Abstract
There is increasing evidence suggesting a wider biological role of erythropoietin (Epo) and Epo receptor (EpoR) not related to erythropoiesis, such as the detection of EpoR in other cells, i.e. polymorphonuclear leukocytes, megakaryocytes, endothelial, myocardial and neural cells. In this study, by using a mouse model (designated tg6) that constitutively overexpresses human Epo in an oxygen-independent manner, we have investigated mast cell and macrophage number and distribution in duodenal mucosa using immunohistochemical, morphometric and image analysis methods. The results showed that tryptase-positive mast cells and BM8-positive macrophages were more numerous in duodenal mucosa specimens of tg6 mice compared with wild-type mice. Moreover, whereas in wild-type specimens both mast cells and macrophages were generally scattered throughout the villus, in tg6 specimens they were aligned along the axis of the villus. Morphometric analysis confirms this observation, and the quantitative analysis of the spatial distribution of the cells in duodenal villi indicated that in both wild-type and tg6 groups the macrophage and mast cell distribution was characterized by significant deviations from randomness. In addition, an increased number of c-kit-positive cells have been identified in the villus axis of tg6 mice, indicating an expanded compartment of mast cell precursors in the intestinal mucosa of these animals. Finally, we have also demonstrated that in tg6 specimens the number of duodenal epithelial cells positive for Epo were significantly higher as compared to wild type. Overall, these data confirm that Epo, acting as a general stimulator of the hemopoietic compartment, is able to induce an expansion of two effectors of the immune response, mast cells and macrophages, in a specific peripheral site, the duodenal mucosa, in the tg6 mouse experimental model.
Keywords: duodenum, erythropoietin, erythropoietin receptor, macrophages, mast cells, tg6 mouse
Introduction
Erythropoietin (Epo) is a low-molecular weight glycoprotein hormone stimulator of erythropoiesis produced in the fetal liver and subsequently in the adult kidney (Tilbrook & Klinken, 1999). Epo exerts its action through its specific receptor (EpoR), a member of the cytokine receptor superfamily, which is mainly expressed on the erythroid colony-forming unit (Tilbrook & Klinken, 1999). The importance of the Epo-EpoR system in primary and definitive erythropoiesis has been determined by generating lines of mutant mice lacking either the Epo or the EpoR gene (Wu et al. 1995; Lin et al. 1996).
There is increasing evidence suggesting a wider biological role of Epo-EpoR not related to erythropoiesis, such as the detection of EpoR in other cells, i.e. polymorphonuclear leukocytes, megakaryocytes, endothelial, myocardial and neural cells (Buemi et al. 2003; Fraser et al. 1989; Sela et al. 2001; Anagnostou et al. 1994; Ribatti et al. 1999).
A mouse model (designated tg6) that constitutively overexpresses human Epo in an oxygen-independent manner has been generated (Ruschitzka et al. 2000; Wagner et al. 2001; Vogel et al. 2003). Transgenic mice show a 10- to 12-fold elevation of Epo plasma levels leading to hematocrit values of up to 0.9. Considering that excessive erythrocytosis has been observed in various human diseases, such as polycythemia vera, by using this experimental model it has been possible to investigate the effect of an increase in red blood cell number on various organs. Ogunshola et al. (2005) demonstrated that brain vessel density of tg6 mice was significantly reduced and vessel diameter significantly increased compared with wild-type mice. Moreover, tg6 brain vascular endothelial cells appeared to be activated, with increased luminal protrusions. Heinicke et al. (2006) demonstrated degenerative processes in the liver and kidney of tg6 mice, characterized by increased vascular permeability, chronic progressive inflammation (characterized by lymphoid cell infiltrations) and hemosiderin deposition. Moreover, most of the tg6 mice showed severe fiber degeneration of the sciatic nerve, decreased number of neuromuscular junctions, and degeneration of skeletal muscle fibers.
More recently, by using the same experimental model, Katz et al. (2007) demonstrated that Epo affects both T and B populations, and augments B-cell responses, manifested in endogenous normal polyclonal immunoglobulin production, lipopolysaccharide (LPS)-induced proliferation of splenocytes, as well as specific antibody response to antigen stimulation.
