Abstract
Aquaporins are a family of water channel proteins involved in water homeostasis in several tissues. Current knowledge of aquaporin expression in the nervous system is very limited. Therefore the first aim of this study was to assess, by immunohistochemistry and immunoblotting analysis, the presence and localization of aquaporin-2 in the spinal cord and dorsal root ganglia of naïve adult rats. In addition, we evaluated aquaporin-2 expression in response to chronic constriction injury of the sciatic nerve, a model of neuropathic pain. Our results showed that aquaporin-2 expression was not detectable either in the spinal cord or the dorsal root ganglia of naïve rats. However, we showed for the first time an increase of aquaporin-2 expression in response to chronic constriction injury treatment in small-diameter dorsal root ganglia neurons but no expression in the lumbar spinal cord. These data support the hypothesis that aquaporin-2 expression is involved in inflammatory neuropathic nerve injuries, although its precise role remains to be determined.
Keywords: aquaporin-2, chronic constriction injury, dorsal root ganglia, pain, spinal cord
Introduction
Water is quantitatively the major component of organisms. Because its movement across cell membranes is a fundamental property of life, the cloning of aquaporin-1 (AQP1), the archetypal water channel, has stimulated research into water transport and osmoregulation in mammals (Denker et al. 1988).
AQPs are small hydrophobic membrane proteins that facilitate bi-directional water transport across the plasma membrane in a number of tissues, including the nervous system (Manley et al. 2000, 2004). This family of proteins contains at least 13 subtypes, which are ubiquitously distributed (Verkman, 2002a,b;). Despite a common molecular structure, AQPs are classified, according to their substrate specificity, as water-selective AQPs (AQP0-1-2-4-5-6-8-11) or aquaglyceroporins (AQP3-7-9 and 10), which allow the passage of small solutes, such as glycerol and urea (Borgnia et al. 1999; King et al. 2004; Yakata et al. 2007); AQP12 is intracellular and its function is unknown (Ishibashi, 2006).
To date, only a few subtypes have been described in the rodent central and peripheral nervous system. In particular, AQP1 is expressed in the chorioid plexus, where its deletion reduced cerebrospinal fluid production (Frigeri et al. 1995; Oshio et al. 2005) and in glia cells of the peripheral nervous system (Gao et al. 2006). AQP4 is expressed in astroglial cells (Nielsen et al. 1997; Badaut et al. 2001; Oshio et al. 2004) and is correlated with the blood-brain barrier (Saadoun et al. 2002). AQP9 is localized in astroglial cells, endothelial cells of pial vessels and catecholaminergic neurons and participates in water transport across the blood–brain barrier and in neuronal metabolism (Badaut et al. 2001, 2004).
Chronic pain is widely present in the general population but there are few treatments which can provide relief for many patients. Chronic constriction injury (CCI) is used as an animal model of chronic neuropathic pain (Bennet & Xie, 1988) that causes pathophysiological changes in which the ion channel and receptor functions are impaired, causing neurochemical and ultrastructural alterations (Richards et al. 1980; Schafers et al. 2003; Nesic et al. 2005).
A number of studies have provided indirect evidence of osmosis contributing to altered pain perception (Solenov et al. 2002; Oshio et al. 2006; Shields et al. 2007). Forty years ago Hitchcock (1969) reported that intrathecal injections of cold saline in patients with intractable pain led to immediate relief. In another study, Jawett & King (1971) used an in vivo preparation of dorsal roots from either monkeys or cats to examine the effects of saline of various osmolalities on the firing of A- and C-fibers. Interestingly, they found that distilled water or hypotonic saline resulted in differential blockade of C-fibers, with little or no effect on A-fibers. It has been proposed that differences in water flux underlie the preferential conduction block of C-fibers. Although anatomical differences among A- and C-fibers were offered as a possible explanation, Oshio et al. (2006) suggested that the differential expression of AQPs in C-fibers may account for these results and provided a molecular basis for osmosis in the pain pathway.
Recently, Oshio et al. (2006) described AQP1 immunoreactivity in the superficial dorsal horn and primary afferent neurons of the dorsal root ganglia (DRG). In particular, behavioural analyses demonstrated that AQP1 appears to contribute to the processing of two principal types of acute pain (thermal and chemical-capsaicin). In addition, AQP1 deletion in mice led to a substantial reduction in the rate of swelling of the dorsal horn after exposure to hypotonic medium (Solenov et al. 2002). Moreover, these authors showed that AQP1 could be involved in the peripheral transduction of the noxious signal, nerve conduction or synaptic transmission in the superficial dorsal horn. Each of these processes is characterized by net ion fluxes that can cause osmotic gradients, resulting in the rapid redistribution of water between intracellular and extracellular compartments (Oshio et al. 2006).
