Abstract
The generation of transgenic cell lines is acquired by facilitating the uptake and integration of DNA. Unfortunately, most of the systems generating stable expression systems are cost and time-consuming and transient expression is optimized to generate milligram amounts of the recombinant protein. Therefore we improved and compared two transfection systems, one based on cationic liposomes consisting of DOTAP/DOPE and the second one on polyethylenimine (PEI). Both systems have been used as chemically defined transfection systems in combination with serum-free cultivated host cell line. At first we had determined the toxicity and ideal ratio of DNA to PEI followed by determination of the optimal transfection conditions in order to achieve maximum transfection efficiency. We then directly compared DOTAP/DOPE and PEI in transient transfection experiments using enhanced green fluorescence protein (EGFP) and a human monoclonal antibody, mAb 2F5, as a model protein. The results which were achieved in case of EGFP were more than 15% transfectants at a viability of 85%. Despite the fact that expression of the mAb was found negligible we used both techniques to generate stable mAb 2F5 expressing cell lines that underwent several cycles of screening and amplification with methotrexate, and resulted in cell lines with similar volumetric production titers. These experiments serve to demonstrate the potential of stable cell lines even in case where the transient systems did not show satisfying results.
Keywords: Serum-free transfection, DOTAP/DOPE, PEI, Antibody expression
Introduction
Recombinant protein expression in mammalian cell technology relays on efficient DNA transfer crossing the endosomal or lysosomal membrane, reaching the cytoplasm and finally entering the nucleus (Wattiaux et al. 2000). Based on the existence of several different endocytic pathways their own specific characteristics need to be taken into account (Khalil et al. 2006). Cationic lipids with positive net charge on the surface bind to the cell by electrostatic interactions with negatively charged membrane components (van der Aa et al. 2007), destabilize lipid bilayers and thus enable the DNA to escape (Wattiaux et al. 1997). Wrobel and Collins (1995) were first to demonstrate the fusion between cationic liposomes and endocytic vesicles in cell cultures, but still both, the exact mechanism of the endosomal membrane crossing (Zuhorn et al. 2005) and the DNA transfer across the nuclear membrane remain unidentified (Escriou et al. 2001).
In contrast, PEI mediated DNA transfer is assigned to the caveolae pathway enabling to by-pass the degradation through endocytosis (Le and Nabi 2003) and DNA permeates via the clathrin dependent pathway which routes the polyplex into the early and late endosomes and promotes escape thereof (Takei and Haucke 2001).
The goal of this current study was the comparison of the cost-efficient and less complicated PEI transfection system to the liposomal system in a serum-free adapted host cell line. Both methods have been applied for transient expression and stable clone selection. We could generate and characterize tailor-made liposomes consisting of DOTAP/DOPE (DD) as described previously (Reisinger et al. 2007) and, in addition, we used PEI, a cationic polymer for the transient and stable transfection of Chinese Hamster Ovary (CHO) cells to investigate its toxicity and applicability for transient transfection experiments. Finally we used both systems in order to determine their suitability and potential to generate stable antibody expressing cell lines.
Materials and methods
Expression plasmids and host cell line
The plasmid pEGFP-N3 (Clontech, Palo Alto, CA, USA) served for transient transfection experiments as reporter gene. For transient and stable expression experiments mAb 2F5 LC and HC cDNAs (Kunert et al. 1998, 2000) were used in standard eukaryotic expression plasmids with viral promoters. For stable cell line development a p2_dhfr plasmid was co-transfected for the amplification via MTX (Alt et al. 1978). Plasmid DNA from transformed E.coli Top10 cells was purified using a Qiagen Maxi-prep kit. Isolated plasmids were stored in water to avoid interaction between buffer salts and cationic transfectants during the formation of complexes. Purity and concentration of the plasmids were determined by measuring the absorption at 260 nm spectrophotometrically (Biophotometer, Eppendorf). The A260/A280 ratio was typically between 1.7 and 1.8.
Serum-free cultivated dihydrofolate-reductase (dhfr) deficient CHO-cells DUKX-B11, ATCC CRL-9096 (Urlaub and Chasin 1980) were cultivated in Dulbecco’s modified Eagle’s medium (Biochrom KG, Berlin, Germany) containing 4 mM l-glutamine (Life Technologies, Grand Island, NY, USA), 0.25% Soya-peptone/UF (HY-SOY/UF Quest International GmbH, Erfstadt-Lechenich, Germany), 0.1% Pluronic-F68 (Sigma–Aldrich Handels GmbH, Vienna, Austria), PF-supplement (Polymun Scientific, Vienna, Austria) and HT (Hypoxanthine and Thymidine: Sigma–Aldrich Handels GmbH, Vienna, Austria), subsequently called “cultivation medium”.