In this study, using the tg6 model, we have investigated the impact of excessive Epo production on mast cells and macrophages in duodenal mucosa. For this purpose we have studied the immunohistochemical expression of tryptase, a specific mast cell marker, BM8, a specific macrophage marker, c-kit, an indicator of mast cells and mast cell precursors, and Epo-EpoR in duodenal mucosa of tg6 and wild-type mice. Furthermore, we have analyzed their spatial distribution utilizing an image analysis system and a mathematical model as a quantitative approach.
Materials and methods
Mice
The tg mice were generated by pronuclear microinjection of the full-length human Epo cDNA driven by the human platelet-derived growth factor (PDFG) B-chain promoter as previously described (Ruschitzka et al. 2000). The resulting tg mouse line TgN (PDGFBEPO) 321ZbZ (termed tg6) showed increased Epo levels in plasma and brain (Wiessner et al. 2001) and was bred by mating hemizygous males to wild-type C57BL/6 females. Half of the offspring were hemizygous for the transgene (n = 15), and the other half were wild-type (n = 15) and served as controls. Male and female mice were used at 4–5 months of age.
Immunohistochemistry
After fixation in 10% (w/v) phosphate-buffered formaldehyde solution, the tissues were processed, trimmed, embedded in paraffin wax, sectioned at 4 μm thickness. Two murine monoclonal antibodies (MAb) against the mast cell marker tryptase (MAb AA1, Dako, Glostrup, Denmark) and the pan-macrophage marker BM8 (Bachem Biosciences King of Prussia, PA, USA; Malorny et al. 1986), and three rabbit polyclonal antibodies against CD117/c-kit (Dako), Epo and EpoR (sc-73963 and sc-5624, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and were used. In brief, sections were collected on 3-amino-propyl-triethoxysilane-coated slides, deparaffinized by the xylene-ethanol sequence, rehydrated in a graded ethanol scale and in Tris-buffered saline (TBS, pH 7.6), and incubated overnight at 4 °C with the MAbs (diluted 1 : 100 in TBS) and the polyclonal antibodies (diluted 1 : 200 in TBS) after prior antigen retrieval by enzymatic digestion with Ficin (Sigma, St. Louis, MO, USA) for 30 min at room temperature. The immunoreaction was performed with alkaline phosphatase anti-alkaline phosphatase (APAAP; Dako) and Fast Red as chromogen, followed by hematoxylin counterstaining. Tissue sections processed in the absence of primary or secondary antibody, or with a blocking antibody (Dako) displayed no appreciable nonspecific stain.
Cell counts
Tryptase-positive mast cells, BM8-positive macrophages, c-kit-, Epo- and EpoR-positive cells counts were carried out in well-orientated sections cut perpendicularly to the mucosa. The counts were performed on a Zeiss Axioskop light microscope (Carl Zeiss Italy, Arese, Italy), using a micrometer grid fitted in a ×10 eyepiece at ×100 objective magnification. In all, 15–25 contiguous, nonoverlapping rectangular areas (each area measured 0.0117 mm2) of three sections per sample for wild-type and tg6 specimens, were examined. Mean, median and standard deviation were determined for each group of samples.
Image analysis methods
Computer-assisted image analysis was performed to characterize the spatial distribution of the cells in the villi of the duodenum, starting from 30 images obtained from 10 wild-type and 10 tg6 specimens of these structures taken at a primary magnification of ×200. Images were analyzed according to a previously detailed procedure (see Guidolin et al. 2006). In brief, the study area within each image was defined as the area corresponding to the villus profile and the central axis of the villus was interactively traced. To discriminate the cells, a colour thresholding procedure was applied, followed by further processing steps to remove artefacts and provide a deagglomeration of the cell profiles. The distance of each profile from the central axis of the villus was then estimated.
In the same study area, 100 random (Poisson) point patterns were finally computer generated. Each pattern had a number of points equal to the number of observed cell profiles. They provided Monte Carlo estimates (Besag & Diggle, 1997) of the distances from the central axis in the case of a random spatial distribution of the cells in the villus.
