Abstract
Estrogens exert rapid, non-genomic effects, which are mediated by plasma membrane-associated estrogen receptors (mER) mERα and mERβ, and the intracellular transmembrane G protein-coupled estrogen receptor (GPER). Membrane-initiated responses contribute to transcriptional activation, resulting in a complex interplay of nuclear and extra-nuclear mechanisms that mediate the acute physiological responses to estrogens. Non-genomic estrogen signaling also activates a variety of intracellular estrogen signaling pathways that regulate vascular function and cell growth involving rapid but also long-term effects. This review discusses recent advances in understanding of the mechanisms of non-genomic estrogen receptor signaling in the vascular wall.
Keywords: Endothelial Cell, Estradiol, Estrogen Receptor, Genomic Signaling, GPER, GPR30, Non-Genomic Signaling, Plasma Membrane, Vascular Smooth Muscle Cell
1. Genomic and Non-Genomic Estrogen Signaling
Traditionally, cellular responses to estrogens and estrogenic compounds have been considered to be mediated by the two “classical” nuclear estrogen receptors α(ERα) and β (ERβ), known as “genomic” estrogen signaling (Katzenellenbogen et al., 2000; Nilsson et al., 2001). Both receptors function as ligand-activated transcription factors that reside in the cytosol and translocate into the nucleus upon ligand-binding, where they form receptor homo- or heterodimers, and interact with estrogen response elements (ERE) in the promoter region of target genes (Figure 1) (Barton, 2001; Katzenellenbogen et al., 2000; Matthews and Gustafsson, 2003; Nilsson et al., 2001). Nuclear ER-estrogen complexes also modulate the function of other classes of transcription factors through protein-protein interactions, thus regulating gene expression even without direct binding to DNA (O’Lone et al., 2004). In both scenarios, cell-specific recruitment of co-activators or displacement of co-repressors to the sites of DNA binding enhances or represses gene activation and thereby modulates cell function (Figure 1) (Levin, 2005).
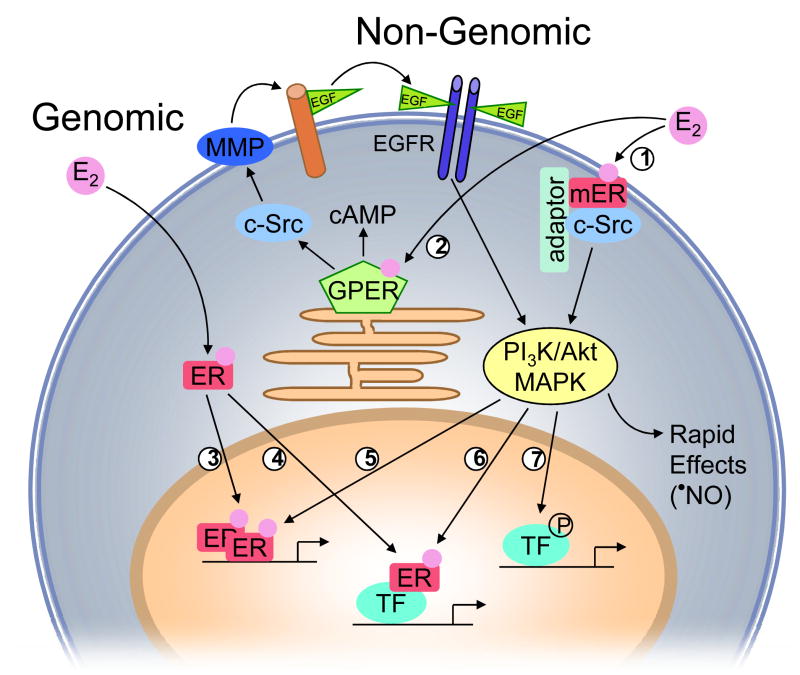
Figure 1.
Proposed concept of non-genomic and genomic estrogen receptor (ER) signaling in vascular cells. Endogenous estrogens (E2) can activate a subpopulation of ER at the plasma membrane (mER) that interacts with adaptor proteins (adaptor) and signaling molecules such as c-Src (1), which is critical for down-stream ER-induced rapid signaling via PI3K/Akt and MAPK pathways. E2 also binds to the G protein-coupled estrogen receptor GPER, which is primarily localized to the endoplasmic reticulum (2). GPER activates downstream effectors, such as adenylate cyclase (resulting in cAMP production), and c-Src. c-Src, in turn, activates matrix metalloproteinases (MMP), which cleave pro-heparin-bound-epidermal growth factor (EGF), releasing free EGF that can then transactivate epidermal growth factor receptors (EGF-R). EGFR activation leads to multiple downstream events, including activation of MAPK and PI3K. Once activated, PI3K/Akt and MAPK cascades can either induce rapid effects, such as E2-induced NO release in endothelial cells, or ultimately influence gene transcription. E2 also activates nuclear ER, inducing nuclear translocation, receptor dimerization, and binding of receptor dimers within the promoter region of target genes (3). Alternatively, activated ER modulates the function of other classes of transcription factors (TF) through protein-protein interactions (4). ER transcriptional activity may be further enhanced by phosphorylation (5), or other transcription factors may be activated that either directly interact with ER (6), or bind independent of ER (7) within the promoter region of the target gene.
A variety of cellular responses to physiological concentrations of estrogens occur rapidly within seconds to few minutes, which cannot be mediated by transcription and protein synthesis (Haynes et al., 2002; Levin, 2005; Meyer et al., 2006; Pietras and Szego, 1977; Simoncini et al., 2004). Instead, these rapid estrogen-mediated effects are transmitted via enzymatic pathways and ion channels through activation of membrane-associated ER (mER), and are referred to as “non-genomic” (Aronica et al., 1994; Boonyaratanakornkit and Edwards, 2007; Cheskis et al., 2007; Haynes et al., 2002; Levin, 2005; Meyer et al., 2006; Moriarty et al., 2006; Simoncini et al., 2004). It should be noted that the distinction between transcriptional and non-genomic effects is entirely arbitrary since some of the intracellular signaling pathways converge and activate nuclear transcription factors (Bjornstrom and Sjoberg, 2005). As a consequence, the combination of these actions at multiple response elements allow fine-tuning of estrogen-dependent regulation of gene transcription (Figure 1) (Bjornstrom and Sjoberg, 2005). In addition, nuclear ER also represent targets of phosphorylation by mitogen-activated protein kinases (MAPK) (Kato et al., 1995), and their function may be affected by cross-talk between ERα and ERβ (Matthews and Gustafsson, 2003; Traupe et al., 2007). Thus, the cellular response to estrogens results from a complex interplay of transcriptional and non-transcriptional events.
Endogenous estrogens protect from vascular disease and atherosclerosis prior to menopause involving beneficial effects on the cholesterol profile and blood pressure (Mendelsohn and Karas, 1999; Meyer et al., 2006). Indeed, estrogens have direct effects on the vascular wall, including inhibition of vascular smooth muscle cell proliferation, powerful vasodilator activity, inhibition of inflammation, antioxidant properties, and accelerated endothelial cell recovery after vascular injury (Mendelsohn and Karas, 1999; Meyer et al., 2006). Nuclear ERα and ERβ mediate several of these effects, presumably by regulating distinct and largely non-overlapping sets of atheroprotective and atheropromoting ERE-containing genes (Mendelsohn and Karas, 1999; O’Lone et al., 2007). In addition, estrogens activate numerous signaling pathways in cardiovascular cells which are thought to either acutely affect cellular function, or to regulate transcription factors through protein kinase-mediated phosphorylation independent of classical ERE (Bjornstrom and Sjoberg, 2005). We will discuss how non-nuclear estrogen receptors contribute to rapid and long-term vascular effects.