Lipoplex preparation and transfection
The liposomes consisted of DOTAP and DOPE. DOTAP was obtained from Merck Eprova AG (Darmstadt, Germany) and DOPE was purchased from Lipoid (Ludwigshafen, Germany). Liposomes were prepared and characterized as previously described (Reisinger et al. 2007). Liposomes and plasmid were diluted in HBS to a total volume of 100 μL for the lipoplex formation. In the first step 11 μg DOTAP/DOPE and 1 μg DNA were added to HBS and incubated at 22 °C for 30 min, resulting in the formation of lipoplexes with a molar charge ratio of 2.5:1 (cationic lipid to DNA) (Regelin et al. 2000) and used for transient experiments with 5 × 105 cells in 1 mL cultivation medium. The lipoplexes were added by gentle pipetting and incubated at 37 °C for 4 h, before the cultivation medium was changed. 72 h post-transfection the cells were screened for the EGFP or mAb 2F5 HC and LC expression by FACS analysis. For the stable transfection experiments 5 × 106 cells in 10 mL were transfected with 10 μg DNA and 110 μg DD. Selection was started after 24 h in 96 well plates and clones growing in HT free medium were adapted to 0.19 μM MTX for gene amplification. In a second sub-cloning round the MTX level was raised to 0.38 μM MTX to select the stable cell line 2F5/DD.
Polyplex preparation and transfection
PEI (PolySciences Inc., Warrington, Pennsylvania, USA, linear, 25 kDa) stock solution (1 mg mL−1) has been prepared by dissolving PEI powder in water. Polyplexes of 90 μg PEI and 2.5 μg DNA were formed in 200 μL HBS buffer at 22, 37 and 60 °C for 10, 30 and 60 min. 5 × 105 cells in 1 mL cultivation medium were used for transient transfections, while for the generation of stable cell lines a tenfold upscale, with respect to cells, PEI and DNA was performed. The polyplexes were added to the cells by gentle pipetting and incubated at 37 °C for 4 h before the cultivation medium was changed. 72 h post-transfection the cells were then screened for the EGFP or mAb 2F5 HC and LC by FACS analysis in transient experiments. Stable recombinant clones were selected using the same procedure as with lipoplexes to generate the final and stable cell line 2F5/PEI.
Batch experiments
The two stable cell lines, 2F5/DD and 2F5/PEI, were cultivated in amplification medium with 0.38 μM MTX and passaged twice a week. For determination of specific titers and intracellular content of heavy and light chain a 10 days batch had been applied.
Determination of specific productivity
The cell concentration was determined via a MultisizerTM 3 Coulter Counter® (Beckman Coulter) and the secreted antibody was quantified using ELISA. Viability was determined via trypan blue vital stain using a Bürker-Türk chamber. For the quantification of antibody titer, plates were pre-coated with goat anti-human γ-chain specific antiserum (Sigma) and detected with HRP conjugated goat anti-human κ-chain specific antiserum (Sigma) and stained with OPD (orthophenylediamine). Purified mAb 2F5 served as a standard starting with 200 ng mL−1 and 1:2 dilution series of the samples and standard were analyzed.
Flow cytometry
The relative fluorescence of 10,000 cells per sample was determined with a FACS-Calibur flow cytometer (Becton–Dickinson, NJ, USA, 5 W argon laser). Viable single cells were gated by size (FSC-H) and granularity (SSC) using the Cell Quest Pro software to eliminate dead cells and cell debris from further analysis. These viable single cells were then analyzed for the EGFP expression, since FACS-analyses do not result in absolute fluorescence values and we refrained from the use of any standard curve. The samples of all experiments had been collected and the relative fluorescence units (rfu) been immediately compared with identical FACS settings.
For determination of intracellular LC and HC content fixed cells (70% ethanol, stored at 4 °C) were washed twice in Tris buffer (100 mM Tris–HCl, 2 mM MgCl2, pH 7.4) containing 20% FCS, resuspended in 100 μL Tris–FCS containing Quantum Red conjugated mouse anti-human κ-chain antiserum (1:60, Sigma) and FITC conjugated goat anti-human-γ chain antiserum (1:60, Sigma) and incubated for 1 h at 37 °C. CHO host cells served as negative controls.
