Abstract
Recent advances in transient transfection protocols using polyethylenimine (PEI) as a transfection reagent have led to the development of economical methods that provide yields sufficient for industrial production of proteins for many preclinical needs. There are many variables that can be optimized to improve protein expression in transient transfection, and one of the most critical is the medium in which the cells are grown. While transfection with PEI works well in media containing serum, the biopharmaceutical industry is moving away from animal-derived components in media. A number of serum-free media have been found to allow transient transfection, but many others do not for reasons that are not clear. Thus, knowledge of the components of serum-free media that can cause inhibition of PEI-mediated transient transfection would be useful for media development. In this study, an analysis was performed of various components of a serum-free medium used for Chinese hamster ovary cells in which PEI-mediated transient transfection was inhibited. We found that an iron supplement added to the medium was responsible for the inhibition. Further investigation showed that iron (III) citrate, a common iron chelator found in serum-free medium, was the specific component that caused the effect. Further, we showed that inhibition of transient transfection was caused by iron (III) citrate specifically, rather than citrate or iron alone. Finally, we showed that various iron chelators in serum-free media other than iron (III) citrate do not inhibit antibody expression.
Keywords: Transient transfection, Polyethylenimine (PEI), Iron (III) citrate, Recombinant protein expression, Monoclonal antibody, Chinese hamster ovary (CHO) cell line
Introduction
The use of monoclonal antibodies for therapeutics has risen dramatically in the past few years. Due to the need for post-translational modifications for optimal function of the antibody, mammalian cells are most commonly used for the production of monoclonal antibodies. In order to produce sufficient quantities of antibody for clinical and commercial needs, stable cell lines expressing these molecules must be generated. However, considerable time and resources are required to generate even small amounts of material by this approach. Therefore, production of monoclonal antibodies by transient transfection is becoming increasingly popular for the production of material for research and development, and for in vivo studies. This approach allows for rapid delivery of milligram to gram levels of antibody while stable cells lines are being generated for clinical and commercial production.
One of the most commonly used and economical methods of transient gene expression uses polyethylenimine (PEI) as the transfection reagent (Boussif et al. 1995). PEI condenses DNA into particulate complexes, which are then believed to be taken up by the cell through endocytosis (Kopatz et al. 2004). Currently, most reports of transient transfection using PEI have used either HEK 293 or Chinese hamster ovary (CHO) cells (Derouazi et al. 2004; Schlaeger and Christensen 1999; Tait et al. 2004). It appears that higher titers may be achieved using 293 cells, due to the ability of these cells to utilize the EBNA protein for replicating plasmids (Shen et al. 1995). However, CHO cells are used in most manufacturing cell lines, and so it is desirable to use CHO cells for transient transfections to maintain the same post-translational modifications that would be found in the clinical or commercial product for applications that are sensitive to these modifications.
One of the most critical parameters affecting transient transfection efficiency is the medium in which the cells are grown. In general, production of recombinant proteins is performed in serum-free medium to facilitate purification. However, studies testing serum versus serum-free media have shown that higher titers could be obtained in serum-containing media (Durocher et al. 2002), which suggests that there may be components in serum-free media that inhibit PEI-mediated transient transfection or that serum-free media lacks components necessary for successful transfections. Despite this observation, there have been reports of serum-free medium used with both 293 and CHO cells that allow levels of recombinant protein expression in the range of hundreds of milligrams per liter, with a recent study reporting over 1 g/L of recombinant monoclonal antibody produced in 293 cells (Backliwal et al. 2008). In this study, we have examined several different media for their compatibility with PEI-mediated transient transfection in CHO cells. We found one serum-free medium formulation that prevented expression of antibody in CHO cells, and further investigation revealed that the presence of iron (III) citrate in the medium was responsible for the inhibition. In addition, we have found several substitutes for iron (III) citrate that do not inhibit antibody expression.