To characterize the relationship between the cells and the central axis of the villus an approach based on ‘spatial statistics’ (Diggle, 1983) was used. It involves the calculation of the cumulative frequency distribution [G(d)] of all the observed cell-to-axis distances. To interpret spatial relationship of the cells to the central axis statistically, G(d) has to be compared with the value [G0(d)] estimated on the random (Poisson) point patterns (Ripley, 1979). If G(d) is significantly greater than G0(d) for any range of d, then the cells are closer to the center of the villus than expected by chance. Conversely, if G(d) is significantly lower than G0(d), then short cell-to-central axis distances are less frequent than expected by chance, i.e. the cells tend to place themselves at the periphery of the villus (Philimonenko et al. 2000).
Statistics
The significance of differences in cell counts between tg6 mice and their wild-type littermates was assessed using prism software. Values are means ± SD. Statistical significance (P < 0.05%) was calculated using an unpaired t-test of independent samples. The public domain statistical software r 2.6.0 (R Development Core Team, 2005) was used to obtain estimators of G(d) and G0(d) (together with the 95% confidence limits) from the observed and simulated cell-to-central axis distances, respectively.
Results
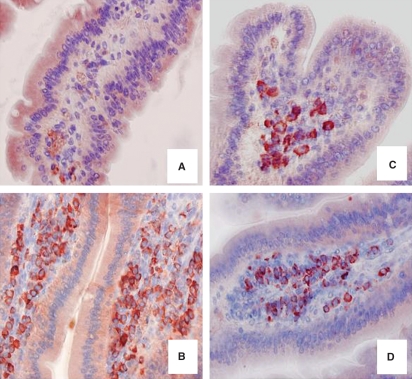
As shown in Fig. 1, tryptase-positive mast cells and, respectively, BM8-positive macrophages were more numerous in duodenal mucosa specimens of tg6 mice compared with wild-type mice. Whereas in wild-type specimens both mast cells and macrophages were generally scattered throughout the villus, in tg6 specimens they were aligned along the axis of the villus.
Fig. 1.
Histochemical pictures of tryptase-positive mast cells (A,B), BM8-positive macrophages (C,D) in duodenum of wild-type (A, C) and tg6 (B, D) mice. Note a higher number of mast cells in tg6 specimens. Original magnification: A–D, ×200.
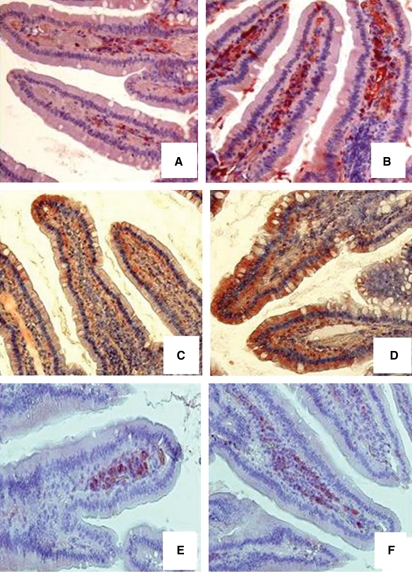
Moreover, the density of c-kit-positive mast cell precursors in the lamina propria of Epo-overexpressing tg6 mice is higher than in wild-type mice; as well, the Epo-positive epithelial and stromal cells and EpoR-positive stromal cells were more numerous in tg6 as compared to wild-type specimens (Fig. 2). Morphometric analysis confirmed these findings (Table 1). There were significant differences (P < 0.001) in all counts between the two groups.
Fig. 2.
Histochemical pictures of c-kit-positive cells in duodenum of wild-type (A) and tg6 (B) mice; Epo-positive epithelial and stromal cells (C,D) and EpoR-positive stromal cells (E,F) in duodenum of wild-type (C,E) and tg6 (D,F) mice. Note a higher number of c-kit-positive cells and an higher number of Epo- and EpoR-positive cells in tg6 specimens. Original magnification: A,B, ×200; C–F, ×160.
Table 1.
Cell counts in the duodenum of wild-type and tg6 mice
| Wild type (n = 15) | tg6 (n = 15) | |
|---|---|---|
| Mast cells | 6 ± 2* | 44 ± 7** |
| C-kit positive cells | 12 ± 3 | 60 ± 12** |
| Macrophages | 12 ± 5 | 36 ± 6** |
| Epithelial cells positive for Epo | 8 ± 4 | 32 ± 7** |
| Stromal cells positive for Epo | 4 ± 1 | 14 ± 3** |
| Stromal cells positive for EpoR | 7 ± 2 | 40 ± 8** |
The values are expressed per unit area (0.0117 mm2).