Results
Optimization of PEI transfection conditions
The optimization of the PEI/DNA (w/w) ratio has been found to be an important prerequisite step since various media compositions contain differently charged components that interfere with the complex and thereby influence the transfection efficiency and cell viability because of unbound PEI. To test the toxicity, DNA was kept constant at 2.5 μg, while the amount of PEI was constantly increased in transfection experiments with 5 × 105 CHO dhfr− cells. The viability decreased dramatically, resulting in complete cell death when the DNA to PEI concentration exceeded a ratio of 1:48, while complexes below this level of PEI showed viabilities above 80% (Table 1). The cell number demonstrated that growth occurred soon after transfection (Table 1 column 2), while other labs dealing with transient transfection normally have to cope with low viabilities after transfection and delayed cell growth (Galbraith et al. 2006).
Table 1.
Determination of PEI toxicity
| DNA:PEI ratio | Cell number (×105 cells) | Viability (%) |
|---|---|---|
| 1:12 | 9.5 | 83 |
| 1:24 | 8.8 | 90 |
| 1:36 | 12.2 | 87 |
| 1:48 | 8.5 | 80 |
| 1:60 | 4 | 0.0 |
| 1:72 | 3.3 | 0.0 |
| 1:84 | 4.9 | 0.0 |
| Neg. | 6 | 88 |
To determine which DNA (pEGFP) to PEI ratio (w/w) is lethal for the cells the PEI concentration was stepwise raised at constant DNA concentration of 2.5 μg and added to 5 × 105 CHO cells. The cell concentration and viability were determined 72 h post-transfection
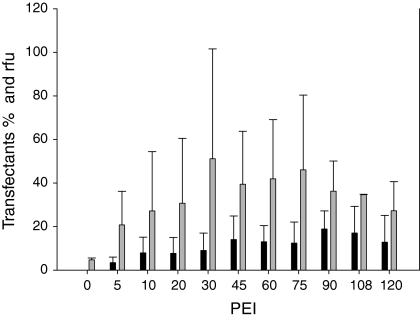
The next step was to determine the most efficient DNA to PEI ratio to gain maximal transfection efficacy. Again, DNA was kept constant at 2.5 μg per 5 × 105 cells and the PEI concentration was raised from 5 μg PEI to 120 μg. After 72 h of cultivation we determined the amount of living cells and the fluorescence of EGFP expressing cells during three independent experiments (Fig. 1). The highest rate of EGFP expression was achieved with 90 μg PEI transfecting an average of 19% of cells while 108 and 120 μg PEI resulted in 17 and 13%, respectively. At lower PEI concentrations lower transfection rates were found despite the relative fluorescence intensity was similar or even higher. However, the results at lower PEI concentrations turned out to be more inconsistent. Therefore we decided to proceed with 90, 120 and 150 μg PEI per transfection because of the higher transfection efficiency and consistency found and then studied different complex formation times (10 or 30 min) and polyplex formation temperatures (22 or 37 °C) keeping plasmid DNA (pEGFP) constant. Table 2 confirms that 90 μg PEI transfections resulted in highest numbers of transfectants, while higher ratios showed no improvement. An incubation time of 30 min showed higher efficiency than 10 min incubation. Additionally, complex formation at 22 °C showed higher transfection efficiencies in combination with highest relative fluorescence. Based on these results we decided to use 2.5 μg DNA and 90 μg PEI for the transfection of 5 × 105 CHO dhfr− cells, which led to 19% positive transfectants, a rfu of 59 and a viability of 71% (Table 2). Additionally, the influence of the storage temperature of PEI was tested as well. First the PEI stock solution was stored at 22 °C and used immediately for polyplex formation. Then the PEI stock solution was pre-heated to 60 °C for 5 min in order to shift the PEI molecules into a more relaxed and more accessible shape before polyplex formation. In a third approach we stored the PEI stock solution at −20 °C and treated it like in the second approach. Table 3 describes experiments clustered into pre-warming of PEI (22 and 60 °C) and conditions for polyplex formation (22, 37, 60 °C; 30 and 60 min). Seventy two hours post transfection the cell concentration was increased to 1.5- to 2-fold of the seeded cells and viabilities were above 85%. Table 3 indicates that the PEI transfections performed better when the polyplex formations lasted for 30 min instead of 60 min, an effect that might be based on the aggregation of the DNA/PEI complex reducing the probability of cellular uptake. Twenty seven percent positive transfectants (rfu of 42) and 29% transfectants (rfu of 41) were reached after pre-heating PEI to 60 °C before mixing with DNA. Summarizing all these experiments we decided to store PEI at 22 °C, pre-heat it to 60 °C and mix DNA and PEI for 30 min at 22 °C for complex formation.