Materials and methods
Chemicals and media supplements
Iron (III) citrate was obtained from Acros Organics (Geel, Belgium) and prepared in water as a 20 mM stock solution. Citric acid and iron (III) chloride hexahydrate were obtained from Sigma-Aldrich and prepared in water as 25 and 35 mM stock solutions, respectively. Sodium citrate, potassium citrate and ammonium iron (III) citrate were obtained from Fisher Scientific and prepared as 25 mM stock solutions in water. The holo form of bovine transferrin was obtained from Calbiochem and prepared in water as a 1 mg/mL stock solution. FeNaEDTA was purchased from Irvine Scientific (Santa Ana, CA).
Cell lines and media
The DXB11 CHO cell line was obtained from Lawrence Chasin at Columbia University (Graf and Chasin 1982; Urlaub and Chasin 1980). Attached DXB11 cells were grown in DMEM (Gibco) supplemented with 10 mL/L of 45% glucose (Sigma) and 10% characterized fetal bovine serum (FBS, Hyclone Lot# ARC26080). CHO-S cells were purchased from Gibco, and grown in Medium A, which consists of a customized medium manufactured by Sigma (C5467-44), and supplemented with 6 mM l-glutamine, 10 mL/L HT supplement containing 10 mM sodium hypoxanthine and 1.6 mM thymidine (Gibco), Trace Elements A & B (Mediatech, Inc., Herndon, VA), and CHO Kit 2 Iron Chelator Solution (Sigma), or Medium B, which is CD-CHO medium (Gibco), supplemented with 10 mL/L HT supplement containing 10 mM sodium hypoxanthine and 1.6 mM thymidine (Gibco) and 8 mM l-glutamine. Cells were grown in a humidified incubator at 37 °C and 7.5% CO2 unless otherwise indicated.
Polyethylenimine
Stock solutions of linear 25 kD PEI (Polysciences, Warrington, PA) were prepared in water at a concentration of 1 mg/mL. The pH was adjusted to 7.0 with HCl, the solutions were sterilized through a 0.22 μm filter, and stored at −20 °C.
Transfections
For transfections of attached cells in six-well plates, DXB11 cells were seeded onto the wells for 70–80% confluency the next day. To prepare DNA/PEI complexes, 4 μg of pEGFP-N1 DNA (Clontech) was added to 150 μL 150 mM NaCl per well. Next, 16 μg of PEI was added, and the mixture was incubated at room temperature for 20 min. Finally, the transfection mixture was added to the cells. Cells were assessed for GFP expression by fluorescent microscopy or flow cytometry 24–48 h post transfection. For transfections of recombinant antibody in suspension cells, CHO-S cells were seeded at 2 × 106 cells/mL in 30 mL of the indicated media. Vectors expressing heavy and light chain were made using pUC-based vectors in which transcription was driven by the cytomegalovirus (CMV) promoter. Heavy chain and light chain plasmid DNA at a 1:1 ratio was diluted at a concentration of 2.5 μg/mL of culture into 1.5 mL of 150 mM NaCl, and then PEI was added to get to a DNA:PEI ratio of 1:4. The solution was incubated at room temperature for 20 min, and the entire mixture was added directly to the cells. The next day, cultures were shifted to 34 °C. Antibody concentration in the cell culture media was assessed by ELISA 7 days after transfection. Sandwich ELISAs were performed using Biocoat Secondary Assay Coated Plates (Becton-Dickinson, Franklin Lakes, NJ) and ImmunoPure peroxidase conjugated goat anti-human IgG antibody (Pierce, Rockford, IL) for detection.