Conversely, genetic deletion of AQP1 does not alter nociceptive responses to a variety of pain stimuli (Shields et al. 2007). This could be due to the different experimental model used, which could give a different type of pain, acute or chronic pain, or the de novo expression of other AQPs.
Many studies suggest that AQP2 is exclusively expressed in the renal collecting duct (Kwon et al. 2001). Nevertheless, there is increasing evidence that AQP2 is also expressed in several extra-renal locations (Stevens et al. 2000), including the peripheral nervous system (Mobasheri et al. 2005).
As there are no data showing any AQP2 expression in the rat spinal cord or DRG, our aims were to evaluate its presence in the spinal cord and DRG of naïve rats and its possible expression in an experimental model of neuropathic pain.
Materials and methods
Animal maintenance and preparation
Experiments were carried out on 18 male Sprague–Dawley rats (200 g body weight) both for immunohistochemistry and immunoblotting analyses. To minimize circadian variations, the animals were housed in individual cages with food and water ad libitum and kept in an animal house at a constant temperature of 22 °C with a 12-h alternating light–dark cycle. The experiments were performed between 08:00 and 12:00 h. All efforts were made to minimize animal suffering and the number of animals used. The experimental procedures were approved by the Italian Ministry of Health, followed the guidelines for the treatment of animals of the International Association of the Study of Pain (Zimmermann, 1983) and were in line with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and with the Guidelines laid down by the NIH in the US regarding the care and use of animals for experimental procedures.
Experimental groups
The animals were subdivided into three surgical groups (each of six animals) both for immunohistochemistry and immunoblotting analyses. The first group was the control non-operated animals (naïve). The second group was the sham-operated animals; in the third group the left sciatic nerve was tied, producing a chronic constriction injury (CCI).
Surgical procedures
The rats were anesthetized by intraperitoneal injection of Zoletil (60 mg kg−1; Virbac, France), and the right sciatic nerve was exposed at the level of the mid-thigh by blunt dissection and separated from the adhering tissue immediately proximal to its trifurcation. Four ligatures were then loosely tied around the nerve at 1–2 mm distance using 4–0 chromic gut suture material according to the method described by Bennet & Xie (1988). In the sham-operated animals the left sciatic nerve was exposed at the same level, without ligature.
Behavioural tests
All behavioural testing was performed blind at established time points: day 0, the last session before operation, which represented the baseline value for the tests between pre- and post neuropathic injury, and 3, 7 and 14 days after surgery. Animals were acclimatized to the testing for 30–60 min before starting. The order of behavioural tests was therefore defined as follows: plantar and mechanical. Animals were left for 30 min undisturbed between each assay to habituate them to the testing environment.
Plantar (Hargreaves) test
Animals were placed in an acrylic box with glass-pane floor and the plantar surface of their hind paw was exposed to a beam of infrared radiant heat (Hargreaves et al. 1988). The paw withdrawal latencies were recorded twice per session, separated by a minimum interval of 5 min. Minimum and maximum cut-offs were assigned at 1 and 30 s, respectively. Again, paw withdrawals due to locomotion or weight shifting were not counted and the trials were repeated.
Von Frey test of mechanical threshold
Mechanical sensitivity of the hind paw was measured by determining withdrawal thresholds to the Von Frey filaments, according to Chaplan et al. (1994). The respective bending forces were in the range of 0.005–125.892 g. The animals were placed individually in a small plastic cage with an open wire mesh bottom. Before testing, the rats were left in the test cages for 15–20 min so that their grooming and exploratory behaviours ceased and all four paws were placed on the ground. Von Frey filaments were applied perpendicular to the planter surface of the paw with an upward force just sufficient to bend the microfilament. Paw withdrawals due to locomotion or weight shifting were not counted and such trials were repeated.
Immunohistochemical analysis
Fourteen days after the surgical procedures the animals were anesthetized with an intraperitoneal injection of Zoletil (60 mg kg−1) and transcardially perfused with saline followed by 1 L of 4% paraformaldehyde in phosphate-buffered saline (PBS, 0.1 m pH 7.4). After fixation, kidney, spinal cord (L4–L6), dorsal root ganglia (DRG) L4–L5–L6 corresponding to the sciatic afferent fibers and sciatic nerve proximal to the ligatures of each animal were removed, post-fixed in 4% paraformaldehyde in PBS for 2 h and cryoprotected overnight in 30% sucrose at 4 °C. The lumbar L4–L6 segment was determined using a dissecting microscope and measuring the distance between the points of entry of the most rostral and most caudal rootlets of the L4, L5 and L6 dorsal root. Frozen serial transverse sections (35 μm thick) were cut by cryostat (Leica 1900) and collected in PBS.