Fig. 1.
Determination of the optimal DNA/PEI (w/w) ratio to maximize the amount of transfected cells. 5 × 105 Cells were transfected with 2.5 μg pEGFP-N3, while the PEI concentration was varied. Relative fluorescence units (rfu) ( ) and transfected cells (■) were determined 72 h post-transfection. Three independent experiments were carried out to determine the mean and the standard deviation
) and transfected cells (■) were determined 72 h post-transfection. Three independent experiments were carried out to determine the mean and the standard deviation
Table 2.
Determination of the ideal transfection conditions and PEI concentration
| Complex formation temperature (°C) | Incubation | PEI amount (μg) | Cell number (1 × 105 cells) | SD | Transfectants (%) | SD | rfu | SD | Via (%) | SD |
|---|---|---|---|---|---|---|---|---|---|---|
| 22 | 10′ | 90 | 12.5 | 7 | 16 | 6 | 46 | 19 | 65 | 25 |
| 120 | 6.8 | 5 | 11 | 16 | 23 | 31 | 20 | 35 | ||
| 150 | 6.6 | 3 | 8 | 12 | 20 | 26 | 16 | 27 | ||
| 37 | 10′ | 90 | 10.2 | 8 | 13 | 14 | 45 | 35 | 63 | 31 |
| 120 | 6.6 | 5 | 12 | 19 | 25 | 34 | 30 | 38 | ||
| 150 | 5.9 | 2 | 8 | 11 | 19 | 23 | 17 | 28 | ||
| 22 | 30′ | 90 | 9.8 | 5 | 19 | 10 | 59 | 19 | 71 | 22 |
| 120 | 8 | 4 | 17 | 26 | 28 | 39 | 24 | 30 | ||
| 150 | 7.9 | 1 | 15 | 23 | 30 | 42 | 19 | 34 | ||
| 37 | 30′ | 90 | 13.4 | 8 | 16 | 6 | 61 | 18 | 72 | 21 |
| 120 | 6.7 | 4 | 14 | 22 | 32 | 46 | 27 | 39 | ||
| 150 | 5.9 | 2 | 11 | 17 | 29 | 41 | 19 | 32 | ||
| Negative control | 0 | 13 | 7 | 0 | – | 5 | – | 91 | 3 |
5 × 105 cells were transfected with different amounts of PEI and 2.5 μg DNA under varying polyplex formation times and temperatures. The cell number, positive transfectants, rfu and viability were determined 72 h post-transfection. All data were determined by three independent experiments
Table 3.
Determination of transfection protocol
| Storage/reassociation of PEI | Complex formation temperature (°C) | Incubation | Transfectants (%) | rfu | Viability (%) |
|---|---|---|---|---|---|
| 22/22 (°C) | 22 | 30′ | 21 | 28 | 95 |
| 37 | 30′ | 9 | 24 | 90 | |
| 60 | 30′ | 10 | 24 | 88 | |
| 22 | 60′ | 15 | 26 | 92 | |
| 37 | 60′ | 16 | 28 | 93 | |
| 60 | 60′ | 13 | 25 | 90 | |
| 22/60 (°C) | 22 | 30′ | 27 | 42 | 93 |
| 37 | 30′ | 17 | 36 | 93 | |
| 60 | 30′ | 10 | 24 | 94 | |
| 22 | 60′ | 15 | 30 | 93 | |
| 37 | 60′ | 15 | 30 | 93 | |
| 60 | 60′ | 8 | 22 | 90 | |
| −20/60 (°C) | 22 | 30′ | 13 | 25 | 91 |
| 37 | 30′ | 29 | 41 | 90 | |
| 60 | 30′ | 6 | 18 | 88 | |
| 22 | 60′ | 14 | 25 | 87 | |
| 37 | 60′ | 12 | 28 | 89 | |
| 60 | 60′ | 14 | 30 | 90 | |
| Control | 22 | 30′ | 0 | 3 | 91 |
After determining the ideal ration between DNA and PEI, the influence of PEI pre-treatments and different incubation temperatures for polyplex formation onto transfection were compared. 5 × 105 cells were transfected with 2.5 μg pEGFP-N3 and 90 μg PEI with varying PEI treatment
Additionally we compared circular and linear DNA in transient transfection and found that the transfection efficiency of linearized plasmids was poor ranging from 1 to 2% (data not shown) in all experiments and therefore we proceeded with circular plasmids.
