Abstract
The aim of this study was to record growth-related changes in collagen network organization and proteoglycan distribution in intermittently peak-loaded and continuously lower-level-loaded articular cartilage. Cartilage from the proximal phalangeal bone of the equine metacarpophalangeal joint at birth, at 5, 11 and 18 months, and at 6–10 years of age was collected from two sites. Site 1, at the joint margin, is unloaded at slow gaits but is subjected to high-intensity loading during athletic activity; site 2 is a continuously but less intensively loaded site in the centre of the joint. The degree of collagen parallelism was determined with quantitative polarized light microscopy and the parallelism index for collagen fibrils was computed from the cartilage surface to the osteochondral junction. Concurrent changes in the proteoglycan distribution were quantified with digital densitometry. We found that the parallelism index increased significantly with age (up to 90%). At birth, site 2 exhibited a more organized collagen network than site 1. In adult horses this situation was reversed. The superficial and intermediate zones exhibited the greatest reorganization of collagen. Site 1 had a higher proteoglycan content than site 2 at birth but here too the situation was reversed in adult horses. We conclude that large changes in joint loading during growth and maturation in the period from birth to adulthood profoundly affect the architecture of the collagen network in equine cartilage. In addition, the distribution and content of proteoglycans are modified significantly by altered joint use. Intermittent peak-loading with shear seems to induce higher collagen parallelism and a lower proteoglycan content in cartilage than more constant weight-bearing. Therefore, we hypothesize that the formation of mature articular cartilage with a highly parallel collagen network and relatively low proteoglycan content in the peak-loaded area of a joint is needed to withstand intermittent stress and shear, whereas a constantly weight-bearing joint area benefits from lower collagen parallelism and a higher proteoglycan content.
Keywords: cartilage, collagen, horse, maturation, parallelism, proteoglycans
Introduction
The hyaline cartilage that covers the articular surfaces of diarthrodial joints is a highly specialized connective tissue with biomechanical characteristics that make it particularly suitable for load-bearing, transition of forces and shock absorption (Herzog & Federico, 2006). The physical properties of the tissue are dictated by the structure and organization of the macromolecules in the extracellular matrix (Cohen et al. 1998). A sparse population of chondrocytes is distributed throughout the extracellular matrix, which consists mainly of collagen, proteoglycans (PGs) and water. Experiments with enzymatic digestions of either the collagen network or PGs have clarified the functional interactions of PGs and collagen in articular cartilage. Partial digestion of the collagen network leads to loss of the tensile properties of articular cartilage, whereas PG removal reduces the viscous and compressive properties of the tissue (Bader et al. 1992; Rieppo et al. 2003).
Whereas articular cartilage collagen and PG metabolism are relatively active during growth and adolescence, in adult individuals the metabolism of cartilage is more sluggish. This is especially true for the collagen component of the extracellular matrix of cartilage; the collagen turnover time in adult human articular cartilage has been estimated to be 100–200 years (Maroudas et al. 1992; Verzijl et al. 2001). This makes the early juvenile period in which the collagen network is moulded under the influence of biomechanical loading into a crucial period for the determination of the ultimate biomechanical characteristics of articular cartilage (Brama et al. 2000, 2002).
Benninghoff (1925)originally described the spatial organization of the collagen network in articular cartilage using plane-polarized light microscopy. The collagen fibril network of articular cartilage was divided into a tangential (parallel), a transitional (arcading) and a radial (perpendicular) zone according to the preferential direction of the fibrils in relation to the articular cartilage surface. Later reports have either supported this model (Aspden & Hukins, 1981; Dunham et al. 1988; Hwang et al. 1992) or pointed to a more complex organization pattern (Nieminen et al. 2001; Xia et al. 2003). The numbers of collagen fibrils that constitute the collagen network, their thickness (Langsjo et al. 1999) and their organization vary in the different zones of articular cartilage (Benninghoff, 1925; Aspden & Hukins, 1981; Hwang et al. 1992). In addition, the collagen fibril organization varies in different mammalian species (Clark, 1991; Kaab et al. 1998). It appears that the species and age of the subject (Kaab et al. 1998; Hyttinen et al. 2001), frequency and mode of joint loading all modulate the spatial architecture of the collagen network, especially in the superficial articular cartilage (Arokoski et al. 1996; Hyttinen et al. 2001).