Some sections were Nissl-stained for morphological control. The sections were incubated in normal goat serum for immunoreactivity (10% in PBS containing 0.1% Triton X-100) for 30 min and then incubated in rabbit polyclonal primary antibodies against AQP1 and AQP2 (Chemicon, #AB3272, #AB3274; Temecula, CA, USA) diluted, respectively, 1 : 200 and 1 : 500 in PBS containing 3% normal goat serum and 0.1% Triton X-100, for 24 h at 4 °C. After incubation in the primary antibodies, the sections were sequentially incubated in biotinylated goat anti-rabbit immunoglobulins and avidin–biotin peroxidase complex (Vector Labs., Burlingame, CA, USA), according to the manufacturer’s instructions. The reaction product was visualized using 0.01% hydrogen peroxide and 0.05% 3,3-diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO, USA) as chromogen. The immunohistochemistry control was performed by omitting the primary antibody and incubating the sections with non-immune rabbit serum and with isotype-matched irrelevant rat IgGs as negative control. The positive controls using kidney slices showed the immunopositivity localized in renal collecting ducts.
Double immunofluorescence analysis
The sections were incubated in bovine serum albumin (BSA) 5% diluted in PBS containing 0.1% Triton X-100 for 30 min and then incubated in rabbit polyclonal primary antibody directed against AQP2 (1 : 200, Chemicon, #AB3274) mixed with mouse monoclonal primary antibodies NeuN (1 : 100, Chemicon, #MAB377), CD31 (1 : 50, #550300; BD Biosciences, Franklin Lakes, NJ, USA) and S100 (1 : 1000, Genetex, GTX11178, Irvine, CA, USA) diluted in PBS containing 5% BSA and 0.1% Triton X-100, for 24 h at 4 °C. After incubation in the primary antibodies, the sections were sequentially incubated with appropriated fluorescent secondary antibodies diluted in PBS 1 : 200 (anti-rabbit Alexa-Fluor 555, red fluorescent dye and anti-mouse Alexa-Fluor 588, green fluorescent dye; Invitrogen, Carlsbad, CA, USA).
Quantitative analyses of AQP1 and AQP2 expression
The immunohistochemical data of AQP1 and AQP2 expression were evaluated quantitatively. For the analysis, immunostaining intensity was evaluated blind, using an optical microscope (Olympus, Hamburg, Germany) at a final magnification of 20×. Digitally fixed images of slices were analyzed using an image analyzer (Image Pro-Plus, Milan, Italy) and were calculated as integrated optical density (IOD) for arbitrary areas (0.01 mm2), measuring the neurons with a nucleus in five fields with the same area for each section (Borsani et al. 2009). In particular, small, medium and large neurons were distinguished according to parameters reported by Study & Kral (1996). The data were pooled to give a mean value ± SD and a statistical analysis was applied to compare the results obtained from the different groups.
Immunoblotting analysis
Fourteen days after the surgical procedures the animals were killed; the spinal cord (lumbar L4–L6 segment) and dorsal root ganglia (DRG) L4–L5–L6 corresponding to the sciatic afferent fibers of each animal were removed and immediately frozen in liquid nitrogen and stored at −80 °C until use. The tissues were homogenized in a buffer system and centrifuged at 13 254 g at 4 °C for 15 min, and the supernatants were processed using immunoblotting analysis. Protein concentration was assessed using Albumin Standards (Pierce, Rockford, IL, USA) according to the manufacturer’s instruction. Twenty micrograms of the samples were analyzed by 10% SDS–PAGE and electro-transferred to a nitro-cellulose membrane (pore size 0.45 μm; BioRad) by wet blotting (100 V for 1 h). The membrane was blocked with 5% non-fat dry milk in Tris-buffered saline Tween-20 (TTBS) at 4 °C. After washing with TTBS, proteins were exposed overnight at 4 °C to rabbit polyclonal anti-AQP2 antibody (Chemicon #AB3274) diluted 1 : 1000. These were detected using a proper biotinylated secondary antibodies (Dakopatts, Glostrup, Denmark) and an avidin–peroxidase complex according to the manufacturer’s instructions (ABC kit; Dakopatts), with a solution of 0.05% DAB (3,3-diaminobenzedine tetrahydrochloride) and 0.03% hydrogen peroxide. For the quantitative analyses, the bands were evaluated as IOD, using Gel Pro 3.1 software, and the data obtained were statistically examined.
Statistical analysis
The results obtained for all analyses are presented as means ± SD and the statistical significance of differences among experimental groups was estimated using the anova test corrected by Bonferroni, with P<0.05 considered significant.