2. Current Concepts of Rapid Vascular Estrogen Signaling
2.1. Plasma Membrane-Associated Estrogen Receptors (mER) mERα and mERβ
In the late 1960s, Clara Szego and associates reported increases in uterine cyclic AMP concentrations within minutes (Szego and Davis, 1969). These investigators later also identified estrogen binding sites in isolated plasma membranes of uterine endometrium (Pietras and Szego, 1977). Similarly, increases in vascular cyclic AMP concentrations in response to 17β-estradiol have been reported (Kishi and Numano, 1982; Mügge et al., 1993). Since then, a concept of rapid activation of intracellular signaling pathways via mER has emerged (Boonyaratanakornkit and Edwards, 2007; Cheskis et al., 2007; Haynes et al., 2002; Levin, 2005; Meyer et al., 2006; Moriarty et al., 2006; Simoncini et al., 2004). In both native human umbilical vein endothelial cells and human endothelial cell lines, cell surface estrogen binding sites have been detected using antibodies against epitopes of ERα (Russell et al., 2000). In addition, results from transfection studies suggest that both the nuclear and plasma membrane-associated form of ERα and ERβ respectively, are derived from one transcript (Razandi et al., 1999), Endogenous ERα and ERβ are detectable in plasma membranes of primary human endothelial cells, but not in endothelial cells derived from ERα/ERβ combined-deleted (DERKO) mice (Razandi et al., 2004). In certain vascular endothelial and smooth muscle cells and endothelial cell lines, a role for mER activating MAPK and Akt signaling has been suggested using membrane-impermeable 17β-estradiol-BSA linked to fluorescent dyes (Chen et al., 2004; Haynes et al., 2000; Russell et al., 2000; Somjen et al., 2004). It should be kept in mind, however, that 17β-estradiol-BSA is reportedly prone to artifacts due to contamination with unconjugated 17β-estradiol (Taguchi et al., 2004), thus any findings need to be interpreted with caution. More recently, work from Levin’s laboratory has proposed that in certain cancer cells, which are immortalized by nature, both membrane and nuclear 17β-estradiol-binding proteins could be identical with the “classical” human ER by comparing nuclear- and plasma membrane-localized ER using ER protein isolation, digestion, and tandem array mass spectrometry (Pedram et al., 2006). Whether and how a subpopulation of classical ERα and ERβ also translocates from the cytosol to the plasma membrane of vascular cells in vivo is unclear.
2.2. Mechanisms of mERα and mERβ-Mediated Rapid Estrogen Signaling
Several recent studies identified potential mechanisms leading to translocation of an ER subpopulation to the plasma membrane, as well as several scaffold proteins involved in this process (Boonyaratanakornkit and Edwards, 2007; Cheskis et al., 2007; Moriarty et al., 2006). Caveolin-1 is a primary structural protein of caveolae (Okamoto et al., 1998), vesicular invaginations of the plasma membrane that represent specialized membrane signaling organelles, which are the preferential site of mER-centered signaling complexes in endothelial cells (Chambliss et al., 2000; Chambliss et al., 2002; Razandi et al., 2002). Characteristically, ERα co-localizes with caveolin-1 and activates endothelial nitric oxide synthase (eNOS) in caveolae of endothelial cells (Chambliss et al., 2000; Razandi et al., 2002). Moreover, overexpression of striatin, an ERα-binding protein facilitating the assembly of signaling molecules required for rapid estrogen signaling, enhances localization of ERα to the plasma membrane of the human aortic EAhy926 endothelial cell line (Lu et al., 2004). In line with this data, ERα also associates with caveolin-1 at the plasma membrane of primary vascular smooth muscle cells upon stimulation with 17β-estradiol (Figure 2) (Razandi et al., 2002).
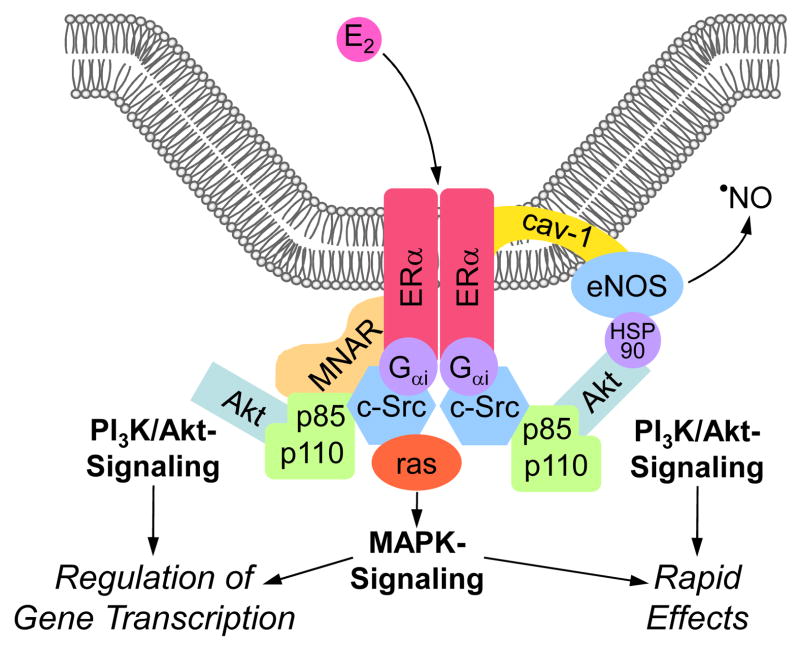
Figure 2.
Integrative model of putative mechanisms of mERα-centered non-genomic estrogen (E2) signaling at the plasma membrane. Caveolae, invaginations of the plasma membrane, are signaling processing centers providing localization for the various molecules involved. The “modulator of non-genomic activity of the ER” (MNAR), a scaffold protein, promotes complex formation with ERα, c-Src, and p85, the regulatory subunit of PI3K (depicted as subunits p85 and p110), thus facilitating activation of PI3K/Akt-signaling. Alternatively, c-Src activates the monomeric GTPase p21ras (ras), which is capable of recruiting downstream kinases of the MAPK-pathway. Direct interaction of the G protein Gαi with ERα is essential for the activation of c-Src. Once activated, both PI3K/Akt- and MAPK-pathways can modulate gene transcription. Alternatively, activation of PI3K/Akt-signaling in endothelial cells leads to the phosphorylation of eNOS protein, which is localized to caveolae through interaction with caveolin-1 (cav-1), a protein that also targets ERα. The molecular chaperone Hsp90 enhances the PI3K/Akt-eNOS interaction. Once eNOS is activated, the release of NO induces rapid cellular effects. Figure modified from (Moriarty et al., 2006).
These findings have been extended by several studies using non-vascular cell lines. Overexpression of caveolin-1 increases ERα translocation to the plasma membrane in MCF-7 breast cancer cells (Razandi et al., 2002). In addition, the adaptor protein Shc appears to interact with and promote the translocation of ERα to the plasma membrane of these cells (Song et al., 2004). In addition to scaffold protein-mediated translocation, palmitoyl acyl transferase-dependent palmitoylation within a highly conserved 9 amino acid motif in the ligand-binding domain that is detectable in ERα and ERβ, as well as in the androgen and progesterone receptor proteins, promotes ER association with the plasma membrane and interaction with caveolin-1 in different cell types (Acconcia et al., 2005; Pedram et al., 2007).