P < 0.001 vs. wild type.
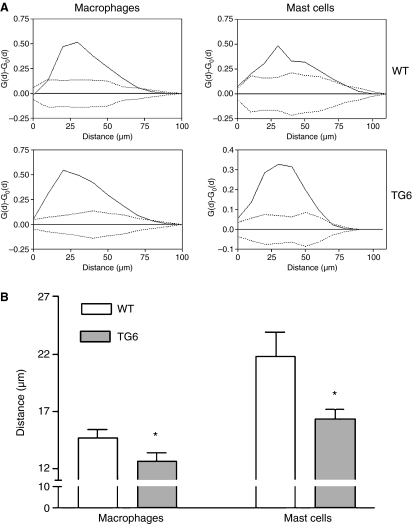
The quantitative analysis of the spatial distribution of the cells in duodenal villi indicated that in both wild-type and tg6 groups the macrophage and mast cell distribution was characterized by significant deviations from randomness. In particular, the cell patterns were in both cases preferentially clumped around the central axis of the villus, as indicated by a G(d) significantly higher than expected under complete spatial randomness, expression of a trend towards clustering (Fig. 3A). However, when compared to the samples from wild-type mice, macrophages from tg6 mice were significantly (P < 0.05) closer to the central axis of the villi, as indicated by their lower mean cell-to-axis distance (Fig. 3B).
Fig. 3.
Morphometric evaluation of the spatial distribution of macrophages and mast cells in the central axis of duodenal villus. (A) Representative results of the analysis based on spatial statistics. In each plot the difference G(d)–Go(d) is drawn, together with the 95% confidence limits around the value 0 for such a difference. The spatial relationship between cells and the central axis of the villus has been reported. Solid lines indicate the difference between the observed distribution of cell-to-axis distances [G(d)] and the estimated distribution [Go(d)] under the hypothesis of complete spatial randomness (CSR). Dotted lines indicate the 95% confidence envelope for CSR. In both groups both cell types are localized around the central axis of the villi, as indicated by a frequency of short distances significantly higher than expected by chance. (B) Mean distances between cells and the central axis of the villi. When compared to the samples from wild-type mice, macrophages from tg6 mice are significantly (P < 0.05) closer to the central axis of the villi, as indicated by their lower mean cell-to-axis distance. Error bars are SD. *P < 0.05 (two-sample Student’s t-test).
Discussion
In this study we have demonstrated that in the duodenal mucosa of Epo-overexpressing tg6 mice, mast cells and macrophages are more numerous as compared with wild-type mice. Mast cells and macrophages are inflammatory cells which exert fundamental functions in the physiopathology of the intestinal mucosa. Mast cells are primarily located in the lamina propria, where under normal conditions they account for 2–3% of cells (Bischoff et al. 1996). Macrophages are present in the small intestine in a subepithelial position, where they can interact directly with bacteria in the lumen and detect any microbes or microbial products that may cross the epithelial monolayer (Platt & Mowat, 2008). Thus, both mast cells and macrophages play a crucial role in the processes of local defence as well as tissue homeostasis and remodelling.