Results
Behavioural tests
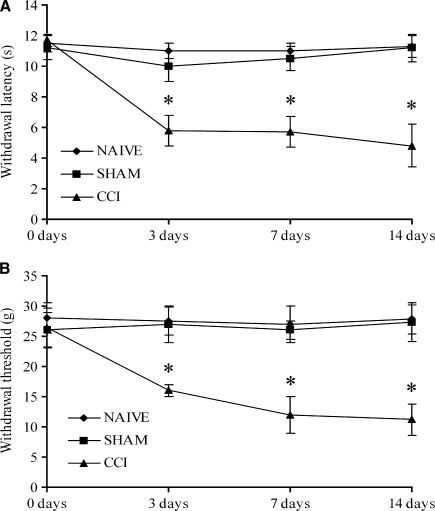
The behavioural testing was used to determine the allodynic state induced by CCI treatment. The evaluation was estimated before (0 days) and after surgery (3, 7, 14 days). The results obtained by plantar and Von Frey tests showed a similar response in naïve and sham-operated rats. Animals with CCI treatment had a decreased threshold, which was stable throughout the post-surgical period, as reported in Fig. 1.
Fig. 1.
Behavioural analysis of pain response to thermal and mechanical stimuli. (A) Withdrawal latency after plantar test in naïve, sham-operated and CCI rats. (B) Withdrawal threshold after Von Frey test in naïve, sham-operated and CCI rats. Data are expressed means ± SEM in six animals per group, *P<0.05 naïve and sham group.
Immunohistochemical and immunofluorescence analysis
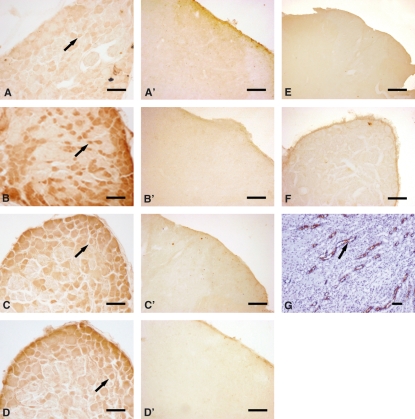
The immunohistochemical analyses of AQP2 expression in both DRG and spinal cord of naïve and sham-operated rats showed very weak and negative staining, respectively (Fig. 2A,A′). CCI treatment induced a significant increase of AQP2 expression restricted to DRG (Fig. 2B), whereas it showed a similar pattern to those observed for naïve and sham-operated rats in all laminae of spinal cord (Fig. 2B′).
Fig. 2.
Immunohistochemical analysis: AQP2 expression in the DRG (A) and spinal cord (A′) of naïve rats and in DRG (B) and spinal cord (B′) of CCI rats; AQP1 expression in the DRG (C) and spinal cord (C′) of naïve rat and in DRG (D) and spinal cord (D′) of CCI rats. Negative controls were shown in (E,F). Positive control (kidney) was shown in (G). Arrows indicate positive staining. Scale bar = 100 μm.
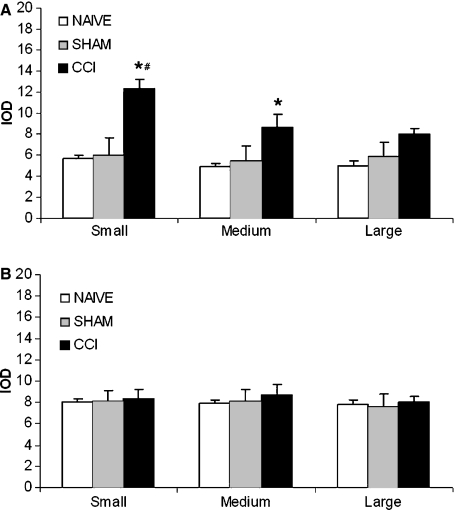
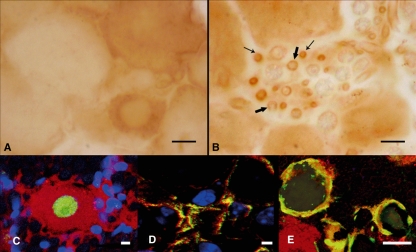
In DRG of CCI-treated animals, we observed immunopositive neurons, scattered throughout the ganglia, showing a variable degree of staining. In particular, the most intensely stained cells were small in diameter, as calculated by IOD (Fig. 3A). The staining was localized mainly in the cytoplasm, whereas the nucleus appeared unstained (Fig. 4A). Some Schwann cells were also weakly stained. Moreover, several small vessels scattered in ganglia were strongly immunopositive (Fig. 4B). Double immunofluorescence confirmed the data previously reported (Fig. 4C–E), showing a co-localization of AQP2 with CD31 (marker of endothelial cells) in small vessels (Fig. 4D) and AQP2 with S100 a marker of Schwann cells (Fig. 4E).