The exact role of mERα and mERβ in cell signaling is currently not clear. Although mERs do not have intrinsic kinase activity, they represents a central component of a membrane “signalosome” (Moriarty et al., 2006), where numerous molecules potentially important for mediating rapid signaling cascades, such as G-proteins, tyrosine kinase c-Src, modulator of non-genomic activity of the ER (MNAR), caveolin-1, and heat shock protein 90 (Hsp90) interact in response to estrogen (Figure 2) (Boonyaratanakornkit and Edwards, 2007; Cheskis et al., 2007; Moriarty et al., 2006). In bovine aortic endothelial cells, direct interaction of the G protein Gαi with ERα is essential for the activation of c-Src (Kumar et al., 2007). This interaction represents one of the initial steps in mER-mediated rapid cell signaling (Migliaccio et al., 2002). MNAR is an important scaffold molecule that is preferentially expressed in rapidly proliferating cells (Vadlamudi and Kumar, 2007). It facilitates and stabilizes the mER-c-Src interaction, a critical step for sufficient activation of c-Src and subsequent MAPK signaling (Vadlamudi and Kumar, 2007). Moreover, MNAR coordinates and promotes a complex formation with ERα, c-Src and p85, the regulatory subunit of phosphatidylinositol-3-kinase (PI3K), in MCF-7 cells upon treatment with 17β-estradiol (Greger et al., 2007). Overexpression of MNAR potentiates estrogen-mediated cell proliferation, suggesting that MNAR is an important regulator of cell cycle progression via MAPK and PI3K/Akt pathways (Cheskis et al., 2008; Greger et al., 2007; Vadlamudi and Kumar, 2007). The role of MNAR for estrogen-related vascular physiology and pathophysiology is currently not known.
2.3. The Intracellular Transmembrane G Protein-Coupled Estrogen Receptor (GPER)
GPER, formerly known as the G protein-coupled orphan receptor GPR30, is a member of the seven-transmembrane G protein-coupled receptor family and has been shown to activate rapid signaling cascades following estrogen binding (Figure 1) (Filardo et al., 2000; Haas et al., 2009; Prossnitz et al., 2008; Revankar et al., 2005; Thomas et al., 2005). Using fluorescent estrogen derivatives (E2-Alexas), confocal microscopy revealed that GPER is primarily localized to the endoplasmic reticulum and that this expression correlates with endogenous GPER expression (Revankar et al., 2005). Although in contrast to the expected localization of GPER at the plasma membrane (Filardo et al., 2007; Kanda and Watanabe, 2003; Thomas et al., 2005), intracellularly expressed GPER is capable of initiating cellular signaling (Revankar et al., 2007). This discrepancy may be explained by the observation that G protein-coupled receptors traffic between the endoplasmic reticulum and the plasma membrane during receptor biogenesis and internalization in response to agonist stimulation (Kleuser et al., 2008). Upon agonist binding, GPER activates heterotrimeric G proteins that initiate several effectors, including adenylate cyclase and c-Src (Filardo et al., 2002; Thomas et al., 2005). Rapid increases in cAMP tissue content following stimulation by 17β-estradiol occur in vascular and non-vascular tissues (Aronica et al., 1994; Mügge et al., 1993; Revankar et al., 2005). The protein c-Src activates matrix metalloproteinases, which are involved in transactivation of epidermal growth factor receptors (Filardo et al., 2000). This results in multiple down-stream events, including activation of MAPK and PI3K (Filardo et al., 2000; Revankar et al., 2005; Thomas et al., 2005). Kinase activation, in turn, may activate nuclear proteins involved in transcriptional regulation of genes whose promoters do not necessarily contain an ERE (Figure 1) (Prossnitz et al., 2008). Interestingly, antagonists of the “classic” ER, tamoxifen, raloxifene and ICI182,780, are agonists of GPER (Filardo et al., 2000; Thomas et al., 2005), as are certain phytoestrogens such as genistein and quercetin (Maggiolini et al., 2004). These GPER-agonistic activities may have important implications for the clinical use of these drugs.
2.4. Mechanisms of GPER-Mediated Rapid Estrogen Signaling
Expression of GPER can be detected in tissues from malignant tumors, and its role for human cancer is currently being studied (Prossnitz et al., 2008). GPER is overexpressed in certain “high-risk” breast and endometrial carcinoma cells (Filardo et al., 2006; Smith et al., 2007). However, GPER activation may inhibit tumor cell growth in certain types of breast cancer cells (Kleuser et al., 2008). In addition, GPER transcripts are widely distributed in human tissues, including the brain, liver, and male and female reproductive tract. In the latter, GPER expression is differentially regulated during the estrous cycle (Prossnitz et al., 2008). We have recently found high expression levels of GPER in arterial vascular smooth muscle cells and intact arteries of patients with coronary atherosclerosis (Haas et al., 2007). Recent studies in cardiomyocytes have suggested that estrogen signaling involves ERα and ERβ-independent pathways (Ullrich et al., 2008). This observation is supported by the finding that activation of GPER by ICI 182,780 or tamoxifen inhibits growth of cardiomyocytes and fibroblasts (Mercier et al., 2003). Although there was evidence for rapid calcium signaling mediated by GPER (Revankar et al., 2005), which is crucial for regulation of vascular tone (Feletou and Vanhoutte, 2006), and although genetic linkage studies in human have suggested a potential role of the GPER locus on chromosome 7p22 in the susceptibility to low-renin hypertension (Lafferty et al., 2000), the role of GPER for vascular homeostasis remained unclear. We therefore set out a number of experiments to study its role in vascular cells, as well as in rodents and humans in vitro and in vivo (Haas et al., 2009). We found, using selective ligands of GPER, that this receptor indeed controls vascular tone by directly and indirectly promoting vasodilation and growth inhibition. Accordingly, injection of a GPER agonist (G-1) into normotensive rats was associated with a marked reduction in blood pressure (Figure 3). Of particular interest was the observation that the time course of changes in intracellular calcium in human vascular smooth muscle cells depends on whether the agonist was applied extra- or intracellularly, the latter causing much faster responses in calcium increases than after extracellular application (Haas et al., 2009). These data are consistent with the notion that GPER indeed is an intracellular G protein-coupled receptor, in line with its localization on the endoplasmic reticulum (Prossnitz et al., 2008). Acute stimulation of human vascular smooth muscle cells devoid of ERα and ERβ stimulated with the GPER agonist G-1 increases MAPK phosphorylation (Haas et al., 2009). Selective activation of GPER using different agonists also potently inhibits human vascular smooth muscle cell growth (Figure 3, panel F), consistent with potent growth-inhibitory effects of tamoxifen in rat cardiomyocytes and fibroblasts (Mercier et al., 2003). Vascular effects of GPER-activation investigated were absent in animals deficient of GPER (Figure 3, panel B); surprisingly, we found that GPER deficiency is associated with marked abdominal obesity in both male and female mice (Figure 3, panel C), indicating that estrogen signaling via this receptor contributes to adipocyte and metabolic function independent of gender. The mechanisms underlying the obesity phenotype have been extensively studied and results will be presented in an upcoming publication (Dr. D.J. Clegg, personal communication). Similarly, abnormal glucose tolerance – though in the absence of an obesity phenotype – have been linked to GPER-deficiency (Mårtensson et al., 2009). Taken together, GPER is a novel and important regulator of vascular tone, vascular smooth muscle cell growth, and obesity.