The increased density of mast cells and macrophages in the villous lamina propria of Epo-overexpressing tg6 mice is an intriguing finding. The unique condition we have documented here is probably due to an increase in mast cell and macrophage production. This is certainly true for mast cells. In fact, it emerges from our study that the density of c-kit-positive mast cell precursors in the lamina propria of Epo-overexpressing tg6 mice is higher than in wild-type mice (60 ± 12 vs. 12 ± 3 cells per unit area, P < 0.001) Remarkably, the number of c-kit-positive cells in the lamina propria of Epo-overexpressing tg6 mice is higher than the number of tryptase-positive mast cells in the same transgenic animals, indicating that the increased production of Epo causes an expansion of the compartment of mast cell precursors in the intestinal lamina propria (60 ± 12 vs. 44 ± 7, P < 0.001). These data are in keeping with literature data, which indicate a proliferative expansion and an increased activity of different cell lineages upon Epo stimulation. In fact, it has been demonstrated previously in both Epo-treated wild-type and tg6 mice that Epo induces a higher level of splenocyte proliferative response induced by LPS (Katz et al. 2007). Moreover, these mice had increased production of anti-dinitrophenyl (DNP) antibodies following immunization with DNP-keyhole limpet hemocyanin. Finally, Epo-treated mice showed an enhanced immune response to clinically relevant hepatitis B surface antigen. More recently, in vivo experiments in Epo-injected mice and in tg6 mice showed an increased splenic dendritic cell population with a higher cell surface expression of CD80 and CD86 (Lifshitz et al. 2009). Overall, these data suggest a potential use of Epo as an immunomodulator. Bogdanova et al. (2007) demonstrated that compared with wild-type animals, the erythrocyte lifespan is 70% shorter in tg6 mice. Transgenic mice have a younger erythrocyte population which harbors characteristics of accelerated aging. Moreover, in vitro experiments showed that tg6 macrophages are more active than wild-type macrophages and that tg6 erythrocytes are more attractive for macrophages than wild-type ones. Accelerated erythrocyte aging together with an increased number and activity of macrophages resulted in an enhanced erythrocyte clearance. As a consequence of an enhanced elimination of older erythrocytes, whole blood viscosity decreases. Interestingly, several features of tg6 mice, including excessive erythrocytosis and splenomegaly, resemble the symptoms observed in patients suffering from polycythemia vera, in which erythrocytosis is responsible for severe thrombotic events that eventually lead to death (Spivak, 2002).
Both mast cells and macrophages originate from bone marrow precursors, circulate in the bloodstream as distinct elements and reach their complete differentiation once they have entered peripheral homing tissues. Remarkably, mast cells are the only bone marrow-derived cells that maintain the stem cell factor (SCF) receptor c-kit when completely differentiated. SCF represents the main survival and developmental factor for mast cells (Broudy, 1997). Lack of expression of a functional c-kit receptor, as occurs in genetically mast cell-deficient WBB6F1-c-kitW-c-kitW-v mice (W/Wv mice), results in a virtual absence of tissue mast cells (Kitamura et al. 1978). Of note, the Vickid mouse mutant that, because of the lack of c-kit, exhibits hematopoietic defects causing perinatal death, could be rescued by breeding with the Epo-overexpressing tg6 mice (Waskow & Rodewald, 2002). Indeed, introduction of an Epo transgene into a c-kitW/W background yielded Epo-transgenic c-kitW/W mice, about half of which survived long term (Waskow et al. 2002). These mutant mice, termed WEPO, demonstrate that Epo receptor signals can partially by-pass the strict requirement for c-kit signalling in erythropoiesis in the absence of c-kit in vivo (Waskow et al. 2004). Interestingly, former investigations demonstrated that Epo down-regulates levels of c-kit mRNA expressed by mast cells and stem cell progenitors (Welham & Schrader, 1991).
These data are relevant in the light of our findings that display a significant increase of mast cell density in the villus axis of Epo-overexpressing tg6 mice. This result suggests that, under certain conditions, Epo signalling may play a role in the development of mast cell lineage as well. We do not know, at present, what these conditions are. Some local factors, however, are likely to intervene, because the expansion of mast cell population was not generalized but was limited to the mucosa of the small intestine. As previously stated, in the present study we have found an increased population of c-kit-positive mast cell precursors localized in the villus lamina propria of Epo-overexpressing tg6 mice. This suggests that the increased levels of intestinal mast cells observed in these animals are the result of an overproduction of these cells. Remarkably, we did not find specific signs of inflammation in the duodenum of Epo-overexpressing tg6 mice. In particular, no distinct neutrophil, eosinophil or lymphocyte infiltration of the villus lamina propria and epithelium could be identified. This would exclude any significant allergic response or local inflammation as the causative agent of the increased density of mast cells and macrophages observed in tg6 transgenic mice.