Fig. 3.
Quantitative analysis of AQP2 (A) and AQP1 (B) immunopositivity as IOD in naïve, sham-operated and CCI rat DRG. The values have been evaluated in each class of immunopositive neurons (small, medium and large sized). Data are expressed means ± SEM, *P<0.05 vs. naïve; #P<0.05 vs. medium and large sized neurons.
Fig. 4.
Immuohistochemical analysis of AQP2 expression in the DRG of CCI rats. (A) neuronal immunostaining was mainly intracytoplasmatic, whereas the nucleus appeared unstained. (B) Schwann cells were weakly stained (thick arrows) and several small vessels were strongly immunopositive (thin arrows). Double immunofluorescence analysis of AQP2 (red)/NeuN (green) (C) AQP2 (red)/CD31 (green) (D) and AQP2 (red)/S100 (green) expression in DRG of CCI rats. Nuclei were stained in blue. Scale bars (A,B) 10 μm, (C–E) 1 μm.
AQP1 immunopositivity both in DRG and spinal cord was also evaluated. Our results showed AQP1 immunoreactivity associated to cell bodies of a large number of neurons of DRG both in naïve/sham-operated (Fig. 2C) and CCI-treated rats (Fig. 2D). Quantitative analysis of AQP1 expression, as calculated by IOD, showed a similar degree of staining in small, medium and large neurons without any significant difference among the different experimental groups (Fig. 3B).
Similarly, we did not observe any AQP1-positive staining in the dorsal horn of spinal cord of any of the experimental groups (Fig. 2C′,D′). The immunohistochemical analysis of AQP1 and AQP2 expression in sciatic nerve showed negative staining in all experimental groups (Fig. 5).
Fig. 5.
Immunohistochemical analysis of AQP2 and AQP1 expression in the sciatic nerve proximal to the ligatures of naïve/sham (A and D, respectively) and CCI-treated rats (B and E, respectively). Negative controls are shown in (C, F). Scale bar = 100 μm.
Immunoblotting analysis
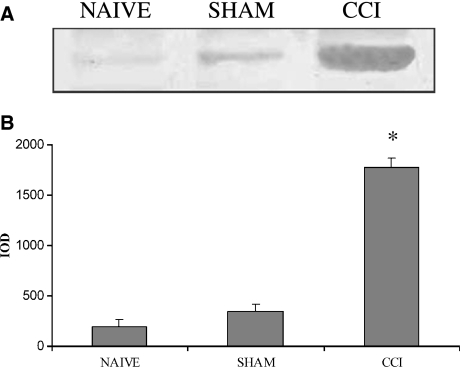
Immunoblotting analysis confirmed all data previously observed by immunohistochemistry. In particular, we did not observe any signals for AQP2 immunoblotting in the spinal cord (data not shown), whereas the limited AQP2 expression in DRG was supported by the presence of a protein migrating at ∼ 30 kDa, the predicted molecular weight of AQP2, only in CCI-treated rats. A weak signal was visible for both naïve and sham-operated rats (Fig. 6A). The quantitative assay of immunoblotting results is plotted in a graph reported in Fig. 6B.
Fig. 6.
Immunoblotting analysis of AQP2 expression. (A) 30 kDa bands corresponding to AQP2 isoform in rat DRG. (B) Quantitative evaluation of immunoblotting in naïve, sham and CCI rats. Data are expressed means ± SEM, *P<0.05 vs. naïve.
Discussion
To date, knowledge about AQP expression in the nervous system is limited only to certain members of this family. Among the AQP subtypes cloned in mammals only some of these could be related to pain transmission (Nielsen et al. 1993; Kobayashi et al. 2001; Verkman, 2002a; Amiry-Moghaddam & Ottersen, 2003). In particular, AQP1 identified in DRG nociceptive neurons as well as in afferent sensory nerve fibers both in the central and peripheral nervous system seem to contribute to the processing of pain messages, facilitating water transport in selected modalities of acute pain pathway (Oshio et al. 2006).
AQP2 is a vasopressin-regulated water channel of the distal nephron and is responsible for regulating the volume and osmolarity of urine (Sasaki et al. 1994, 2000). There is increasing evidence that it is expressed also in selected parts of the central and peripheral nervous system in human (Mobasheri et al. 2005). Nevertheless, no data about the expression of this protein in the rat nervous system and its modulation in pain pathway have been reported.