Results
Effect of serum-free media on PEI transient transfection
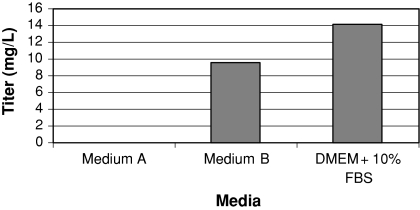
In an attempt to identify a serum-free medium to support PEI-mediated transient transfection, we tested different media that had been used previously to support CHO cells for the production of monoclonal antibodies in stable cell lines. Transient transfections were performed on suspension CHO-S cells grown in three different media: Medium A and Medium B, two serum-free media (see “Materials and methods”), and DMEM supplemented with 10% FBS and 0.1% Pluronic-F68. We found that while the PEI transfections in DMEM with serum and Medium B media produced reasonable levels of antibody, the cells grown in Medium A, which consists of a customized medium called C5467-44, produced no detectable amount of antibody, despite the fact that this medium supports growth and antibody production well in stably transfected cell lines (Fig. 1).
Fig. 1.
Transient expression of an IgG in suspension CHO cells in various media. CHO-S cells were grown in two different serum-free media, Medium A or Medium B (see “Materials and methods” for details), or DMEM with 10% serum and 0.1% Pluronic F-68. Cells were transfected with the heavy and light chains of a monoclonal antibody using PEI, as detailed in “Materials and methods”. Seven days after transfection, samples were taken and antibody expression was determined by ELISA
Inhibition of PEI transfection by iron carrier supplements
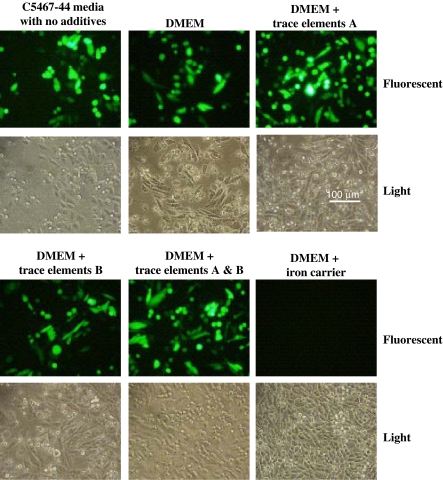
The fact that the medium B was amenable to transient transfection while the medium A was not suggests that either some components of the medium A were inhibiting the process, or it is lacking an ingredient needed for successful transfection. Several supplements are added to the C5467-44 base medium (see “Materials and methods”), including two solutions that provide trace elements, and an iron supplement. We decided to determine if any of these supplements could inhibit a PEI-mediated transfection in CHO cells grown in serum containing media. For this experiment, the trace element solutions and iron supplements were added individually to CHO cells grown in DMEM with 10% FBS in attached mode on six-well plates, and the cells were transfected with a plasmid expressing GFP using PEI as described in “Materials and methods” (Fig. 2). Of the supplements tested, only the iron supplement inhibited the transfection. In addition, when the medium was exchanged for C5467-44 with no additives, GFP expression was seen at a level comparable to that of DMEM with serum (Fig. 2, compare upper left picture to upper middle picture). These results indicate that the iron supplement contains an inhibitor of the PEI transfection.
Fig. 2.