In the past, Isogai et al. (2006) demonstrated that dermal mast cells express EpoR, by double staining with anti-EpoR antibody and anti-tryptase antibody. In addition, immunoreactivity for EpoR has been demonstrated in the widespread population of tissue macrophages in the developing human fetus (Juul et al. 1998). These findings have been questioned by Elliott et al. (1996), who claimed that the C-20 (sc 695) anti-EpoR antibody used in these studies is a nonselective marker because it gives a false–positive signal in the absence of EpoR. In our present study, we have used a different antibody against EpoR and, alongside this, we have immunostained our samples with an anti-Epo antibody for Epo detection. Our data indicate a significant increase of EpoR positivity in the stromal cells of the villus axis in Epo-overexpressing tg6 mice (40 ± 8 vs. 7 ± 2 cells per unit area, P < 0.001). Most remarkably, the increased levels of EpoR are accompanied by a parallel expansion of the population of Epo-positive stromal cells localized in the villus axis in the same animals (14 ± 3 vs. 4 ± 1 cells per unit area, P < 0.001). In addition, we have found increased values of Epo positivity in the villus-lining epithelial cell population of tg6 transgenic mice (32 ± 7 vs. 8 ± 4 cells per unit area, P < 0.001). These data suggest that an expanded population of stromal cells in the villus axis expresses the EpoR in tg6 mice and that stromal cells in the same anatomical site contain higher levels of Epo in their cytoplasm. Given the high density of both mast cells and macrophages in the villus lamina propria of Tg6 mice, we cautiously conclude that a percentage of Epo-positive and EpoR-positive stromal cells are realistic candidates to represent both cell types. Interestingly, former immunohistochemical studies for Epo detection in capillary hemangioblastomas have demonstrated positive staining for mast cells (Kamitani et al. 1987; Tachibana et al. 1991).
It should also be emphasized that Epo has an intrinsic anti-apoptotic activity. For instance, it has recently been reported that Epo protects cardiomyocytes from ischemic injury (Hirose et al. 2007) and has a trophic effect on neurons under ischemic and degenerative conditions (Sakanaka et al. 1998). EpoR have been identified in various brain regions, both on capillary endothelial cells as well as neuronal cells (Digicalylioglu et al. 1995). Under hypoxic conditions, EpoR is synthesized in astrocytes and neurons (Bernaudin et al. 2000). Thus, we speculate that the increased amount of mast cells and macrophages in the villus lamina propria may in part depend upon the pro-surviving function linked to Epo overexpression.
In conclusion, these data confirm that Epo, acting as a general stimulator of the hemopoietic compartment, is able to induce an expansion of two effectors of the immune response, such as mast cells and macrophages, in specific peripheral sites, such as duodenal mucosa, as occurs in the tg6 mouse experimental model.
Acknowledgments
Supported in part by MIUR (PRIN 2007), Rome, and Fondazione Cassa di Risparmio di Puglia, Bari, Italy.
References
- Anagnostou A, Liu Z, Steiner M, et al. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci USA. 1994;91:3974–3978. doi: 10.1073/pnas.91.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernaudin M, Bellail A, Marti HH, et al. Neurons and astrocytes express EPO mRNA: oxygen-sensing mechanisms that involve the redox-state of the brain. Glia. 2000;30:271–278. [PubMed] [Google Scholar]
- Besag J, Diggle PJ. Simple Monte Carlo tests for spatial pattern. Appl Stat. 1997;26:327–333. [Google Scholar]
- Bischoff SC, Machel H, Stolte M, et al. Quantitative assessment of intestinal eosinophils and mast cells in inflammatory bowel disease. Histopathology. 1996;28:1–13. doi: 10.1046/j.1365-2559.1996.262309.x. [DOI] [PubMed] [Google Scholar]