In this study, we used immunohistochemistry and immunoblotting analysis to investigate the expression and immunolocalization of AQP2 in adult rat spinal cord and DRG and to evaluate the possible modulation of its expression in a model of neuropathic pain (CCI) after 14 days, a time point in which the neuropathy is consolidated (Bennet & Xie, 1988).
Our results showed, for the first time, that AQP2 expression is induced only in DRG neurons by CCI treatment; in particular, its expression was higher in small-diameter sized neurons, i.e. those that co-label for markers of nociceptors (Oshio et al. 2006; Shields et al. 2007; Borsani et al. 2009). Conversely, its expression was very weak in the DRG of naïve and sham-operated rats and negative in spinal cord and sciatic nerve of all animals. The staining was localized mainly in the cytoplasm of small-diameter sized neurons, although AQPs are tetramers that are found in the lipid membrane (Manley et al. 2000, 2004). These observations led us to consider the distribution of AQPs in the cytoplasm with respect to the membrane compartment for an adaptation in chronic nociceptive conditions with acute pain status. These could be related to the results obtained in the trigeminal ganglia during acute inflammation, in which a redistribution of AQP2 in the neuronal membrane was observed (Borsani et al. 2009).
Although there is evidence that AQP2 mRNA is also present in the human spinal cord (Mobasheri et al. 2005) our results showed AQP2 staining only in DRG, not in the lumbar spinal cord. This pattern of expression was different from the results obtained in other studies in which AQP1 expression was found in small afferent sensory nerve fibers in both the central and peripheral nervous system (Oshio et al. 2006; Shields et al. 2007). This might suggest that in our experimental model, AQP2 is a water-channel protein that is activated and expressed at a peripheral but not a central level, underlining a different modulation in different parts of the nervous system. So, although AQPs could be involved in cellular processes characterized by net ion influx (such as the transduction of signal injury, nerve conduction and synaptic transmission), the functional significance of water channels in sensory neurons needs to be established.
Moreover, we did not observe any change in AQP1 expression or distribution after CCI treatment either in the spinal cord or DRG that could be due to the different animal species and/or experimental model used. Therefore, our results would suggest that AQP2, and not AQP1, is increased by CCI treatment. Nevertheless, because previous studies investigated only AQP1 expression in a model of complete axotomy, which does not have an inflammatory component, it is not known whether a similar decrease in AQP2 would occur in that experimental model.
The exact mechanism by which AQP2 is enhanced is not clear. It is known that this protein translocates in response to arginine vasopressin (AVP) in the renal collecting ducts (Kwon et al. 2001) and some authors reported the presence of AVP and its receptor (V1) in DRG (Horn & Lightman, 1987; Kai-Kai & Che, 1995). In particular, there is evidence that AVP induces hyper-polarization in the membrane of most DRG neurons, which might be caused by K(+) outflow mediated by V1 receptor, and that the membrane conductance of the DRG neurons increased following AVP application (Horn & Lightman, 1987; Hu et al. 2004). Moreover, other results indicate a role of AVP in the regulation of anti-nociception in rats in certain components of the central nervous system but not at the spinal cord level (Yang et al. 2006a,b;).
It is important to consider that CCI itself causes local inflammation (Levy & Zochodne, 1998; Martucci et al. 2008) and Wallerian degeneration with loss of target-derived trophic support (Coleman et al. 1998; Martucci et al. 2008). It is also accompanied by an altered environment so that osmotic changes, rather than nociceptor activation, could be the cause of increased AQP2 expression. Regarding this point, immunohistochemical analysis has enabled us to observe that CCI treatment does not induce AQP2 and AQP1 expression at the CCI injury site but does increase AQP2 expression in some small vessels of DRG. This could be due to the development of peripheral edema associated with this pain model (La Rana et al. 2008). In addition, Kobayashi et al. (2001) found the expression of AQP mRNA in rat cerebral microvessels, suggesting that this family is involved in the regulation of water transport between blood and nervous components.
To fully understand the contribution of AQP2 both in nociceptive processing and in inflammatory and edematous responses to CCI treatment, it will be necessary to generate an inducible deletion that can be triggered in the adult, or to develop a selective blocker of the channel which can be delivered directly to the primary afferent neurons.
Acknowledgments
We would like to thank Dr. Foglio for her technical assistance and Dr. Coates for the English revision. This work was supported by local institutional grants (ex 60% 2007).