There is substantial evidence for the development of the biochemical heterogeneity of the collagen network in different regions of the joint surface in the juvenile period as a result of differences in their biomechanical loading, growth and maturation (Brama et al. 2000, 2002). However, little is known about the effects of these important modulators on the development of the collagen fibril network structure in normal articular cartilage. Such knowledge is, however, indispensable to understanding the potential effects in the short and long term of both physiological and pathological factors on the once-in-a-lifetime process of cartilage maturation. Very recently two studies have specifically focused on structural (re)modelling of the collagen network during growth and maturation in horses (Van Turnhout et al. 2008) and pigs (Rieppo et al. 2009). Van Turnhout et al. (2008) investigated the collagen structure of articular cartilage with quantitative polarized light microscopy and scanning electron microscopy at the distal metacarpus of a relatively small number of young and adult horses. They concluded that structural remodelling of the collagen network in articular cartilage occurs in the first months after birth, in which the collagen network transforms from a parallel arrangement along the articular surface towards the typical Benninghoff architecture of cartilage. Rieppo et al. (2009) revealed a similar remodelling process during maturation and growth in pigs from a parallel arrangement along the articular surface towards a typical Benninghoff arching architecture. However, these studies only studied the lateral facet of the femoral trochlea of the knee joint and did not compare differently loaded sites within the same joint.
The aim of this study was to analyse the growth- and maturation-related alterations in the structure of the collagen fibril network in normal articular cartilage at sites in a joint exposed to either dynamic, intermittent peak-type loads or to a regular static type of weight-bearing. Concurrent spatial changes in the PG distribution, an extracellular matrix component that is known to respond well to compressive loading, were also recorded.
Materials and methods
Experimental design
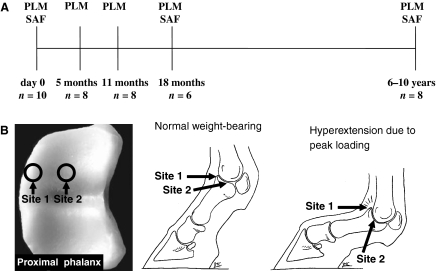
The study design and number of animals are shown in Fig. 1A. Right metacarpophalangeal (MCP) joints were collected from neonatal foals (n= 8), 5-month-old foals raised on pasture (n= 8), yearlings aged 11 months raised on pasture except for the winter period in which a loose housing system was used (n= 8), horses aged 18 months (n= 6) raised and kept on pasture, and adult horses (n= 8, mean age 7.9 years, range 6–10 years). The adult animals were used for dressage and show jumping at recreational level. The animals used in this study were either from control groups of studies approved by the institutional committees for animal welfare at Utrecht University, the Netherlands [5 and 11 month olds (van Weeren & Barneveld, 1999)] or Massey University, New Zealand [18 month olds (Rogers et al. 2008)] or had to be killed for reasons other than MCP joint disease. The right MCP joint was freed from surrounding skin and subcutaneous tissues within 4 h after killing and isolated in its entirety by transecting the proximal phalangeal and third metacarpal bone with a band saw. The joint was then immediately wrapped in cling film and stored at −20 °C until further processing.
Fig. 1.
(A) Study design and time points for quantitative analysis of equine articular cartilage collagen network parallelism and proteoglycan (PG) content in the equine metacarpophalangeal (MCP) joint. PLM, polarized light microscopy of collagen; SAF, digital densitometry of the PG distribution after safranin-O staining. The age range covers newborn (day 0), growing and maturing (5, 11 and 18 months) and adult (6–10 years) animals. n= number of animals. (B) Sampling sites of cylindrical osteochondral plugs for microscopy. Site 1 is situated at the dorsal edge and site 2 in the mid-region (central fovea) of the proximal articular surface of the proximal phalangeal bone.