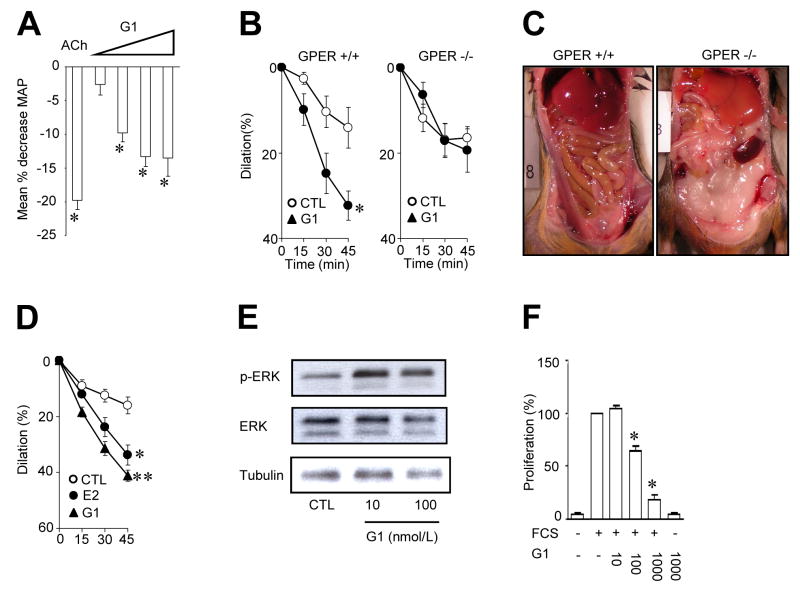
Figure 3.
Involvement of G protein-coupled estrogen receptor in regulation of blood pressure, vascular function, obesity and vascular smooth muscle signaling and growth. Intravenous injection of the GPER-agonist G-1 at increasing concentrations acutely reduces mean arterial blood pressure in normotensive rats. For comparison, the response to acetylcholine (ACh) is shown (A). In precontracted arterial vascular preparations from mice (carotid artery, B, left panel) and humans (internal mammary artery, D), G-1 causes acute dilation, which requires about 45 minutes to reach a maximum. In the internal mammary artery, the dilator effect of G-1 is even stronger than that of 17β-estradiol (E2). Dilatory effects of G-1 are absent in carotid arteries of mice lacking the GPER gene (B, right panel), which also display abdominal obesity (C). In human vascular smooth muscle cells, G-1 causes rapid phosphorylation of extracellular signal-regulated kinases 1/2 (p-ERK, E). GPER activation potently and concentration-dependently inhibits growth of these cells (F). Figure reproduced in part from Circulation Research 2009; 104: 288-291 ©2009 American Heart Association.
These findings are possibly relevant to the vascular protective effects of estrogens in humans, as well as to hormone therapy. Indeed, selective estrogen receptor modulators such as raloxifene or tamoxifen, or ICI 182,780 are potent agonists of GPER while simultanously blocking the “classical” estrogen receptors ERα and ERβ (Filardo et al., 2000; Thomas et al., 2005). Although the Raloxifene Use for The Heart (RUTH) trial found any effects of treatment on cardiac events in elderly women with coronary artery disease or multiple cardiovascular risk factors (Barrett-Connor et al., 2006), it remained unclear whether there could be beneficial effects suggested by the preclinical data (Haas et al., 2009). Indeed, a most recent publication including an age-dependent sub-analysis of the RUTH trial found a significantly lower incidence of cardiovascular events only if menopausal women were younger, i.e. below 60 years of age (Collins et al., 2009), consistent with the recently proposed hypothesis that aging might limit the vasculoprotective effect of estrogen receptor activation (Barton et al., 2007). Further studies are required to determine whether GPER activation could become a novel therapeutic approach in cardiovascular medicine.
2.5. Rapid Estrogen Signaling via the Actin Cytoskeleton
Rapid changes in cell shape and size confer basal physiological functions of many cell types, including neurons and podocytes, and involve changes of the morphological plasticity of the actin cytoskeleton (Faul et al., 2007; Schubert and Dotti, 2007). Rearrangement of the actin cytoskeleton generally depends on binding of external stimuli to membrane-associated receptors, and is mediated via subsequent rapid action of different protein phosphatases and kinases (Faul et al., 2007; Schubert and Dotti, 2007). Accordingly, movement as well as growth and migration of vascular cells are characterized by dynamic remodeling of the actin cytoskeleton. These processes are thought to be required for intracellular signaling, growth, angiogenesis, but also for repairing injured vascular areas and maintaining the functional integrity of the endothelium (Giretti and Simoncini, 2008). Interestingly, rapid signaling cascades activated by sex steroids have been implicated in these processes (Fu and Simoncini, 2007; Giretti and Simoncini, 2008). For example, 17β-estradiol-induced MAPK signaling contributes to the preservation of endothelial cell shape by preventing the stress-induced disruption of the actin cytoskeleton (Razandi et al., 2000). Moreover, exposure of isolated human endothelial cells to 17β-estradiol induces rapid remodeling of the actin cytoskeleton, a process that requires the interaction of ERα and the G protein Gα13 (Simoncini et al., 2006). In this context, non-genomic estrogen signaling may represent a mechanism, which enables the cell to rapidly adapt to surrounding stimuli.
3. Non-Genomic Estrogen Signaling and Regulation of Vascular Cell Function
3.1. Endothelial Cell Function and Nitric Oxide
In the human vascular system, the prototypical non-transcriptional action of estrogens is the induction of direct, i.e. endothelium-independent vasodilation (Mügge et al., 1993). In addition to the regulation of ion fluxes (Fu and Simoncini, 2007; Simoncini et al., 2004), 17β-estradiol rapidly activates PI3K/Akt with subsequent phosphorylation and activation of eNOS. These effects are mediated by ERα but not ERβ (Chambliss et al., 2000; Haynes et al., 2000; Simoncini et al., 2000; Traupe et al., 2007). c-Src is a physiologically relevant modulator of estrogen-induced vasodilation (Li et al., 2007), and is considered a critical upstream regulator of the interaction of ERα and the p85α regulatory subunit of PI3K (Haynes et al., 2003). As these activation events occur in caveolae (Chambliss et al., 2000; Li et al., 2003), ER-mediated activation of eNOS is in part caveolin-1-dependent (Razandi et al., 2002), but may also be facilitated by adaptor proteins such as MNAR, Shc, and striatin (Boonyaratanakornkit and Edwards, 2007; Fu and Simoncini, 2007; Moriarty et al., 2006). The subsequent immediate eNOS activation and release of NO causes rapid, endothelium-dependent vasodilation, while simultaneously inhibiting vascular smooth muscle cell proliferation, leukocyte adhesion, and platelet aggregation (Simoncini et al., 2000). Interestingly, the estrogen-activated PI3K/Akt-eNOS signaling cascade in isolated endothelial cells is also required for transcriptional activation of human telomerase, a protein which affects angiogenesis and aging (Grasselli et al., 2008). In this context, there is evidence to suggest that active eNOS and ERα co-localize in the nucleus and are recruited onto the ERE of a specific telomerase promoter site (Grasselli et al., 2008). This provides yet another possible mechanism whereby rapid estrogen signaling pathways can affect transcriptional and thus long-term regulation of cell growth and function.