An iron supplement in Medium A inhibits PEI-mediated transfection. CHO DXB11 cells were plated onto a six well plate in DMEM with 10% FBS. The next day, the medium in one of the wells was replaced with medium A with no supplements, and the remaining wells contained DMEM and 10% FBS with the indicated supplement. The cells were transfected with the vector pEGFP-N1 with PEI as detailed in “Materials and methods”. After 48 h, the cells were examined by fluorescent microscopy to determine expression of GFP. The experiment was performed three times with similar results
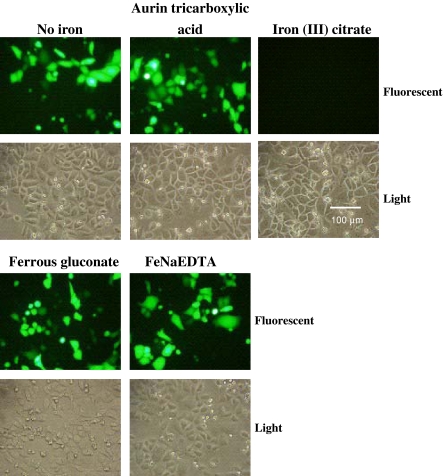
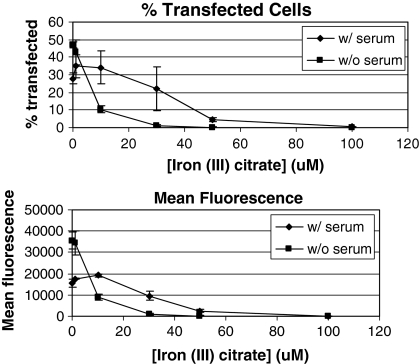
The iron supplement used with the C5467-44 medium is a proprietary mixture of iron chelators, thus the information needed to establish which chemical or chemicals in the solution is responsible for the inhibition of PEI transfection is not readily available. However, there are a number of known chelators that are commonly used to deliver iron to cells. Among them are ferrous gluconate, iron-EDTA, iron (III) citrate, and aurintricarboxylic acid (Bertheussen 1993; Glahn et al. 2000). These chelators were tested for their ability to inhibit GFP expression in DXB11 CHO cells. Of all the iron chelators tested, only iron (III) citrate inhibited transfection (Fig. 3). In addition, a dose response test was performed to determine how increasing amounts of iron (III) citrate affects transfection efficiency in both serum-containing and serum-free medium. Cells were transfected with a plasmid expressing GFP in the presence of increasing levels of iron (III) citrate, and after 24 h the percentage of transfected cells was determined by flow cytometry. An iron (III) citrate concentration of 50 μM almost completely inhibited GFP expression in both serum-containing and serum-free medium (Fig. 4). However, media containing serum were more resistant to inhibition of transfection by iron (III) citrate, in that a noticeable decline in transfection efficiency was not observed until iron (III) citrate concentration was 30 μM. In contrast, transfection efficiency in serum-free media was affected by levels of iron (III) citrate as low as 1 μM, and at 10 μM a significant decrease in the percentage of cells expressing GFP was observed. Thus it appears that serum contains some factor(s) that prevents iron (III) citrate from inhibiting PEI-mediated transfection, which may help to explain the higher levels of protein expression seen when using serum-containing medium for PEI transfections.
Fig. 3.
Iron (III) citrate inhibits PEI-mediated transfection of CHO cells. CHO DXB11 cells were plated onto a six well plate in DMEM with 10% FBS. The following day, the indicated iron chelator was added to the media at a final concentration of 20 μM and cells were transfected with the vector pEGFP-N1 using PEI as detailed in “Materials and methods”. After 48 h, the cells were examined by fluorescent microscopy to determine expression of GFP. The experiment was performed three times with similar results
Fig. 4.
Titration of iron (III) citrate inhibition of PEI-mediated transfection. CHO DXB11 cells were plated onto two-six well plates in DMEM with 10% FBS. The following day, the media of one of the plates was replaced with serum-free Medium A with no iron supplement. Then iron (III) citrate was added at a concentration of 0, 1, 10, 20, 50 or 100 μM to the wells of each plate, and the cells were transfected with pEGFP-N1 as detailed in “Materials and methods”. After 48 h, the cells were analyzed by flow cytometry to determine the percentage of cells transfected (top) and the mean fluorescence (bottom) of each cell population. The experiment was performed three times
Test of other citrate salts on PEI transfection
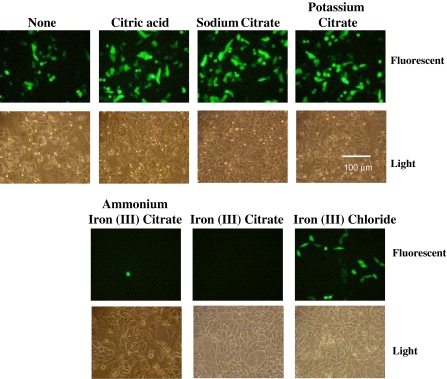
The fact that only iron (III) citrate inhibits PEI transfection out of all the iron carriers tested suggests that citrate itself may be inhibiting the PEI/DNA complex formation. If this is the case, then other citrate salts may also cause inhibition of the transfection. To test this, PEI transfection of pEGFP-N1 was carried out in the presence of citric acid and a number of citrate salts, including sodium citrate, potassium citrate, ammonium iron (III) citrate, and iron (III) citrate. Of all the different citrate compounds, only those containing iron inhibited GFP expression (Fig. 5). In addition, we also wished to see if iron in the form of the ferric iron would also inhibit PEI transfections, as in this experiment and previous experiments (Fig. 3), the iron was in a complex with various chelators. Thus, the PEI transfection was also carried out in the presence of iron (III) chloride, and no significant loss of transfection efficiency was seen (Fig. 5, lower right panel compared to top left panel).