Sample collection
After thawing, the joint was opened and inspected visually to exclude macroscopic pathology. Subsequently, osteochondral plugs (diameter 4 mm) were taken perpendicular to the articular surface from two predefined locations (Fig. 1B) on the articular surface of the proximal phalanx, using a custom-built hollow drill. One plug was taken from the dorsal edge of the proximal articular surface of the proximal phalanx (site 1, Fig. 1B). This site is exposed to dynamic, intermittent peak-type loading during athletic activities such as cantering or jumping but is not loaded during standing or when the animal is moving at slow speed (Brama et al. 2001). Another plug was harvested from the mid-region of the medial joint surface (site 2, Fig. 1B), a site that is constantly loaded whenever the limb is weight-bearing but not subjected to the peak loads and shear forces to which site 1 is exposed (Brama et al. 2001). The harvested osteochondral plugs were randomly divided into two halves using a dentist’s drill equipped with a 100-μm-thick sawing disc. One half was selected for analysis.
The tissue was fixed in 10% (v/v) phosphate-buffered neutral formalin, pH 7.4, for 48 h. After fixation, the specimens were demineralized in 10% EDTA-formalin at room temperature (20°C), pH 7.4, for 14 days. After alcohol dehydration and xylene treatment, the specimens were embedded in Paraplast Plus® wax (Sherwood Medical, St Louis, MO, USA) (Kiviranta et al. 1985; Kiraly et al. 1997). Vertical sections (3 μm thick) were cut with a Historange rotary microtome (LKB, Bromma, Sweden) for digital densitometry of PGs from a randomly selected cutting plane. Sections (7 μm thick) were cut for polarized light microscopy analysis of the collagen fibril network from a randomly selected cutting plane and another plane exactly perpendicular to the first. The additional 90° slices eliminated the potential effect of the direction of the cartilage sample in relation to the joint surface on collagen network measurements.
Polarized light microscopy of the collagen network
A methodological description of the polarized light microscopy technique used has recently been published by Rieppo et al. (2008) with a detailed technical appendix. Dewaxed 7-μm-thick sections were digested with testicular hyaluronidase and left unstained prior to DePeX embedding (BDH, Poole, UK) and quantitative polarized light microscopy of the collagen network (Kiraly et al. 1997). The degree of parallelism of the fibrils in the collagen network was analysed with a novel quantitative polarized light microscopic method (Rieppo et al. 2008) and expressed as the parallelism index (PI). When the course of collagen fibrils is random, i.e. parallelism is lacking, the value of the PI is 0%. Conversely, the PI is 100% in the case of perfect collagen fibril parallelism. The PI from the articular cartilage surface to the osteochondral junction was measured from two orthogonal articular cartilage section planes and the mean of the two measurements was used as outcome parameter. To visualize depth-related change, the parallelism profiles from the cartilage surface to the osteochondral junction were divided into 10 fractions. The pixel side length was 9.0 μm and the width of the measuring area in each section was 1350 μm.
Semiquantitative estimation of proteoglycan content and distribution
Anionic groups, predominantly sulphate and carboxyl moieties of PGs, of articular cartilage were stained at pH 4.6 with cationic safranin-O, which binds stoichiometrically to anionic groups of PGs (Kiviranta et al. 1985; Lammi & Tammi, 1988; Király et al. 1996). The stain distribution was analysed by digital densitometry at 593 nm from 3-μm-thick sections (Hyttinen et al. 2001). The pixel side length was 6.9 μm and the width of the measuring region was 1380 μm for absorbance profile calculations. The amount of safranin-O stain bound to PGs in sites 1 and 2 was expressed using the mean area-integrated optical density (Hyttinen et al. 2001) and for graphic representation the section profiles through the articular cartilage depth were divided into four fractions.
Statistics
The statistical significance of differences between the individual fractions of sites 1 and 2 was computed with Wilcoxon’s signed ranks test for paired observations (fractional P-values were adjusted with the Bonferroni method), which was also used in comparison of the collagen PI and PG distributions of sites 1 and 2. The statistical software package SPSS 12.0.2 (SPSS, Chicago, IL, USA) was used for all statistical calculations. A P-value of <0.05 was deemed significant.