References
- Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci. 2003;4:991–1001. doi: 10.1038/nrn1252. [DOI] [PubMed] [Google Scholar]
- Badaut J, Hirt L, Granziera C, et al. Astrocyte-specific expression of aquaporin-9 in mouse brain is increased after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:477–482. doi: 10.1097/00004647-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Badaut J, Petit JM, Brunet JF, et al. Distribution of Aquaporin 9 in the adult rat brain: preferential expression in catecholaminergic neurons and in glia cells. Neuroscience. 2004;128:27–38. doi: 10.1016/j.neuroscience.2004.05.042. [DOI] [PubMed] [Google Scholar]
- Bennet GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Borgnia M, Nielsen S, Engel A, et al. Cellular and molecular biology of the aquaporin water channels. Annu Rev Biochem. 1999;68:425–458. doi: 10.1146/annurev.biochem.68.1.425. [DOI] [PubMed] [Google Scholar]
- Borsani E, Bernardi S, Albertini R, et al. Alterations of AQP2 expression in trigeminal ganglia in a murine inflammation model. Neurosci Lett. 2009;449:183–188. doi: 10.1016/j.neulet.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Conforti L, Buckmaster EA, et al. An 85-kb tandem triplication in the slow Wallerian degeneration (Wlds) mouse. Proc Natl Acad Sci U S A. 1998;95:9985–9990. doi: 10.1073/pnas.95.17.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker BM, Smith BL, Kuhajda FP, et al. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J Biol Chem. 1988;263:15634–15642. [PubMed] [Google Scholar]
- Frigeri A, Gropper MA, Turk CW, et al. Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proc Natl Acad Sci U S A. 1995;92:4328–4331. doi: 10.1073/pnas.92.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, He C, Fang X, et al. Localization of Aquaporin-1 water channel in glia cells of the human peripheral nervous system. Glia. 2006;53:783–787. doi: 10.1002/glia.20336. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hitchcock E. Osmolytic neurolysis for intractable facial pain. Lancet. 1969;1:434–436. doi: 10.1016/s0140-6736(69)91479-2. [DOI] [PubMed] [Google Scholar]
- Horn AM, Lightman SL. Vasopressin-induced turnover of phosphatidylinositol in the sensory nervous system of the rat. Exp Brain Res. 1987;68:299–304. doi: 10.1007/BF00248795. [DOI] [PubMed] [Google Scholar]
- Hu HY, Sun ZP, Zhao YM, et al. Effect of arginine vasopressin on membrane potential of dorsal root ganglion neurons in rats. Sheng Li Xue Bao. 2004;56:107–111. [PubMed] [Google Scholar]
- Ishibashi K. Aquaporin subfamily with unusual NPA boxes. Biochim Biophys Acta. 2006;1758:989–993. doi: 10.1016/j.bbamem.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Jawett DL, King JS. Conduction block of monkey dorsal rootlets by water and hypertonic saline solution. Exp Neurol. 1971;33:225–237. doi: 10.1016/0014-4886(71)90116-6. [DOI] [PubMed] [Google Scholar]
- Kai-Kai MA, Che YM. Distribution of arginine-vasopressin in the trigeminal, dorsal root ganglia and spinal cord of the rat; depletion by capsaicin. Comp Biochem Physiol A Physiol. 1995;110:71–78. doi: 10.1016/0300-9629(94)00145-j. [DOI] [PubMed] [Google Scholar]
- King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5:687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Minami S, Itoh S, et al. Aquaporin subtypes in rat cerebral microvessels. Neurosci Lett. 2001;297:163–166. doi: 10.1016/s0304-3940(00)01705-5. [DOI] [PubMed] [Google Scholar]
- Kwon TH, Hager H, Nejsum LN, et al. Physiology and pathophysiology of renal aquaporins. Semin Nephrol. 2001;21:231–238. doi: 10.1053/snep.2001.21647. [DOI] [PubMed] [Google Scholar]
- La Rana G, Russo R, D’Agostino G, et al. AM404, an anandamide transport inhibitor, reduces plasma extravasation in a model of neuropathic pain in rat: role for cannabinoid receptors. Neuropharmacology. 2008;54:521–529. doi: 10.1016/j.neuropharm.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Levy D, Zochodne DW. Local nitric oxide synthase activity in a model of neuropathic pain. Eur J Pain. 1998;10:1846–1855. doi: 10.1046/j.1460-9568.1998.00186.x. [DOI] [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, et al. Aquaporin-4 deletion in mice reduces brain edema following acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Manley GT, Binder DK, Papadopoulos MC, et al. New insight into water transport and edema in the central nervous system from phenotype analysis of AQP4-null mice. Neuroscience. 2004;129:981–989. doi: 10.1016/j.neuroscience.2004.06.088. [DOI] [PubMed] [Google Scholar]