As a consequence of structural and functional endothelial cell damage induced by hypertension, diabetes, and hypercholesterolemia, reduced NO bioavailability enhances proliferation and migration of vascular smooth muscle cells (Barton and Haudenschild, 2001). In contrast, early restoration of endothelial cell integrity by estrogens may attenuate the response to vascular smooth muscle cell injury by increasing the availability of NO (Garg and Hassid, 1989). Accordingly, NO attenuates the endothelin-1-induced activation of MAPK as well as protein synthesis in vascular smooth muscle cells (Bouallegue et al., 2007). Thus, constitutive endothelial production of NO is critical for vascular health, as it maintains the mitogenic quiescence of vascular smooth muscle cells by tonic generation of NO, which is partly derived from ERα-mediated non-genomic signaling. In immortalized human endothelial cells, it has been shown that a 46kDa splice variant of ERα (ER46) associates with the plasma membrane and triggers estrogen-stimulated phosphorylation of membrane eNOS more efficiently than full-length ERα (ER66) (Li et al., 2003). In contrast, ER66 more efficiently mediates ERE-dependent eNOS-gene transactivation (Figtree et al., 2003; Li et al., 2003). Thus, differential regulation of expression of ER46 and ER66 could provide the endothelial cell with a stringent tool to regulate between these genomic and non-genomic pathways in health as well as in disease.
3.2. Regulation of Vascular Cell Growth
Natural estrogens differently regulate vascular cell growth, including inhibition of vascular smooth muscle cell proliferation and acceleration of endothelial cell growth, which contributes to the protective effects of estrogens on the cardiovascular system (Mendelsohn and Karas, 1999). Treatment of vascular smooth muscle cells with growth factors induces proliferation and activates MAPK signaling via extracellular signal-related kinases (ERK)-1/2 (Force and Bonventre, 1998), effects that are reversed by 17β-estradiol in isolated human, porcine, and rat vascular smooth muscle cells (Dubey et al., 2000; Geraldes et al., 2002; Geraldes et al., 2003; Haas et al., 2007; Morey et al., 1997). Interestingly, the inhibitory effect of 17β-estradiol on ERK1/2 activity and vascular smooth muscle cell growth are blocked by an ER antagonist (Dubey et al., 2000), indicating that these effects are mediated by ER. Treatment of porcine aortic vascular smooth muscle cells with antisense oligonucleotide sequences complementary to ERβ mRNA abrogates the inhibitory effect of 17β-estradiol on ERK1/2 activity and proliferation, indicating that in pigs these effects are primarily mediated by ERβ (Geraldes et al., 2003). In contrast, in endothelial cells of pigs and mice, 17β-estradiol acutely stimulates ERK1/2 activity and cell proliferation (Geraldes et al., 2002; Geraldes et al., 2003; Pedram et al., 2006), and studies using antisense oligomers indicate that ERα may mediate these effects (Geraldes et al., 2003). This illustrates that in these two neighboring vascular cell types estrogen-signaling cascades activated by different ER have opposite effects on cellular proliferation. The variable ability of 17β-estradiol to modulate ER-caveolin-1 association in different cell types has also been implicated in these differential effects of estrogen on ERK1/2 activity and proliferation (Razandi et al., 2002).
Expression of GPER, which also mediates ERK1/2 signaling via trans-activation of the epidermal growth factor receptor (Filardo et al., 2000), can be detected in several cell types (Prossnitz et al., 2008). A number of reports indicate that estrogen-mediated proliferation of certain cancer cells appears to be dependent on GPER via MAPK-dependent signaling (Albanito et al., 2007; Vivacqua et al., 2006a; Vivacqua et al., 2006b). However, a more recent report indicates that GPER can also inhibit cell growth in breast cancer cells, an effect involving transforming growth factor-β/Smad signaling (Kleuser et al., 2008). Because of the high GPER expression levels observed in human vascular smooth muscle cells (Haas et al., 2007), this receptor could also be involved in estrogen-dependent regulation of vascular cell proliferation. Indeed, when activated with GPER-selective ligands vascular smooth muscle cell growth is strongly inhibited (Haas et al., 2009).
3.3. Vascular Apoptosis and Cell Survival
Estrogens have been implicated in the regulation of vascular cell survival (Barton and Kockx, 2002). Apoptosis occurs during atherogenesis, and may be required by certain vascular smooth muscle cells as a physiological regulator in order to accommodate for the altered structural situation in hypertensive vascular hypertrophy or restenosis (Kockx and Herman, 2000). However, apoptosis of vascular smooth muscle cells may also transform a previously stable plaque into a lesion prone to rupture (Kockx and Herman, 2000). Indeed, vascular smooth muscle cells produce most of the interstitial collagen fibers, which are important for the tensile strength of the fibrous cap (Kockx and Herman, 2000).
Rapid signaling through mERα and mERβ not only regulates cellular proliferation, but is also involved in mechanisms regulating cell survival (Moriarty et al., 2006). Importantly, signaling via different MAPK pathways enables the cell to dynamically balance between these opposing events: on the one hand the ERK1/2 pathway contributes to cell proliferation, while on the other hand activation of the p38 MAPK pathway has been linked to apoptosis (Cheng et al., 2008; Xia et al., 1995). In bovine aortic endothelial cells, estrogens have been shown to mediate cell survival via activation of ERK1/2 and inhibition of p38 signaling (Liu et al., 2002; Razandi et al., 2000). Interestingly, 17β-estradiol-BSA is able to activate p38, which is reversed by ER antagonists, suggesting that the mERs mediate these effects (Razandi et al., 2000). In contrast to endothelial cells, treatment of synthetic vascular smooth muscle cells with 17β-estradiol rapidly activates the p38 signaling cascade and induces apoptosis, while inhibiting ERK1/2 phosphorylation (Mori-Abe et al., 2003). In rat aortic vascular smooth muscle cells, selective activation of ERα rapidly increases ERK phosphorylation and cell proliferation, whereas selective activation of ERβ rapidly induces p38 activity and apoptosis (Cheng et al., 2008). Thus, vascular smooth muscle cell survival may also be regulated by differential expression of ERα and ERβ in order to affect the balance between proliferation and apoptosis as required for maintaining the homeostasis of the vascular wall. Whether this also plays a role in humans is unknown. In addition, studies investigating mechanisms of apoptosis in human keratinocytes (Kanda and Watanabe, 2003) and murine thymocytes (Wang et al., 2008) demonstrated an important role for estrogen-signaling via GPER, indicating that this receptor may also have a potential role in regulation of cell survival in the cardiovascular system.