Fig. 5.
Effect of different citrate salts on PEI-mediated transient transfection in CHO cells. CHO DXB11 cells were plated onto the wells of a six well plate in DMEM with 10% FBS. The following day, the media of each well was replaced with Medium A supplemented with 6 mM l-glutamine and Trace Elements A & B (Mediatech Inc.) and the indicated citrate salt, or iron (III) chloride, was added at a concentration of 50 μM. Cells were transfected with pEGFP-N1 as detailed in “Materials and methods”. After 48 h, cells were examined under a fluorescent microscope to determine expression of GFP. The experiment was performed three times with similar results
Rescue of PEI transfection inhibition by disruption of the iron (III) citrate complex
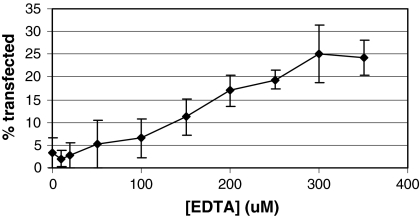
The fact that only iron (III) citrate inhibited PEI-mediated transfections suggests that disruption of the iron (III) citrate complex would prevent inhibition of transfection. By incubating iron (III) citrate with a chelator that has a higher affinity for iron than citrate (KD = 10−11), such as EDTA (KD = 10−25; Bertheussen 1993), the level of iron (III) citrate should decrease, leading to an increase in transfection efficiency. To test this, iron (III) citrate was incubated for 16–20 h with various concentrations of EDTA prior to addition to cells that were transfected with pEGFP-N1 using PEI. As seen previously, no GFP-positive cells were observed in 50 μM iron (III) citrate without any EDTA present (Fig. 6). However, as the iron (III) citrate was incubated with increasing concentrations of EDTA, higher transfection efficiencies were observed, with incubation of iron (III) citrate with 350 μM EDTA yielding 24.3 ± 3.9% GFP-positive cells (Fig. 6). Without any iron (III) citrate present, approximately 64.6 ± 8.2% of the cells expressed GFP (data not shown). Thus while EDTA did not completely prevent iron (III) citrate from inhibiting transfection, the fact that increasing amounts of EDTA led to increasing numbers of GFP-positive cells suggests that disruption of iron (III) citrate complex can relieve its ability to inhibit PEI-mediated transfection. This result may also explain why higher levels of iron (III) citrate are required to inhibit transient transfection in serum-containing media (Fig. 4). Serum contains transferrin, which, like EDTA, has a higher affinity for iron than citrate. Thus, the transferrin may be offsetting the inhibiting effect of iron (III) citrate by disrupting the iron (III) citrate complex at lower iron (III) citrate concentrations.
Fig. 6.