Comparison of DOTAP/DOPE and PEI
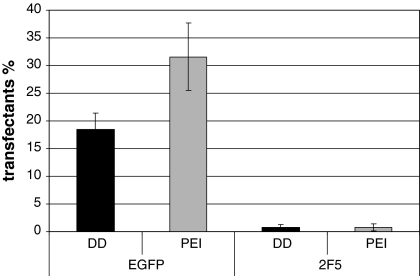
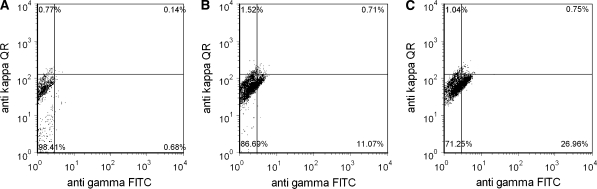
The next step was to compare the adapted PEI protocol with our in-house developed DOTAP/DOPE transfection method in the EGFP and the mAb 2F5 approach. During transient EGFP transfection, PEI performed better than DD as shown in Fig. 2 concerning the number of positive transfectants and fluorescence intensity (92 rfu for PEI transfection and 66 rfu for DD transfection, data not shown). Both systems were also used for transient expression experiments with mAb 2F5. Seventy two hours after transfection of LC and HC plasmids the viable cells producing mAb 2F5 were determined via flow cytometry (Fig. 3). Both transfection methods resulted in a satisfying amount of HC positive cells (11% for DD and 27% for PEI) while only a rare number of LC transfectants was detected. This was the reason for a very limited number of double positive cells with 0.71 and 0.75%, respectively. Although the reason for such a low rate of LC transfectants was not analyzed in detail it might be the cause for the low secretion rate after 3 days (data not shown). Despite such poor transient transfection results (lower than 50 ng mL−1) we have been able to keep high viability and thus, providing a better starting position for the generation of stable recombinant cell lines.
Fig. 2.
Comparison of DD (■) and PEI ( ) using EGFP (pEGFP) and mAb 2F5 (LC and HC) to determine the % transfectants of viable cells and rfu (not shown) 72 h post-transfection via flow cytometry. Negative control not shown
) using EGFP (pEGFP) and mAb 2F5 (LC and HC) to determine the % transfectants of viable cells and rfu (not shown) 72 h post-transfection via flow cytometry. Negative control not shown
Fig. 3.
Intracellular LC and HC content using flow cytometry. The light chain was labeled with an anti kappa Quantum red labeled antibody and the heavy chain was labeled with an anti gamma FITC labeled antibody to investigate the intracellular content of negative control cells (a) and cells transfected with mAB 2F5 using DD (b) or PEI (c). Negative cells and single stained cell lines served as controls to set the gate and adjust the compensation
Stable cell lines expressing mAb 2F5
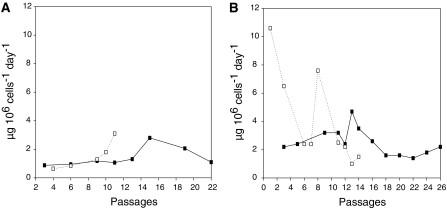
To generate stable cell lines producing mAb 2F5, we used the same methods as for transient transfection but continued after 24 h with limiting dilution sub-cloning in selection medium. Screening of growing clones was done by IgG specific ELISA and best producers were adapted to 0.19 μM MTX and afterwards 0.38 μM MTX for amplification. In case of 2F5/PEI the second sub-cloning step was carried out during passage 11 while the best producer of the 2F5/DD transfection was sub-cloned much later in passage 22 after a rather slow MTX adaptation process. Figure 4 demonstrates that 2F5/PEI was initially the better producing clone but it showed more inconsistency in production and after ten passages it levelled off to the same specific titers as the 2F5/DD clone. The inconsistency might be referred to a loss of high producers due to fast adaptation to MTX.