- Bogdanova A, Mihov D, Lutz H, et al. Enhanced erytro-phagocytosis in polycythemic mice overexpressing erythropoietin. Blood. 2007;110:762–769. doi: 10.1182/blood-2006-12-063602. [DOI] [PubMed] [Google Scholar]
- Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–1364. [PubMed] [Google Scholar]
- Buemi M, Cavallaro E, Floccari F, et al. The pleiotropic effects of erythropoietin in the central nervous system. J Neuropathol Exp Neurol. 2003;62:228–236. doi: 10.1093/jnen/62.3.228. [DOI] [PubMed] [Google Scholar]
- Diggle PJ. Statistical Analysis of Spatial Point Patterns. London: Academic Press; 1983. [Google Scholar]
- Digicalylioglu M, Bichet S, Marti HH, et al. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc Natl Acad Sci U S A. 1995;92:3717–3720. doi: 10.1073/pnas.92.9.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott S, Lorenzini T, Chang J, et al. Fine-structure epitope mapping of antierythropoietin monoclonal antibodies reveals a model of recombinant human erythropoietin structure. Blood. 1996;87:2702–2713. [PubMed] [Google Scholar]
- Fraser JK, Tan AS, Lin FK, Berridge MV. Expression of specific high-affinity binding sites for erythropoietin on rat and mouse megakaryocytes. Exp Hematol. 1989;17:10–16. [PubMed] [Google Scholar]
- Guidolin D, Crivellato E, Nico B, et al. An image analysis of the spatial distribution of perivascular mast cells in human melanoma. Int J Mol Med. 2006;17:981–987. [PubMed] [Google Scholar]
- Heinicke K, Baum O, Ogunshola OO, et al. Excessive erythrocytosis in adult mice overexpressing erythropoietin leads to hepatic, renal, neuronal, and muscular degeneration. Am J Physiol Regul Integr Comp Physiol. 2006;291:R947–R956. doi: 10.1152/ajpregu.00152.2006. [DOI] [PubMed] [Google Scholar]
- Hirose S, Takahashi M, Ogawa R, et al. Erythropoietin attenuates the development of experimental autoimmune myocarditis. Cardiovasc Drugs Ther. 2007;21:17–27. doi: 10.1007/s10557-007-6005-7. [DOI] [PubMed] [Google Scholar]
- Isogai R, Takahashi M, Aisu K, et al. The receptor for erythropoietin is present on cutaneous mast cell. Arch Dermatol Res. 2006;297:389–394. doi: 10.1007/s00403-005-0615-3. [DOI] [PubMed] [Google Scholar]
- Juul SE, Yachnis AT, Christensen RD. Tissue distribution of erythropoietin and erythropoietin receptor in the developing human fetus. Early Hum Dev. 1998;52:235–249. doi: 10.1016/s0378-3782(98)00030-9. [DOI] [PubMed] [Google Scholar]
- Kamitani H, Masuzawa H, Sato J, et al. Erythropoietin in haemangioblastoma: immunohistochemical and electron microscopy studies. Acta Neurochir (Wien) 1987;85:56–62. doi: 10.1007/BF01402372. [DOI] [PubMed] [Google Scholar]
- Katz O, Gil L, Lifshitz L, et al. Erythropoietin enhances immune responses in mice. Eur J Immunol. 2007;37:1584–1593. doi: 10.1002/eji.200637025. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Go S, Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978;52:447–452. [PubMed] [Google Scholar]
- Lifshitz L, Prutchi-Sagiv S, Avneon M, et al. Non-erythroid activities of erythropoietin: functional effects on murine dendritic cells. Mol Immunol. 2009;46:713–721. doi: 10.1016/j.molimm.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Lin CS, Lim SK, D’Agati V, Costantini F. Differential effects of an erythropoietin receptor gene disruption on primitive and definitive erythropoiesis. Genes Dev. 1996;10:154–164. doi: 10.1101/gad.10.2.154. [DOI] [PubMed] [Google Scholar]
- Malorny U, Michels E, Sorg C. A monoclonal antibody against an antigen present on mouse macrophages and absent from monocytes. Cell Tissue Res. 1986;243:421–428. doi: 10.1007/BF00251059. [DOI] [PubMed] [Google Scholar]