Rescue of iron (III) citrate inhibition of PEI-mediated transient transfection. A series of 100 μL solutions were prepared containing iron (III) citrate at 1.5 mM and increasing concentrations of Na4EDTA as indicated, and the solutions were incubated at room temperature overnight. CHO DXB11 cells were plated onto six well plates in DMEM with 10% FBS. The following day, the media were replaced with Medium A supplemented with 6 mM l-glutamine and Trace Elements A & B, and the iron (III) citrate/EDTA solutions were added, bringing the final iron (III) citrate concentration to 50 μM. In addition one well of cells with no iron (III) citrate was included as a control. The cells were transfected with pEGFP-N1 as detailed in “Materials and methods”. After 48 h, cells were trypsinized and analyzed by flow cytometry to determine the percentage of cells expressing GFP. In the well containing no iron (III) citrate, 64.6 ± 8.2% of the cells expressed GFP (data not shown). The experiment was performed three times
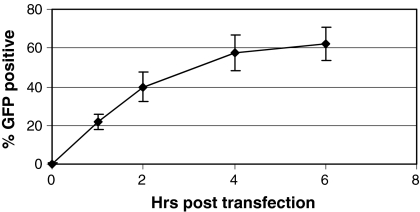
Iron (III) citrate inhibits PEI transfection at an early stage of the transfection process
Introduction of DNA into a cell by transfection is a multi-step process, which includes the formation of DNA-carrier complexes, binding and entry into the cell, and translocation to the nucleus and dissolution of the DNA-carrier complex. Iron (III) citrate may inhibit PEI transfection at any stage of this process. In order to understand the mechanism by which iron (III) citrate inhibits transfection, a time course was performed where iron (III) citrate was added at various times following addition of PEI-DNA complexes to the cells, from immediately following transfection to 6 h after the transfection (Fig. 7). We found that adding iron (III) citrate at later times following transfection results in decreasing inhibition by this compound, and that adding iron (III) citrate 4–6 h after transfection results in transfection efficiencies approximately the same as that seen with no iron citrate added (61.8%, data not shown). These results suggest that iron (III) citrate is affecting an early stage in the transfection process.
Fig. 7.
Iron (III) citrate inhibits PEI-mediated transfection at an early stage in the transfection process. CHO DXB11 cells were plated onto six well plates in DMEM with 10% FBS. The following day, the media were replaced with Medium A supplemented with 6 mM l-glutamine and Trace Elements A & B. Cells were transfected with pEGFP-N1 as detailed in “Materials and methods”, and iron (III) citrate was added at a final concentration of 50 μM at the indicated times following transfection. After 48 h, cells were trypsinized and analyzed by flow cytometry to determine the percentage of cells expressing GFP. In the well containing no iron (III) citrate, 61.8 ± 9.3% of the cells expressed GFP (data not shown). The experiment was performed three times
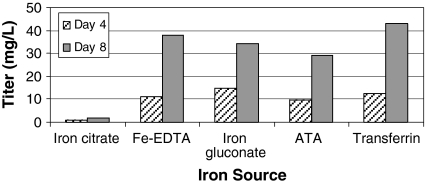
Production of monoclonal antibody with alternate iron chelators
PEI transfections of CHO-S suspension cells with vectors encoding the heavy and light chains of a monoclonal antibody (see “Materials and methods”) were performed with different iron chelators to assess the effect of these compounds on recombinant monoclonal antibody production. CHO-S cells were cultured in C5467-44 media containing 50 μM iron (III) citrate, 50 μM iron (III) sodium EDTA, 50 μM iron (II) gluconate, aurintricarboxylic acid plus 50 μM iron (III) chloride, or purified bovine transferrin at 20 μg/mL. Heavy and light chain genes on separate vectors (see “Materials and methods”) were transfected at a 1:1 ratio, and the DNA:PEI ratio was 1:4. Samples were taken from each transfection at four and 8 days post transfection, and antibody concentration in the media was assessed by ELISA (Fig. 8). As expected, only a minimal level of antibody was produced in the medium containing iron (III) citrate. However, media containing the other iron carriers produced antibody at quantities ranging from 29 to 43 mg/L after 8 days post transfection. Note that these values are significantly better than those seen with CHO-S cells grown in medium B or DMEM (Fig. 1). In the case of medium B, this result may be due to the presence of iron citrate in the medium at a level low enough to allow some protein production, but high enough to prevent maximum protein production. Protein production may be lower in DMEM because this medium is not optimized for cell growth in suspension. Thus it appears that a number of iron carriers may be used in place of iron (III) citrate for transient transfection of suspension CHO cells in serum-free media without affecting recombinant protein expression.