Fig. 4.
Comparison of DD and PEI transfections to produce a human antibody. After sub-cloning the best performing clone of each transfection was propagated in T25 flasks and cultivated in 0.19 μM MTX and analyzed for IgG titer (a). Furthermore the cells were adapted to 0.38 μM MTX, sub-cloned, selected for the best producer clone and long term stability was determined (b); ■ specific titer of 2F5/DD; □ specific titer of 2F5/PEI
Cultivation in spinner flasks demonstrated that the volumetric titer of both final cell lines was nearly the same with 10 μg/mL during continuous passaging confirming long term stability of the generated cell lines (data not shown).
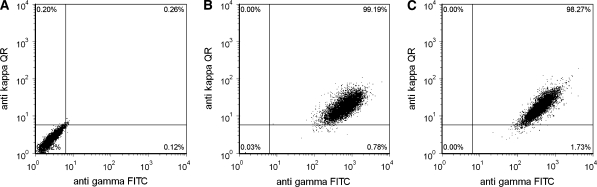
Finally we determined the intracellular LC and HC content of the final cell lines using flow cytometry (Fig. 5). Clone 2F5/DD (Fig. 5b) and clone 2F5/PEI (Fig. 5c) were cultivated in a 10 days batch culture in order to determine the intracellular LC and HC accumulation. Both clones showed a homogeneous double positive population producing intracellular LC and HC and also conferred long-term stability (data not shown). The data indicate that both systems are suitable for stable transfection with 99.2% (Fig. 5b) and 98.3% (Fig. 5c) double positives for 2F5/DD and 2F5/PEI, respectively. This is remarkable as the selection strategy was not adapted to the light chain since we did not use any selection for the LC plasmid. Therefore we postulate that screening with an ELISA system recognizing HC and LC in combination, together with the limiting dilution sub-cloning, is sufficient for selection of homogenous populations expressing the whole antibody despite the initial transfection contains only a very limited number of LC expressing cells.
Fig. 5.
Intracellular light and heavy chain content after 10 days of cultivation of the generated stable cell lines 2F5/DD and 2F5/PEI using flow cytometry. The intracellular light chain was labeled with an anti kappa Quantum red labeled antibody and the heavy chain was labeled with an anti gamma FITC labeled antibody. Negative control (a), 2F5/DD (b) and 2F5/PEI (c). Host cells and single stained cell lines served as controls to set the gate and adjust the compensation
Discussion
The transfection technique is an important parameter in transient gene expression as well as stable cell line generation, but up to now no comprehensive studies have been performed with different model proteins. PEI is a cationic polymer that condenses DNA into small particles or interacts with medium components (Muller et al. 2007). If PEI has higher affinity for these components than for the DNA the transfection efficiency decreases (Godbey et al. 1999). Polyplexes with an overall positive charge bind to the cell surface through electrostatic interactions with negatively charged membrane lipids (van der Aa et al. 2007) but are also responsible for increasing cell death (Tousignant et al. 2000). However, reducing the ± charge ratio of the complexes reduces the transfection efficacy (Plank et al. 1996) and therefore we defined the critical level of free PEI for efficient PEI/DNA for our medium/host system. Concerning the DNA conformation we, as others (Cherng et al. 1999), have found transfection efficacy much lower in case of linearized DNA than with the use of circular DNA. When comparing the transfection abilities of DD and PEI, the latter proofed to be slightly superior in transient transfection concerning EGFP (Fig. 2). By switching the model protein to our in house developed mAb 2F5 we found a tenfold reduction in the percentage of transfectants compared to EGFP. This is assigned to the low number of transfectants in case of the light chain in contrast to heavy chain (Fig. 3) and consequently only very low amounts of mAb 2F5 could be expressed transiently. In general, improvements in transient gene expression are multiplex with the most prominent to be the transfection efficiency, the maximum cell density and the concentration of protein enhancers (Jardin et al. 2008). Other enhancers are sodium butyrate (Leisy et al. 2003; Mahonen et al. 2007; Ping et al. 2006) and trichostatin A (Spenger et al. 2004). More recently proto-oncogenes, cell cycle control genes, growth factor genes and anti-apoptotic genes have been used to improve production hosts or transient expression (Wurm 2004; Backliwal et al. 2008). Finally alternative cultivation methods for suspension cells are applied such as orbital shaker technology working under agitation within an incubator (Muller et al. 2005) which allow cell densities up to 5 × 106 cells mL−1 while our cells reached densities of 1–2 × 106 cells mL−1 only.