- Martucci C, Trovato AE, Costa B, et al. The purinergic antagonist PPADS reduces pain related behaviours and interleukin-1beta, interleukin-6, iNOS and nNOS overproduction in central and peripheral nervous system after peripheral neuropathy in mice. Pain. 2008;137:81–95. doi: 10.1016/j.pain.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Mobasheri A, Wray S, Marples D. Distribution of AQP2 and AQP3 water channels in human tissue microarrays. J Mol Hist. 2005;36:1–14. doi: 10.1007/s10735-004-2633-4. [DOI] [PubMed] [Google Scholar]
- Nesic O, Lee J, Johonson KM, et al. Transcriptional profiling of spinal cord injury-induced central neuropathic pain. J Neurochem. 2005;95:998–1014. doi: 10.1111/j.1471-4159.2005.03462.x. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Smith BL, Christensen EI, et al. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci U S A. 1993;90:7275–7279. doi: 10.1073/pnas.90.15.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Nagelhus EA, Amiry-Moghaddam M, et al. Specialized membrane domains for water transport in glia cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshio K, Binder DK, Yang B, et al. Expression of aquaporin water channels in mouse spinal cord. Neuroscience. 2004;127:685–693. doi: 10.1016/j.neuroscience.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Oshio K, Watanabe H, Song Y, et al. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J. 2005;19:76–78. doi: 10.1096/fj.04-1711fje. [DOI] [PubMed] [Google Scholar]
- Oshio K, Watanabe H, Yan D, et al. Impaired pain sensation in mice lacking Aquaporin-1 water channels. Biochem Biophys Res Commun. 2006;341:1022–1028. doi: 10.1016/j.bbrc.2006.01.062. [DOI] [PubMed] [Google Scholar]
- Richards JS, Meredith RL, Nepomuceno C, et al. Psycho-social aspects of chronic pain in spinal cord injury. Pain. 1980;8:355–366. doi: 10.1016/0304-3959(80)90079-2. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulus MC, Davies DC, et al. Aquaporin-4 expression is increased in oedematous human brain tumours. J Neurol Neurosurg Psychiatry. 2002;72:262–265. doi: 10.1136/jnnp.72.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Fushimi K, Saito H, et al. Cloning, characterization, and chromosomal mapping of human aquaporin of collecting duct. J Clin Invest. 1994;93:1250–1256. doi: 10.1172/JCI117079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Kuwahara M, Yamashita Y, et al. Structure and function of AQP2. Nephrol Dial Transplant. 2000;15:21–22. doi: 10.1093/ndt/15.suppl_6.21. [DOI] [PubMed] [Google Scholar]
- Schafers M, Geis C, Svensson CI, et al. Selective increase of tumour necrosis factor-alpha in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. Eur J Neurosci. 2003;17:791–804. doi: 10.1046/j.1460-9568.2003.02504.x. [DOI] [PubMed] [Google Scholar]
- Shields SD, Mazario J, Skinner K, et al. Anatomical and functional analysis of aquaporin 1, a water channel in primary afferent neurons. Pain. 2007;131:8–20. doi: 10.1016/j.pain.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Solenov EI, Vetrivel L, Oshio K, et al. Optical measurement of swelling and water transport in spinal cord slices from aquaporin null mice. J Neurosci Methods. 2002;113:85–90. doi: 10.1016/s0165-0270(01)00481-2. [DOI] [PubMed] [Google Scholar]
- Stevens AL, Breton S, Gustafson CE, et al. Aquaporin-2 is a vasopressin-independent, constitutive apical membrane protein in rat vas deferens. Am J Physiol Cell Physiol. 2000;278:791–802. doi: 10.1152/ajpcell.2000.278.4.C791. [DOI] [PubMed] [Google Scholar]
- Study RE, Kral MG. Spontaneous action potential activity in isolated dorsal root ganglion neurons from rats with a painful neuropathy. Pain. 1996;65:235–242. doi: 10.1016/0304-3959(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Verkman AS. Physiological importance of aquaporin water channels. Ann Med. 2002a;34:192–200. [PubMed] [Google Scholar]
- Verkman AS. Aquaporin water channels and endothelial cell function. J Anat. 2002b;200:617–627. doi: 10.1046/j.1469-7580.2002.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakata K, Horoaki Y, Ishibashi K, et al. Aquaporin-11 containing a divergent NPA motif has normal water channel activity. Biochim Biophys Acta. 2007;1768:688–693. doi: 10.1016/j.bbamem.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Yang J, Chen JM, Liu WY, et al. Arginine vasopressin in the caudate nucleus plays an antinociceptive role in the rat. Life Sci. 2006a;79:2086–2090. doi: 10.1016/j.lfs.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Yang J, Song CY, Liu WY, et al. Only through the brain nuclei, arginine vasopressin regulates antinociception in the rat. Peptides. 2006b;27:3341–3346. doi: 10.1016/j.peptides.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigation of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]