Fig. 8.
Expression of an IgG in Medium A with various iron carriers. CHO-S cells were seeded at a density of 2 × 106 cells/mL in 30 mL Medium A supplemented with 6 mM l-glutamine, Trace Elements A & B, and the indicated iron carrier at 50 μM (transferrin concentration was 20 mg/L). Cells were transfected with plasmids encoding the heavy and light chain of an IgG on separate plasmids (1:1 Light:Heavy chain ratio) as detailed in “Materials and methods”. At 4 and 8 days post transfection, samples were taken and analyzed by ELISA to determine antibody concentration in the media
Discussion
Iron is an essential element in cellular metabolism. However, free iron in ionic form is highly reactive, and can lead to the formation of free radicals in the cytoplasm. This problem is circumvented in many organisms by the use of the protein transferrin to transport iron to cells. Transferrin is present in animal serum, but in order to eliminate animal products from media for cell lines used to produce products for human use, substitutes for transferrin were developed. Several small molecule chelators can be used to transport iron in cells, including aurintricarboxylic acid, EDTA, gluconate, and citrate (Bertheussen 1993; Glahn et al. 2000). In this study, we have demonstrated that iron (III) citrate inhibits PEI-mediated transient transfection, while none of the other iron chelators we tested show this effect. It has been reported that not all serum-free media are capable of allowing transient expression of recombinant proteins (Pham et al. 2003), and the presence of iron (III) citrate in these media may account for this observation.
The mechanism by which iron (III) citrate inhibits PEI-mediated transfection is not clear. Entry of DNA into the nucleus involves several steps. After DNA/PEI polyplexes are formed, they must bind to the cell, which is believed to involve interactions with heparan sulfate proteoglycans on the cell surface (Kopatz et al. 2004). Bound polyplexes are then taken into the cell through endocytosis into acidified endosomal compartments (Godbey et al. 1999). Release of DNA/PEI polyplexes from the endosome is believed to be due to the “proton sponge” effect from the buffering capacity of PEI (Boussif et al. 1995). From that point, the polyplexes must be translocated into the nucleus and dissociate so that transcription can take place, but the exact mechanism of these later steps are not well characterized. Thus, there are several points in the transfection process where iron (III) citrate could have an effect. For instance, iron (III) citrate may be preventing the formation of DNA/PEI polyplexes, or it may be preventing the polyplexes from binding to cells prior to endocytosis. Alternatively, iron (III) citrate may be acting inside the cell, preventing the DNA/PEI complexes from being released from endosomes, or somehow preventing transport into the nucleus. As the results in Fig. 7 show, iron (III) citrate does not significantly inhibit transfection if it is added more than 2 h after transfection. Thus it appears that iron (III) citrate inhibits an early stage of the transfection process, such as formation of the DNA-PEI complexes, or binding/entry of the complexes to the cell.
In conclusion, we have found that iron (III) citrate, a component of many serum-free media, inhibits PEI-mediated transient transfections. We have also shown that substituting iron (III) citrate for a number of other iron chelators supports expression of proteins in CHO cells by transient transfection. This knowledge should prove useful when designing media for use in transient transfection. It is also worth noting that there may be situations in which medium does not contain iron (III) citrate, but contains an iron salt and citric acid or another citrate salt, leading to the formation of iron (III) citrate and inhibiting PEI transfection. In this case, the addition of another chelator, such as EDTA, may prevent iron (III) citrate formation and allow PEI-mediated transfection. Finally, further studies will be performed to determine the exact mechanism of transfection inhibition by iron (III) citrate.