Results
Polarized light microscopy of the collagen network
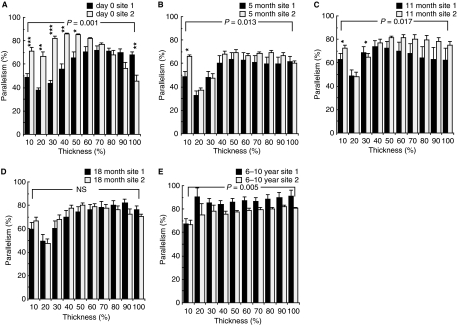
The collagen fibril parallelism varied both with cartilage depth, i.e. between the fractions from the cartilage surface to the osteochondral junction (Fig. 2), and in the two differently loaded joint sites 1 and 2 (Fig. 3A–E). Also, the development of the collagen fibril network varied in the different fractions of the tissue. In general, the PI increased gradually and significantly with age (Fig. 3A–E). The increase in the PI was most evident in the superficial and intermediate fractions of site 1, representing about 30% of the total cartilage thickness. At birth, the lowest PI values were found in these first fractions of site 1, indicating a high degree of random orientation of the fibrils (PI values ranging between 37 and 48%; Fig. 3A). In contrast, in the adult horses the highest absolute PI value (90%) was found in the most superficial layer but one of site 1 (Fig. 3E).
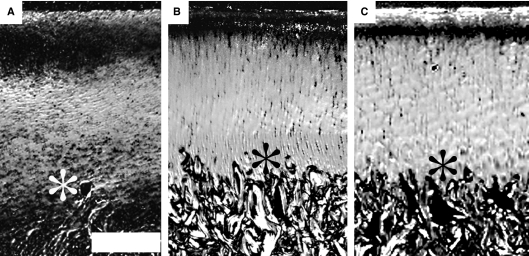
Fig. 2.
Typical example of polarized light microscopy slide from site 1, demonstrating the degree of parallelism of the fibrils in the collagen network at day 0 (A), at 18 months (B) and in the adult animal (8 years) (C). Bright intensity indicates high and dark intensity represents low parallelism of collagen fibrils. The decrease of the dark area indicates that there is increased parallelism in the more superficial cartilage layers over time. The asterisks show the interface between articular cartilage and bone. Scale bar, 500 μm.
Fig. 3.
The average degree of collagen parallelism (mean ± SD) through the cartilage thickness, from the surface to the osteochondral junction, measured with quantitative polarized light microscopy. 0% indicates random course of fibrils [parallelism index (PI) = 0] and 100% indicates perfect parallelism (PI = 100). The cartilage thickness is expressed using a relative scale (10–100%, x-axis) from the cartilage surface to the osteochondral junction. Newborn (day 0), growing and maturing (5, 11 and 18 months), and adult (6–10 years) horses (A–E). *P < 0.05, **P < 0.01, ***P < 0.001 after Bonferroni adjustment for comparison of the individual fractions between sites 1 and 2. The P-values in (A–E) indicate the significance of the overall difference between the parallelism distributions in sites 1 and 2. Wilcoxon’s signed ranks test for paired observations. NS, not significant.
The two cartilage sites exposed to different types of joint loading exhibited clear differences in the PI already at birth (Fig. 3A). Whereas the PI was relatively high at all levels of site 2 except for the two to three fractions closest to the osteochondral junction, it was much lower at most levels at site 1, again except for the deepest layers (Fig. 3A). With growth and maturation, a gradual restructuring process of the collagen network took place at both sites 1 and 2 that is reflected in changes in the PI. At site 1 there was initially a small decrease in the PI at 5 months (Fig. 3B), followed by a gradual increase in the PI over the entire cartilage depth during later development with the most superficial layers following latest (Fig. 3C–E). At site 2 the PI decreased substantially over the larger part of the cartilage thickness during the first few months with an increase only in the deepest layers, to remain relatively stable after that. By older ages a shift had occurred with an overall significantly (P < 0.005) lower PI at site 2 (Fig. 3E) compared with site 1, a situation opposite to that present during the first year of life (Fig. 3A–C). The age of 18 months was apparently some kind of crossing point where the PI of site 1 equalled that of site 2 (Fig. 3D).
Semiquantitative estimation of proteoglycan content and distribution
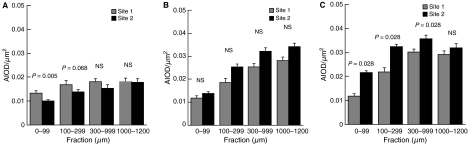
In sites 1 and 2, the PG distributions and contents through the cartilage depth were very different in the newborn, 18-month-old and adult 6- to 10-year-old horses (Fig. 4A–C). At birth, site 1 exhibited a higher PG content than site 2 in the two most superficial zones. However, already at the age of 18 months, the PG content of all zones of site 2 was numerically higher (although not yet statistically significant) than at site 1. The PG accumulation continued in the superficial and intermediate zones of site 2 but not in site 1 in 6- to 10-year-old horses (Fig. 4C), resulting in a significantly higher PG content in all but the deepest zone in site 2 (Fig. 4C). It is to be noted that the superficial zone of site 1 exhibited a rather constant content of PGs from birth to the age of 6–10 years (Fig. 4A–C).