4. Perspectives
Estrogen-dependent regulation of vascular gene expression and protein function is a complex process involving both nuclear and membrane-associated ER signaling pathways. The final gene response to estrogens in a particular cardiovascular cell, however, will depend on a number of conditions, including (a) expression, cellular localization, and affinity of different ER proteins, (b) combination of transcription factors bound to a specific gene promoter, (c) availability of signaling proteins and co-regulatory factors, and (d) nature and intensity of extracellular stimuli (Bjornstrom and Sjoberg, 2005). Moreover, estrogens affect post-transcriptional stability of RNA and post-translational modification of proteins, thereby further affecting cellular phenotype and expression profile (Miller and Duckles, 2008). Regarding the emerging complexity of vascular estrogen action, it is possible that genomic estrogen signaling might be required to maintain basic cellular functions, whereas activation of intracellular signaling pathways may represent mechanisms that allow rapid adaptation of vascular functions in response to changes of the surrounding milieu (Fu and Simoncini, 2007). Rapid estrogen-mediated effects therefore may confer the ability of the cell to dynamically encounter pathological alterations, such as vascular inflammation or atherogenesis (Meyer et al., 2006). Thus, both genomic and non-genomic estrogen actions are likely to contribute to cardiovascular homeostasis. The individual contribution of these pathways, however, to estrogen-dependent vascular effects in health and disease in vivo still remains to be defined. Moreover, there is substantial evidence that function and expression of “classical” ERs is altered during different stages of atherogenesis (Mendelsohn and Karas, 2005; Meyer et al., 2008). It can therefore be assumed that non-genomic and genomic estrogen signaling pathways (Figure 1) are also considerably altered in diseased vessels. This may be one of the factors contributing to the lack of therapeutic benefit in women who received hormone therapy late after menopause (Hulley et al., 1998; Rossouw et al., 2002). At present, we do not know to what extent and how the presence of atherosclerotic vascular disease or the cessation of endogenous estrogen production in postmenopausal women affects vascular estrogen signaling in vivo. However, selective GPER activation might be suitable for the treatment and prevention of adverse clinical events due to cardiovascular disease in the presence of cardiovascular risk factors or even overt atherosclerosis. The first clinical study using raloxifene as a GPER agonist suggests that this appears indeed to be the case, at least in younger postmenopausal women (Collins et al., 2009).
Acknowledgments
Funding
Original work by the authors is supported by Swiss National Science Foundation grants 3200-108258/1 and K-33KO_122504/1 (M.B.), and by NIH grants CA116662 and CA118743 (E.R.P.).
List of Abbreviations
- eNOS
Endothelial nitric oxide synthase
- ERK1/2
Extracellular signal-related kinases-1/2
- ERα
Estrogen receptor alpha
- ERβ
Estrogen receptor beta
- GPER
G protein-coupled estrogen receptor 1
- GPR30
G protein-coupled receptor 30
- Hsp90
Heat shock protein 90
- MAPK
Mitogen-activated protein kinase
- mER
Membrane-associated estrogen receptor (α or β)
- MNAR
Modulator of non-genomic activity of the estrogen receptor
- NO
Nitric oxide
- PI3K
Phosphatidylinositol-3-kinase
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. 2005;16:231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Ando S, Maggiolini M. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 2007;67:1859–1866. doi: 10.1158/0008-5472.CAN-06-2909. [DOI] [PubMed] [Google Scholar]
- Aronica SM, Kraus WL, Katzenellenbogen BS. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc Natl Acad Sci U S A. 1994;91:8517–8521. doi: 10.1073/pnas.91.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, McNabb MA, Wenger NK. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- Barton M. Postmenopausal oestrogen replacement therapy and atherosclerosis: can current compounds provide cardiovascular protection? Expert Opin Investig Drugs. 2001;10:789–809. doi: 10.1517/13543784.10.5.789. [DOI] [PubMed] [Google Scholar]
- Barton M, Haudenschild CC. Endothelium and atherogenesis: endothelial therapy revisited. J Cardiovasc Pharmacol. 2001;38(Suppl 2):S23–25. doi: 10.1097/00005344-200111002-00007. [DOI] [PubMed] [Google Scholar]
- Barton M, Kockx MM. Estrogen and apoptosis in atherosclerosis. In: Samioe G, Skouby S, editors. Midlife Health - Current Concepts and Challanges for the Future; Proceedings of the 5th European Congress on Menopause; Copenhagen, Denmark. July 1–5, 2000; Amsterdam: Elsevier International Congress Series; 2002. pp. 81–93. [Google Scholar]
- Barton M, Meyer MR, Haas E. Hormone replacement therapy and atherosclerosis in postmenopausal women: does aging limit therapeutic benefits? Arterioscler Thromb Vasc Biol. 2007;27:1669–1672. doi: 10.1161/ATVBAHA.106.130260. [DOI] [PubMed] [Google Scholar]
- Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Edwards DP. Receptor mechanisms mediating non-genomic actions of sex steroids. Semin Reprod Med. 2007;25:139–153. doi: 10.1055/s-2007-973427. [DOI] [PubMed] [Google Scholar]
- Bouallegue A, Daou GB, Srivastava AK. Nitric oxide attenuates endothelin-1-induced activation of ERK1/2, PKB, and Pyk2 in vascular smooth muscle cells by a cGMP-dependent pathway. Am J Physiol Heart Circ Physiol. 2007;293:H2072–2079. doi: 10.1152/ajpheart.01097.2006. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RG, Shaul PW. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res. 2000;87:E44–52. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERbeta has nongenomic action in caveolae. Mol Endocrinol. 2002;16:938–946. doi: 10.1210/mend.16.5.0827. [DOI] [PubMed] [Google Scholar]
- Chen DB, Bird IM, Zheng J, Magness RR. Membrane estrogen receptor-dependent extracellular signal-regulated kinase pathway mediates acute activation of endothelial nitric oxide synthase by estrogen in uterine artery endothelial cells. Endocrinology. 2004;145:113–125. doi: 10.1210/en.2003-0547. [DOI] [PubMed] [Google Scholar]
- Cheng B, Song J, Zou Y, Wang Q, Lei Y, Zhu C, Hu C. Responses of vascular smooth muscle cells to estrogen are dependent on balance between ERK and p38 MAPK pathway activities. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2008.02.017. published online. [DOI] [PubMed] [Google Scholar]
- Cheskis BJ, Greger JG, Nagpal S, Freedman LP. Signaling by estrogens. J Cell Physiol. 2007;213:610–617. doi: 10.1002/jcp.21253. [DOI] [PubMed] [Google Scholar]
- Cheskis BJ, Greger J, Cooch N, McNally C, McLarney S, Lam HS, Rutledge S, Mekonnen B, Hauze D, Nagpal S, Freedman LP. MNAR plays an important role in ERa activation of Src/MAPK and PI3K/Akt signaling pathways. Steroids. 2008;73:901–905. doi: 10.1016/j.steroids.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Collins P, Mosca L, Geiger MJ, Grady D, Kornitzer M, Amewou-Atisso MG, Effron MB, Dowsett SA, Barrett-Connor E, Wenger NK. Effects of the Selective Estrogen Receptor Modulator Raloxifene on Coronary Outcomes in The Raloxifene Use for the Heart Trial. Results of Subgroup Analyses by Age and Other Factors. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.108.817577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey RK, Jackson EK, Gillespie DG, Zacharia LC, Imthurn B, Keller PJ. Clinically used estrogens differentially inhibit human aortic smooth muscle cell growth and mitogen-activated protein kinase activity. Arterioscler Thromb Vasc Biol. 2000;20:964–972. doi: 10.1161/01.atv.20.4.964. [DOI] [PubMed] [Google Scholar]
- Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007;17:428–437. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- Figtree GA, McDonald D, Watkins H, Channon KM. Truncated estrogen receptor alpha 46-kDa isoform in human endothelial cells: relationship to acute activation of nitric oxide synthase. Circulation. 2003;107:120–126. doi: 10.1161/01.cir.0000043805.11780.f5. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Graeber CT, Quinn JA, Resnick MB, Giri D, DeLellis RA, Steinhoff MM, Sabo E. Distribution of GPR30, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin Cancer Res. 2006;12:6359–6366. doi: 10.1158/1078-0432.CCR-06-0860. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148:3236–3245. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- Force T, Bonventre JV. Growth factors and mitogen-activated protein kinases. Hypertension. 1998;31:152–161. doi: 10.1161/01.hyp.31.1.152. [DOI] [PubMed] [Google Scholar]
- Fu XD, Simoncini T. Non-genomic sex steroid actions in the vascular system. Semin Reprod Med. 2007;25:178–186. doi: 10.1055/s-2007-973430. [DOI] [PubMed] [Google Scholar]
- Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83:1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldes P, Sirois MG, Bernatchez PN, Tanguay JF. Estrogen regulation of endothelial and smooth muscle cell migration and proliferation: role of p38 and p42/44 mitogen-activated protein kinase. Arterioscler Thromb Vasc Biol. 2002;22:1585–1590. doi: 10.1161/01.atv.0000035393.11854.6a. [DOI] [PubMed] [Google Scholar]
- Geraldes P, Sirois MG, Tanguay JF. Specific contribution of estrogen receptors on mitogen-activated protein kinase pathways and vascular cell activation. Circ Res. 2003;93:399–405. doi: 10.1161/01.RES.0000088640.18462.42. [DOI] [PubMed] [Google Scholar]
- Giretti MS, Simoncini T. Rapid regulatory actions of sex steroids on cell movement through the actin cytoskeleton. Steroids. 2008;73:895–900. doi: 10.1016/j.steroids.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Grasselli A, Nanni S, Colussi C, Aiello A, Benvenuti V, Ragone G, Moretti F, Sacchi A, Bacchetti S, Gaetano C, Capogrossi MC, Pontecorvi A, Farsetti A. Estrogen receptor-alpha and endothelial nitric oxide synthase nuclear complex regulates transcription of human telomerase. Circ Res. 2008;103:34–42. doi: 10.1161/CIRCRESAHA.107.169037. [DOI] [PubMed] [Google Scholar]
- Greger JG, Fursov N, Cooch N, McLarney S, Freedman LP, Edwards DP, Cheskis BJ. Phosphorylation of MNAR promotes estrogen activation of phosphatidylinositol 3-kinase. Mol Cell Biol. 2007;27:1904–1913. doi: 10.1128/MCB.01732-06. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Haas E, Meyer MR, Schurr U, Bhattacharya I, Minotti R, Nguyen HH, Heigl A, Lachat M, Genoni M, Barton M. Differential effects of 17beta-estradiol on function and expression of estrogen receptor alpha, estrogen receptor beta, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension. 2007;49:1358–1363. doi: 10.1161/HYPERTENSIONAHA.107.089995. [DOI] [PubMed] [Google Scholar]
- Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res. 2009;104:288–291. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res. 2000;87:677–682. doi: 10.1161/01.res.87.8.677. [DOI] [PubMed] [Google Scholar]
- Haynes MP, Li L, Russell KS, Bender JR. Rapid vascular cell responses to estrogen and membrane receptors. Vascul Pharmacol. 2002;38:99–108. doi: 10.1016/s0306-3623(02)00133-7. [DOI] [PubMed] [Google Scholar]
- Haynes MP, Li L, Sinha D, Russell KS, Hisamoto K, Baron R, Collinge M, Sessa WC, Bender JR. Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen. J Biol Chem. 2003;278:2118–2123. doi: 10.1074/jbc.M210828200. [DOI] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- Kanda N, Watanabe S. 17beta-estradiol inhibits oxidative stress-induced apoptosis in keratinocytes by promoting Bcl-2 expression. J Invest Dermatol. 2003;121:1500–1509. doi: 10.1111/j.1523-1747.2003.12617.x. [DOI] [PubMed] [Google Scholar]
- Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Montano MM, Ediger TR, Sun J, Ekena K, Lazennec G, Martini PG, McInerney EM, Delage-Mourroux R, Weis K, Katzenellenbogen JA. Estrogen receptors: selective ligands, partners, and distinctive pharmacology. Recent Prog Horm Res. 2000;55:163–193. discussion 194–165. [PubMed] [Google Scholar]
- Kishi Y, Numano F. A study of the mechanism of estrogen as an antiatherosclerotic: the inhibitory effect of estrogen on A23187-induced contraction of the aortic wall. Mech Ageing Dev. 1982;18:115–123. doi: 10.1016/0047-6374(82)90081-1. [DOI] [PubMed] [Google Scholar]
- Kleuser B, Malek D, Gust R, Pertz HH, Potteck H. 17-{beta}-Estradiol inhibits Transforming Growth Factor-{beta} signalling and function in breast cancer cells via activation of Extracellular Signal-Regulated Kinase through the G protein coupled receptor 30. Mol Pharmacol. 2008 doi: 10.1124/mol.108.046854. published online. [DOI] [PubMed] [Google Scholar]
- Kockx MM, Herman AG. Apoptosis in atherosclerosis: beneficial or detrimental? Cardiovasc Res. 2000;45:736–746. doi: 10.1016/s0008-6363(99)00235-7. [DOI] [PubMed] [Google Scholar]
- Kumar P, Wu Q, Chambliss KL, Yuhanna IS, Mumby SM, Mineo C, Tall GG, Shaul PW. Direct Interactions with G alpha i and G betagamma mediate nongenomic signaling by estrogen receptor alpha. Mol Endocrinol. 2007;21:1370–1380. doi: 10.1210/me.2006-0360. [DOI] [PubMed] [Google Scholar]
- Lafferty AR, Torpy DJ, Stowasser M, Taymans SE, Lin JP, Huggard P, Gordon RD, Stratakis CA. A novel genetic locus for low renin hypertension: familial hyperaldosteronism type II maps to chromosome 7 (7p22) J Med Genet. 2000;37:831–835. doi: 10.1136/jmg.37.11.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc Natl Acad Sci U S A. 2003;100:4807–4812. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Hisamoto K, Kim KH, Haynes MP, Bauer PM, Sanjay A, Collinge M, Baron R, Sessa WC, Bender JR. Variant estrogen receptor-c-Src molecular interdependence and c-Src structural requirements for endothelial NO synthase activation. Proc Natl Acad Sci U S A. 2007;104:16468–16473. doi: 10.1073/pnas.0704315104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WL, Guo X, Guo ZG. Estrogen prevents bovine aortic endothelial cells from TNF-alpha-induced apoptosis via opposing effects on p38 and p44/42 CCDPK. Acta Pharmacol Sin. 2002;23:213–218. [PubMed] [Google Scholar]
- Lu Q, Pallas DC, Surks HK, Baur WE, Mendelsohn ME, Karas RH. Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor alpha. Proc Natl Acad Sci U S A. 2004;101:17126–17131. doi: 10.1073/pnas.0407492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, Montanaro D, Musti AM, Picard D, Ando S. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17beta-estradiol and phytoestrogens in breast cancer cells. J Biol Chem. 2004;279:27008–27016. doi: 10.1074/jbc.M403588200. [DOI] [PubMed] [Google Scholar]
- Mårtensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150:687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- Mercier I, Mader S, Calderone A. Tamoxifen and ICI 182,780 negatively influenced cardiac cell growth via an estrogen receptor-independent mechanism. Cardiovasc Res. 2003;59:883–892. doi: 10.1016/s0008-6363(03)00517-0. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Haas E, Barton M. Gender differences of cardiovascular disease: new perspectives for estrogen receptor signaling. Hypertension. 2006;47:1019–1026. doi: 10.1161/01.HYP.0000223064.62762.0b. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Haas E, Barton M. Need for research on estrogen receptor function: importance for postmenopausal hormone therapy and atherosclerosis. Gend Med. 2008;5(Suppl A):S19–33. doi: 10.1016/j.genm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Migliaccio A, Castoria G, Di Domenico M, De Falco A, Bilancio A, Auricchio F. Src is an initial target of sex steroid hormone action. Ann N Y Acad Sci. 2002;963:185–190. doi: 10.1111/j.1749-6632.2002.tb04109.x. [DOI] [PubMed] [Google Scholar]
- Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210–241. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey AK, Pedram A, Razandi M, Prins BA, Hu RM, Biesiada E, Levin ER. Estrogen and progesterone inhibit vascular smooth muscle proliferation. Endocrinology. 1997;138:3330–3339. doi: 10.1210/endo.138.8.5354. [DOI] [PubMed] [Google Scholar]
- Mori-Abe A, Tsutsumi S, Takahashi K, Toya M, Yoshida M, Du B, Kawagoe J, Nakahara K, Takahashi T, Ohmichi M, Kurachi H. Estrogen and raloxifene induce apoptosis by activating p38 mitogen-activated protein kinase cascade in synthetic vascular smooth muscle cells. J Endocrinol. 2003;178:417–426. doi: 10.1677/joe.0.1780417. [DOI] [PubMed] [Google Scholar]
- Moriarty K, Kim KH, Bender JR. Minireview: estrogen receptor-mediated rapid signaling. Endocrinology. 2006;147:5557–5563. doi: 10.1210/en.2006-0729. [DOI] [PubMed] [Google Scholar]
- Mügge A, Riedel M, Barton M, Kuhn M, Lichtlen PR. Endothelium independent relaxation of human coronary arteries by 17 beta-oestradiol in vitro. Cardiovasc Res. 1993;27:1939–1942. doi: 10.1093/cvr/27.11.1939. [DOI] [PubMed] [Google Scholar]
- Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- O’Lone R, Frith MC, Karlsson EK, Hansen U. Genomic targets of nuclear estrogen receptors. Mol Endocrinol. 2004;18:1859–1875. doi: 10.1210/me.2003-0044. [DOI] [PubMed] [Google Scholar]
- O’Lone R, Knorr K, Jaffe IZ, Schaffer ME, Martini PG, Karas RH, Bienkowska J, Mendelsohn ME, Hansen U. Estrogen receptors alpha and beta mediate distinct pathways of vascular gene expression, including genes involved in mitochondrial electron transport and generation of reactive oxygen species. Mol Endocrinol. 2007;21:1281–1296. doi: 10.1210/me.2006-0497. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Szego CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen Signaling through the Transmembrane G Protein-Coupled Receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Levin ER. Estrogen signals to the preservation of endothelial cell form and function. J Biol Chem. 2000;275:38540–38546. doi: 10.1074/jbc.M007555200. [DOI] [PubMed] [Google Scholar]
- Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol Endocrinol. 2002;16:100–115. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER. Plasma membrane estrogen receptors exist and functions as dimers. Mol Endocrinol. 2004;18:2854–2865. doi: 10.1210/me.2004-0115. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Mitchell HD, Field AS, Burai R, Corona C, Ramesh C, Sklar LA, Arterburn JB, Prossnitz ER. Synthetic estrogen derivatives demonstrate the functionality of intracellular GPR30. ACS Chem Biol. 2007;2:536–544. doi: 10.1021/cb700072n. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Russell KS, Haynes MP, Sinha D, Clerisme E, Bender JR. Human vascular endothelial cells contain membrane binding sites for estradiol, which mediate rapid intracellular signaling. Proc Natl Acad Sci U S A. 2000;97:5930–5935. doi: 10.1073/pnas.97.11.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert V, Dotti CG. Transmitting on actin: synaptic control of dendritic architecture. J Cell Sci. 2007;120:205–212. doi: 10.1242/jcs.03337. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncini T, Mannella P, Fornari L, Caruso A, Varone G, Genazzani AR. Genomic and non-genomic effects of estrogens on endothelial cells. Steroids. 2004;69:537–542. doi: 10.1016/j.steroids.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Scorticati C, Mannella P, Fadiel A, Giretti MS, Fu XD, Baldacci C, Garibaldi S, Caruso A, Fornari L, Naftolin F, Genazzani AR. Estrogen receptor alpha interacts with Galpha13 to drive actin remodeling and endothelial cell migration via the RhoA/Rho kinase/moesin pathway. Mol Endocrinol. 2006;20:1756–1771. doi: 10.1210/me.2005-0259. [DOI] [PubMed] [Google Scholar]
- Smith HO, Leslie KK, Singh M, Qualls CR, Revankar CM, Joste NE, Prossnitz ER. GPR30: a novel indicator of poor survival for endometrial carcinoma. Am J Obstet Gynecol. 2007;196:386, e381–389. doi: 10.1016/j.ajog.2007.01.004. discussion 386 e389–311. [DOI] [PubMed] [Google Scholar]
- Somjen D, Kohen F, Gayer B, Sharon O, Baz M, Limor R, Kulik T, Knoll E, Stern N. Role of putative membrane receptors in the effects of estradiol on human vascular cell growth. Am J Hypertens. 2004;17:462–469. doi: 10.1016/j.amjhyper.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor alpha to the plasma membrane. Proc Natl Acad Sci U S A. 2004;101:2076–2081. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szego CM, Davis JS. Inhibition of estrogen-induced cyclic AMP elevation in rat uterus. II: By glucocorticoids. Life Sci. 1969;8:1109–1116. doi: 10.1016/0024-3205(69)90164-7. [DOI] [PubMed] [Google Scholar]
- Taguchi Y, Koslowski M, Bodenner DL. Binding of estrogen receptor with estrogen conjugated to bovine serum albumin (BSA) Nucl Recept. 2004;2:5. doi: 10.1186/1478-1336-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Traupe T, Stettler CD, Li H, Haas E, Bhattacharya I, Minotti R, Barton M. Distinct roles of estrogen receptors alpha and beta mediating acute vasodilation of epicardial coronary arteries. Hypertension. 2007;49:1364–1370. doi: 10.1161/HYPERTENSIONAHA.106.081554. [DOI] [PubMed] [Google Scholar]
- Ullrich ND, Krust A, Collins P, MacLeod KT. Genomic deletion of estrogen receptors ERalpha and ERbeta does not alter estrogen-mediated inhibition of Ca2+ influx and contraction in murine cardiomyocytes. Am J Physiol Heart Circ Physiol. 2008;294:H2421–2427. doi: 10.1152/ajpheart.01225.2007. [DOI] [PubMed] [Google Scholar]
- Vadlamudi RK, Kumar R. Functional and biological properties of the nuclear receptor coregulator PELP1/MNAR. Nucl Recept Signal. 2007;5:e004. doi: 10.1621/nrs.05004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivacqua A, Bonofiglio D, Albanito L, Madeo A, Rago V, Carpino A, Musti AM, Picard D, Ando S, Maggiolini M. 17beta-estradiol, genistein, and 4-hydroxytamoxifen induce the proliferation of thyroid cancer cells through the g protein-coupled receptor GPR30. Mol Pharmacol. 2006a;70:1414–1423. doi: 10.1124/mol.106.026344. [DOI] [PubMed] [Google Scholar]
- Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Ando S, Maggiolini M. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17beta-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol. 2006b;20:631–646. doi: 10.1210/me.2005-0280. [DOI] [PubMed] [Google Scholar]
- Wang C, Dehghani B, Magrisso IJ, Rick EA, Bonhomme E, Cody DB, Elenich LA, Subramanian S, Murphy SJ, Kelly MJ, Rosenbaum JS, Vandenbark AA, Offner H. GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol. 2008;22:636–648. doi: 10.1210/me.2007-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]