- Ogunshola OO, Djonov V, Staudt R, et al. Chronic excessive erythrocytosis induces endothelial activation and damage in mouse brain. Am J Physiol Regul Integr Comp Physiol. 2005;290:R678–R684. doi: 10.1152/ajpregu.00246.2005. [DOI] [PubMed] [Google Scholar]
- Philimonenko AA, Janàcek J, Hozàk P. Statistical evaluation of colocalization patterns in immunogold labeling experiments. J Struct Biol. 2000;132:201–210. doi: 10.1006/jsbi.2000.4326. [DOI] [PubMed] [Google Scholar]
- Platt AM, Mowat AM. Mucosal macrophages and the regulation of the immune response in the intestine. Immunol Lett. 2008;119:22–31. doi: 10.1016/j.imlet.2008.05.009. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna: R foundation for Statistical Computing; 2005. Reference index version 2.0.0 ISBN 3-900051-07-0. Available at: http://www.R-project.org (accessed on 01 July 2005) [Google Scholar]
- Ribatti D, Presta M, Vacca A, et al. Human erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood. 1999;93:2627–2636. [PubMed] [Google Scholar]
- Ripley BD. Tests for randomness for spatial point patterns. J R Statist Soc. 1979;41:368–374. [Google Scholar]
- Ruschitkka FT, Wenger RH, Stallmach T, et al. Nitric oxide prevents cardiovascular disease and determines survival in polyglobulic mice overexpressing erythropoietin. Proc Natl Acad Sci USA. 2000;97:11609–11613. doi: 10.1073/pnas.97.21.11609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka M, Wen TC, Matsuda S, et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela S, Shurtz-Swirski R, Sharon R, et al. The polymorphonuclear leukocyte-a new target for erythropoietin. Nephron. 2001;88:205–210. doi: 10.1159/000045991. [DOI] [PubMed] [Google Scholar]
- Spivak IL. Polycythemia vera: myths, mechanisms, and management. Blood. 2002;100:4272–4290. doi: 10.1182/blood-2001-12-0349. [DOI] [PubMed] [Google Scholar]
- Tachibana O, Yamashima T, Yamashita J. Immunohistochemical study of erythropoietin in cerebellar hemangioblastomas associated with secondary polycythemia. Neurosurgery. 1991;28:24–26. doi: 10.1097/00006123-199101000-00004. [DOI] [PubMed] [Google Scholar]
- Tilbrook PA, Klinken SP. Erythropoietin and erythropoietin receptor. Growth Factors. 1999;17:25–35. doi: 10.3109/08977199909001060. [DOI] [PubMed] [Google Scholar]
- Vogel J, Kiessling I, Heinicke K, et al. Transgenic mice overexpressing erythropoietin adapt to excessive erythrocytosis by regulating blood viscosity. Blood. 2003;102:2278–2284. doi: 10.1182/blood-2003-01-0283. [DOI] [PubMed] [Google Scholar]
- Wagner KF, Kastchinski DM, Hasegawa J, et al. Chronic inborn erythrocytosis leads to cardiac dysfunction and premature death in mice overexpressing erythropoietin. Blood. 2001;97:536–542. doi: 10.1182/blood.v97.2.536. [DOI] [PubMed] [Google Scholar]
- Waskow C, Rodewald HR. Lymphocyte development in neonatal and adult c-Kit-deficient (c-KitW/W) mice. Adv Exp Med Biol. 2002;512:1–10. doi: 10.1007/978-1-4615-0757-4_1. [DOI] [PubMed] [Google Scholar]
- Waskow C, Paul S, Haller C, et al. Viable c-KitW/W mutants reveal pivotal role for c-Kit in the maintenance of lymphopoiesis. Immunity. 2002;17:277–288. doi: 10.1016/s1074-7613(02)00386-2. [DOI] [PubMed] [Google Scholar]
- Waskow C, Terszowski G, Costa C, et al. Rescue of lethal c-KitW/W mice by erythropoietin. Blood. 2004;104:1688–1695. doi: 10.1182/blood-2004-04-1247. [DOI] [PubMed] [Google Scholar]
- Welham MJ, Schrader JW. Modulation of c-kit mRNA and protein by hemopoietic growth factors. Mol Cell Biol. 1991;11:2901–2904. doi: 10.1128/mcb.11.5.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiessner C, Allegrini PR, Ekatodramis D, et al. Increased cerebral infarct volumes in polyglobulic mice overexpressing erythropoietin. J Cereb Blood Flow Metab. 2001;21:857–864. doi: 10.1097/00004647-200107000-00011. [DOI] [PubMed] [Google Scholar]
- Wu H, Liu X, Janisch R, et al. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]