Fig. 4.
Safranin-O stain absorbance (mean ± SD) as a measure of zonal proteoglycan content and distribution at day 0 (A), at 18 months (B) and in adult animals (6–10 years) (C). Fractions of articular cartilage start from the surface and end at the osteochondral interface. The four fractions correspond roughly to the superficial, intermediate, deep and calcified zones of cartilage. AIOD, area-integrated optical density of safranin-O. Wilcoxon’s signed ranks test for paired observations. NS, not significant.
Our results indicate that there is an inverse relationship between the degree of collagen fibril parallelism and PG content over the depth of the cartilage. In newborn foals, polarized light microscopic analysis revealed a low degree of parallelism of collagen fibrils in the superficial and intermediate zones of site 1 (Fig. 3A) but the zones had higher PG contents than at site 2 (Fig. 4A), whereas site 2 showed a high degree of collagen fibril parallelism at birth (Fig. 3A). In mature animals the situation was reversed with a higher collagen parallelism at site 1 (Fig. 3E) and a higher PG content at site 2 (Fig. 4C).
Discussion
The three-dimensional structure of the collagen network of articular cartilage has been the subject of many studies and it has long since become clear that this structure is complex and variable with topographical location, age, and status of health and disease. Polarized light microscopy offers superior performance in the spatial analysis of collagen network specimens compared with X-ray diffraction or electron microscopy techniques (Yarker et al. 1983). It provides a wide view over the tissue section and a straightforward correlation of the results with other histological methods. However, traditional plane-polarized techniques cannot overcome the problem of the unequal detection sensitivity of differently oriented structures. Recently, with the advent of computerized instrumentation of microscope polarizers, and combining data from separate images with Stokes’s calculus, it has proved possible to acquire collagen parallelism data independently of the fibril orientation (Rieppo et al. 2008). This technique was used in the present study.
The results of the study support the concept that the structure of the collagen fibril network of articular cartilage is variable through the articular cartilage thickness as well as between sites of the cartilage surface. The latter variations seem to be load-related. Sites 1 and 2 on the proximal phalangeal bone of the MCP joint that were investigated in this study are exposed to different types of loading during joint flexion and extension along a single axis of movement (the joint is a ginglymus) (Brama et al. 2001). Site 1 is subjected to intermittent, peak-type loading and shear during running or jumping but the site is almost unloaded when the horse is standing or moving slowly. However, site 2 in the mid-region of the medial joint contact area is constantly and moderately loaded during standing and slow movement. The articular cartilage at sites 1 and 2 is almost identical in biochemical composition at birth but the sites subsequently differentiate during maturation, to maintain their phenotype in adulthood unchanged, unless pathological change occurs (Brama et al. 1999b, 2000, 2002). From birth to the age of 5 months, sites 1 and 2 initially have the same total collagen content (about 45% of dry weight) but, by the age of 5 months, the content at site 1 has become higher than at site 2 (about 60 vs. 52%). At the age of 1 year, the collagen content has increased to about 64% of dry weight at site 1. The corresponding figure at site 2 is 56% (Brama et al. 2000). In general, the rate of increase in collagen content during growth is slower at site 2. In mature equine articular cartilage (aged 7–14 years), site 1 maintains its higher collagen content (Brama et al. 1999b). This site also contains a greater number of hydroxylysylpyridinoline cross-links (Brama et al. 1999b).