Despite the disappointing results of transient expression of mAb 2F5, stable cell lines could be selected with both transfection methods showing comparable results at higher consistency (Figs. 4, 5). In case of stable transfections the volumetric titer was similar comparing DD and PEI (Fig. 4) and both systems are suitable for stable transfection (Fig. 5), whereas the performance of transient transfection was not convincing at all. In parallel we used the PEI transfection method for expression of another model antibody. In this case we observed a transfection efficacy of the LC comparable to HC and 8 weeks later titers of 50 μg/mL were obtained in spinner flasks passaged twice a week. This demonstrates that every single antibody behaves differently and results of individual mAbs are not transferable to others.
In conclusion, the two chemically defined systems are suitable for stable serum-free transfection of CHO cells. They are cost efficient and provide easy scalability and are also usable for larger scales. Thus, they can be further improved upon understanding of the details of the molecular mechanism of this process.
Acknowledgments
This research was part of the Pharma-Planta Project (LSHB-CT-2003-503565), kindly funded by an EU FP6 program.
Contributor Information
Hannes Reisinger, Email: hannes.reisinger@gmail.com.
Renate Kunert, Phone: +43-1-360066595, FAX: +43-1-3697615, Email: renate.kunert@boku.ac.at.
References
- Alt FW, Kellems RE, Bertino JR, Schimke RT (1978) Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem 253:1357–1370 [PubMed]
- Backliwal G, Hildinger M, Chenuet S, DeJesus M, Wurm F (2008) Coexpression of acidic fibroblast growth factor enhances specific productivity and antibody titers in transiently transfected HEK293 cells. Nat Biotechnol 25:162–166 [DOI] [PubMed]
- Cherng JY, Schuurmans-Nieuwenbroek NM, Jiskoot W, Talsma H, Zuidam NJ, Hennink WE, Crommelin DJ (1999) Effect of DNA topology on the transfection efficiency of poly((2-dimethylamino)ethyl methacrylate)-plasmid complexes. J Control Release 60:343–353 [DOI] [PubMed]
- Escriou V, Carriere M, Bussone F, Wils P, Scherman D (2001) Critical assessment of the nuclear import of plasmid during cationic lipid-mediated gene transfer. J Gene Med 3:179–187 [DOI] [PubMed]
- Galbraith DJ, Tait AS, Racher AJ, Birch JR, James DC (2006) Control of culture environment for improved polyethylenimine-mediated transient production of recombinant monoclonal antibodies by CHO cells. Biotechnol Prog 22:753–762 [DOI] [PubMed]
- Godbey WT, Wu KK, Hirasaki GJ, Mikos AG (1999) Improved packing of poly(ethylenimine)/DNA complexes increases transfection efficiency. Gene Ther 6:1380–1388 [DOI] [PubMed]
- Jardin BA, Zhao Y, Selvaraj M, Montes J, Tran R, Prakash S, Elias CB (2008) Expression of SEAP (secreted alkaline phosphatase) by baculovirus mediated transduction of HEK 293 cells in a hollow fiber bioreactor system. J Biotechnol 135:272–280 [DOI] [PubMed]
- Khalil IA, Kogure K, Akita H, Harashima H (2006) Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol Rev 58:32–45 [DOI] [PubMed]
- Kunert R, Ruker F, Katinger H (1998) Molecular characterization of five neutralizing anti-HIV type 1 antibodies: identification of nonconventional D segments in the human monoclonal antibodies 2G12 and 2F5. AIDS Res Hum Retroviruses 14:1115–1128 [DOI] [PubMed]
- Kunert R, Steinfellner W, Purtscher M, Assadian A, Katinger H (2000) Stable recombinant expression of the anti HIV-1 monoclonal antibody 2F5 after IgG3/IgG1 subclass switch in CHO cells. Biotechnol Bioeng 67:97–103 [DOI] [PubMed]