Acknowledgments
The authors would like to thank Yung-Shyeng Tsao for helpful discussions and advice about iron carriers used for cell culture, and Russell Condon for expert assistance with the ELISA assay and flow cytometry.
References
- Backliwal G, Hildinger M, Chenuet S, Wulhfard S, De Jesus M, Wurm FM (2008) Rational vector design and multi-pathway modulation of HEK 293E cells yield recombinant antibody titers exceeding 1 g/l by transient transfection under serum-free conditions. Nucleic Acids Res 36(15):e96 [DOI] [PMC free article] [PubMed]
- Bertheussen K (1993) Growth of cells in a new defined protein-free medium. Cytotechnology 11:219–231. doi:10.1007/BF00749873 [DOI] [PubMed]
- Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP (1995) A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA 92:7297–7301. doi:10.1073/pnas.92.16.7297 [DOI] [PMC free article] [PubMed]
- Derouazi M, Girard P, Van Tilborgh F, Iglesias K, Muller N, Bertschinger M, Wurm FM (2004) Serum-free large-scale transient transfection of CHO cells. Biotechnol Bioeng 87:537–545. doi:10.1002/bit.20161 [DOI] [PubMed]
- Durocher Y, Perret S, Kamen A (2002) High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res 30:E9. doi:10.1093/nar/30.2.e9 [DOI] [PMC free article] [PubMed]
- Glahn RP, Rassier M, Goldman MI, Lee OA, Cha J (2000) A comparison of iron availability from commercial iron preparations using an in vitro digestion/caco-2 cell culture model. J Nutr Biochem 11:62–68. doi:10.1016/S0955-2863(99)00078-9 [DOI] [PubMed]
- Godbey WT, Wu KK, Mikos AG (1999) Tracking the intracellular path of poly(ethylenimine)/DNA complexes for gene delivery. Proc Natl Acad Sci USA 96:5177–5181. doi:10.1073/pnas.96.9.5177 [DOI] [PMC free article] [PubMed]
- Graf LH Jr, Chasin LA (1982) Direct demonstration of genetic alterations at the dihydrofolate reductase locus after gamma irradiation. Mol Cell Biol 2:93–96 [DOI] [PMC free article] [PubMed]
- Kopatz I, Remy JS, Behr JP (2004) A model for non-viral gene delivery: through syndecan adhesion molecules and powered by actin. J Gene Med 6:769–776. doi:10.1002/jgm.558 [DOI] [PubMed]
- Pham PL, Perret S, Doan HC, Cass B, St-Laurent G, Kamen A, Durocher Y (2003) Large-scale transient transfection of serum-free suspension-growing HEK293 EBNA1 cells: peptone additives improve cell growth and transfection efficiency. Biotechnol Bioeng 84:332–342. doi:10.1002/bit.10774 [DOI] [PubMed]
- Schlaeger E-J, Christensen K (1999) Transient gene expression in mammalian cells grown in serum-free suspension culture. Cytotechnology 30:71–83. doi:10.1023/A:1008000327766 [DOI] [PMC free article] [PubMed]
- Shen ES, Cooke GM, Horlick RA (1995) Improved expression cloning using reporter genes and Epstein-Barr virus ori-containing vectors. Gene 156:235–239. doi:10.1016/0378-1119(95)00038-8 [DOI] [PubMed]
- Tait AS, Brown CJ, Galbraith DJ, Hines MJ, Hoare M, Birch JR, James DC (2004) Transient production of recombinant proteins by Chinese hamster ovary cells using polyethyleneimine/DNA complexes in combination with microtubule disrupting anti-mitotic agents. Biotechnol Bioeng 88:707–721. doi:10.1002/bit.20265 [DOI] [PubMed]
- Urlaub G, Chasin LA (1980) Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci USA 77:4216–4220. doi:10.1073/pnas.77.7.4216 [DOI] [PMC free article] [PubMed]