Although sites 1 and 2 contain about the same amount of collagen at birth, they show a different degree of collagen network parallelism both at birth and later in life. Interestingly, the initially low collagen parallelism in the superficial and intermediate zones of site 1 is remodelled gradually into a highly organized collagen network, presumably under the influence of intermittent, high-intensity loading as occurs at this site in the freely moving animal. It seems reasonable that the possible combination of high collagen parallelism, high collagen content and high amount of hydroxylysylpyridinoline cross-links all contributes to the articular cartilage at site 1 being load- and strain-resistant at least in tension and most likely shear. Whereas at site 1 the change from intra-uterine motion to postnatal biomechanical loading implies above all a (drastic) change in magnitude of loading, but not in direction of loading, the situation at site 2 is different. This site is subjected to constant, relatively low level, loading in postnatal life due to the influence of gravity, i.e. compressive loading will prevail. During intra-uterine life this type of loading does not exist and fetal movements will result in only relatively small shear forces. These forces will probably only minimally affect the fetal horizontal position of the collagen network (Van Turnhout et al. 2008; Rieppo et al. 2009), which will thus maintain its original high degree of parallelism. After birth, this region is suddenly subjected to compressive loading, urging remodelling of the collagen network structure towards the more classical Benninghoff arcades. Although not macroscopically obvious, it cannot be excluded that the shape of the proximal phalangeal bone and with it the contours of the bony surface of the proximal articular surface undergo some changes in the process of growth and maturation. If so, this factor may also affect stress and strain distribution in the articular cartilage, irrespective of changes in load and hence may contribute to the initiation of collagen remodelling that will effect the physiological age-related changes in the configuration of the collagen network. Additionally, the obvious change in size may alter compressive, tensile and shear stresses on the cartilage. However, any change in shape is trivial compared with the absolutely massive change in loading seen in precocial species immediately after birth, together with the higher forces applied by rapidly developing muscles and tendons to allow a progressively increasing maximum gait speed of a body weight that increases fast (body weight at 1 year equals roughly eight times that at birth).
The remodelling of the collagen network will lead to an initial decrease of the PI before most of the parallelism is regained in the new configuration. Thus, we postulate that the postnatal loading and movement history of the joint governs the local collagen network development and organization. The preferential loading axis and main direction of stress and strain ultimately generate a preferentially organized, functionally optimized collagen network.
The change in loading will also affect the content and distribution of PGs, which are known to respond to compressive loading (Little & Ghosh, 1997; Brama et al. 1999a; Murray et al. 2001). In prenatal life site 1 is, to a certain extent, loaded by compression when the fetus extends its MCP joint. As stated earlier, compressive loading at site 2 is extremely limited at this stage. After birth, the loading pattern reverses with site 2 now under almost constant compressive loading, whereas compressive loading of site 1 retains its occasional character, which may explain the shift in dominance in PG content between the sites during the postnatal period demonstrated in this study.
In summary, the articular cartilage at sites 1 and 2 exhibits specific concurrent but different changes in the parallelism of the collagen network and PG content. We propose that these findings are due to functional adaptation of the joint to different types of loading. This results in different patterns of collagen organization and PG deposition.
In particular, the superficial and intermediate zones of the articular cartilage collagen network show significant responses with growth and maturation. These findings may be related to rapidly increasing physical exercise and gain of body weight. We hypothesize that the formation of mature articular cartilage with a highly parallel collagen network and relatively low PG content in the peak-loaded area of a joint is needed to withstand intermittent stress and shear, whereas a constantly weight-bearing joint area benefits from lower collagen parallelism and a higher PG content.
Acknowledgments
Esa Halmesmäki, Terhi Harjula, Viivi Tolvanen, Ulrika Siitonen, Jarno Rieppo, Eija Rahunen and Kari Kotikumpu are acknowledged for their excellent technical assistance. The study was financially supported by Science Foundation Ireland, the Academy of Finland (project nos 113112, 213548 and 216231) and the Ministry of Education in Finland (project nos 58/722/2004, 61/627/2005 and 25/627/2006).