- Le PU, Nabi IR (2003) Distinct caveolae-mediated endocytic pathways target the golgi apparatus and the endoplasmic reticulum. J Cell Sci 116:1059–1071 [DOI] [PubMed]
- Leisy DJ, Lewis TD, Leong JA, Rohrmann GF (2003) Transduction of cultured fish cells with recombinant baculoviruses. J Gen Virol 84:1173–1178 [DOI] [PubMed]
- Mahonen AJ, Airenne KJ, Purola S, Peltomaa E, Kaikkonen MU, Riekkinen MS, Heikura T, Kinnunen K, Roschier MM, Wirth T et al (2007) Post-transcriptional regulatory element boosts baculovirus-mediated gene expression in vertebrate cells. J Biotechnol 131:1–8 [DOI] [PubMed]
- Muller N, Girard P, Hacker DL, Jordan M, Wurm FM (2005) Orbital shaker technology for the cultivation of mammalian cells in suspension. Biotechnol Bioeng 89:400–406 [DOI] [PubMed]
- Muller N, Derouazi M, Van Tilborgh F, Wulhfard S, Hacker DL, Jordan M, Wurm FM (2007) Scalable transient gene expression in Chinese hamster ovary cells in instrumented and non-instrumented cultivation systems. Biotechnol Lett 29:703–711 [DOI] [PubMed]
- Ping W, Ge J, Li S, Zhou H, Wang K, Feng Y, Lou Z (2006) Baculovirus-mediated gene expression in chicken primary cells. Avian Dis 50:59–63 [DOI] [PubMed]
- Plank C, Mechtler K, Szoka FC Jr, Wagner E (1996) Activation of the complement system by synthetic DNA complexes: a potential barrier for intravenous gene delivery. Hum Gene Ther 7:1437–1446 [DOI] [PubMed]
- Regelin AE, Fankhaenel S, Gurtesch L, Prinz C, von Kiedrowski G, Massing U (2000) Biophysical and lipofection studies of DOTAP analogs. Biochim Biophys Acta 1464:151–164 [DOI] [PubMed]
- Reisinger H, Sevcsik E, Vorauer-Uhl K, Lohner K, Katinger H, Kunert R (2007) Serum-free transfection of CHO-cells with tailor-made unilamellar vesicles. Cytotechnology 54:157–168 [DOI] [PMC free article] [PubMed]
- Spenger A, Ernst W, Condreay JP, Kost TA, Grabherr R (2004) Influence of promoter choice and trichostatin A treatment on expression of baculovirus delivered genes in mammalian cells. Protein Expr Purif 38:17–23 [DOI] [PubMed]
- Takei K, Haucke V (2001) Clathrin-mediated endocytosis: membrane factors pull the trigger. Trends Cell Biol 11:385–391 [DOI] [PubMed]
- Tousignant JD, Gates AL, Ingram LA, Johnson CL, Nietupski JB, Cheng SH, Eastman SJ, Scheule RK (2000) Comprehensive analysis of the acute toxicities induced by systemic administration of cationic lipid: plasmid DNA complexes in mice. Hum Gene Ther 11:2493–2513 [DOI] [PubMed]
- Urlaub G, Chasin LA (1980) Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci U S A 77:4216–4220 [DOI] [PMC free article] [PubMed]
- van der Aa MA, Huth US, Hafele SY, Schubert R, Oosting RS, Mastrobattista E, Hennink WE, Peschka-Suss R, Koning GA, Crommelin DJ (2007) Cellular uptake of cationic polymer-DNA complexes via caveolae plays a pivotal role in gene transfection in COS-7 cells. Pharm Res 24:1590–1598 [DOI] [PMC free article] [PubMed]
- Wattiaux R, Jadot M, Warnier-Pirotte MT, Wattiaux-De Coninck S (1997) Cationic lipids destabilize lysosomal membrane in vitro. FEBS Lett 417:199–202 [DOI] [PubMed]
- Wattiaux R, Laurent N, Wattiaux-De Coninck S, Jadot M (2000) Endosomes, lysosomes: their implication in gene transfer. Adv Drug Deliv Rev 41:201–208 [DOI] [PubMed]
- Wrobel I, Collins D (1995) Fusion of cationic liposomes with mammalian cells occurs after endocytosis. Biochim Biophys Acta 1235:296–304 [DOI] [PubMed]
- Wurm FM (2004) Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol 22:1393–1398 [DOI] [PubMed]
- Zuhorn IS, Bakowsky U, Polushkin E, Visser WH, Stuart MC, Engberts JB, Hoekstra D (2005) Nonbilayer phase of lipoplex-membrane mixture determines endosomal escape of genetic cargo and transfection efficiency. Mol Ther 11:801–810 [DOI] [PubMed]