Nozell S, Chen YF, Hage F, Oparil S. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol. 2009;29:289–95. doi: 10.1161/ATVBAHA.108.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwenke DC. Selective increase in cholesterol at atherosclerosis-susceptible aortic sites after short-term cholesterol feeding. Arterioscler Thromb Vasc Biol. 1995;15:1928–37. doi: 10.1161/01.atv.15.11.1928. [DOI] [PubMed] [Google Scholar]
- 29.Flaherty JT, Pierce JE, Ferrans VJ, Patel DJ, Tucker WK, Fry DL. Endothelial nuclear patterns in the canine arterial tree with particular reference to hemodynamic events. Circ Res. 1972;30:23–33. doi: 10.1161/01.res.30.1.23. [DOI] [PubMed] [Google Scholar]
- 30.Ito Y, Isotani E, Mizuno Y, Azuma H, Hirakawa K. Effective improvement of the cerebral vasospasm after subarachnoid hemorrhage with low-dose nitroglycerin. J Cardiovasc Pharmacol. 2000;35:45–50. doi: 10.1097/00005344-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Zuccarello M, Boccaletti R, Romano A, Rapoport RM. Endothelin B receptor antagonists attenuate subarachnoid hemorrhage-induced cerebral vasospasm. Stroke. 1998;29:1924–9. doi: 10.1161/01.str.29.9.1924. [DOI] [PubMed] [Google Scholar]
- 32.Shi G, Gao G, Zhao Z. Apoptosis of endothelial cells of cerebral basilar arteries in symptomatic cerebral vasospasm rabbit models. Neural Regen Res. 2007;2:479–82. [Google Scholar]
- 33.Dutta A, Dutta SK. Vitamin E and its role in the prevention of atherosclerosis and carcinogenesis: a review. J Am Coll Nutr. 2003;22:258–68. doi: 10.1080/07315724.2003.10719302. [DOI] [PubMed] [Google Scholar]
- 34.Azzi A, Gysin R, Kempná P, Ricciarelli R, Villacorta L, Visarius T, et al. The role of α-tocopherol in preventing disease: from epidemiology to molecular events. Mol Aspects Med. 2003;24:325–36. doi: 10.1016/s0098-2997(03)00028-1. [DOI] [PubMed] [Google Scholar]
- 35.Moreno JJ, Mitjavila MT. The degree of unsaturation of dietary fatty acids and the development of atherosclerosis. J Nutr Biochem. 2003;14:182–95. doi: 10.1016/s0955-2863(02)00294-2. [DOI] [PubMed] [Google Scholar]
- 36.Adam SK, Sulaiman NA, Md Top AG, Jaarin K. Heating reduces vitamin E content in palm and soy oils. Malaysian J Biochem Mol Biol. 2007;15:76–9. [Google Scholar]
- 37.Leong XF, Aishah A, Nor Aini U, Das S, Jaarin K. Heated palm oil causes rise in blood pressure and cardiac changes in heart muscle in experimental rats. Arch Med Res. 2008;39:567–72. doi: 10.1016/j.arcmed.2008.04.009. [DOI] [PubMed] [Google Scholar]