References
- Arokoski JPA, Hyttinen MM, Lapvetalainen T, et al. Decreased birefringence of the superficial zone collagen network in the canine knee (stifle) articular cartilage after long distance running training, detected by quantitative polarised light microscopy. Ann Rheum Dis. 1996;55:253–264. doi: 10.1136/ard.55.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspden RM, Hukins DWL. Collagen organization in articular cartilage, determined by X-ray diffraction, and its relationship to tissue function. Proc R Soc Lond Biol Sci. 1981;212:299–304. doi: 10.1098/rspb.1981.0040. [DOI] [PubMed] [Google Scholar]
- Bader DL, Kempson GE, Egan J, et al. The effects of selective matrix degradation on the short-term compressive properties of adult human articular cartilage. Biochim Biophys Acta – Gen Sub. 1992;1116:147–154. doi: 10.1016/0304-4165(92)90111-7. [DOI] [PubMed] [Google Scholar]
- Benninghoff A. Form und Bau der Gelenkknorpel in ihren Beziehungen zur Funktion. Zweiter teil: Der Aufnau des Gelenkknorpels in seinen Beziehungen zur Funktion. Z Zelforsch Mikrosk Anat. 1925;2:783–862. [Google Scholar]
- Brama PA, Tekoppele JM, Bank RA, et al. Influence of different exercise levels and age on the biochemical characteristics of immature equine articular cartilage. Equine Vet J Suppl. 1999a;31:55–61. doi: 10.1111/j.2042-3306.1999.tb05314.x. [DOI] [PubMed] [Google Scholar]
- Brama PA, TeKoppele JM, Bank RA, et al. Influence of site and age on biochemical characteristics of the collagen network of equine articular cartilage. Am J Vet Res. 1999b;60:341–345. [PubMed] [Google Scholar]
- Brama PA, Tekoppele JM, Bank RA, et al. Functional adaptation of equine articular cartilage: the formation of regional biochemical characteristics up to age one year. Equine Vet J. 2000;32:217–221. doi: 10.2746/042516400776563626. [DOI] [PubMed] [Google Scholar]
- Brama PA, Karssenberg D, Barneveld A, et al. Contact areas and pressure distribution on the proximal articular surface of the proximal phalanx under sagittal plane loading. Equine Vet J. 2001;33:26–32. doi: 10.2746/042516401776767377. [DOI] [PubMed] [Google Scholar]
- Brama PA, TeKoppele JM, Bank RA, et al. Development of biochemical heterogeneity of articular cartilage: influences of age and exercise. Equine Vet J. 2002;34:265–269. doi: 10.2746/042516402776186146. [DOI] [PubMed] [Google Scholar]
- Clark JM. Variation of collagen fiber alignment in a joint surface: a scanning electron microscope study of the tibial plateau in dog, rabbit, and man. J Orthop Res. 1991;9:246–257. doi: 10.1002/jor.1100090213. [DOI] [PubMed] [Google Scholar]
- Cohen NP, Foster RJ, Mow VC. Composition and dynamics of articular cartilage: structure, function, and maintaining healthy state. J Orthop Sports Phys Ther. 1998;28:203–215. doi: 10.2519/jospt.1998.28.4.203. [DOI] [PubMed] [Google Scholar]
- Dunham J, Shackleton DR, Billingham MEJ, et al. A reappraisal of the structure of normal canine articular cartilage. J Anat. 1988;157:89–99. [PMC free article] [PubMed] [Google Scholar]
- Herzog W, Federico S. Considerations on joint and articular cartilage mechanics. Biomech Model Mechanobiol. 2006;5:64–81. doi: 10.1007/s10237-006-0029-y. [DOI] [PubMed] [Google Scholar]
- Hwang WS, Li B, Jin LH, et al. Collagen fibril structure of normal, aging, and osteoarthritic cartilage. J Pathol. 1992;167:425–433. doi: 10.1002/path.1711670413. [DOI] [PubMed] [Google Scholar]
- Hyttinen MM, Arokoski JPA, Parkkinen JJ, et al. Age matters: collagen birefringence of superficial articular cartilage is increased in young guinea-pigs but decreased in older animals after identical physiological type of joint loading. Osteoarth Cart. 2001;9:694–701. doi: 10.1053/joca.2001.0466. [DOI] [PubMed] [Google Scholar]
- Kaab MJ, Gwynn IAP, Notzli HP. Collagen fibre arrangement in the tibial plateau articular cartilage of man and other mammalian species. J Anat. 1998;193:23–34. doi: 10.1046/j.1469-7580.1998.19310023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly K, Hyttinen MM, Lapvetelainen T, et al. Specimen preparation and quantification of collagen birefringence in unstained sections of articular cartilage using image analysis and polarizing light microscopy. Histochem J. 1997;29:317–327. doi: 10.1023/a:1020802631968. [DOI] [PubMed] [Google Scholar]
- Király K, Lapveteläinen T, Arokoski J, et al. Application of selected cationic dyes for the semiquantitative estimation of glycosaminoglycans in histological sections of articular cartilage by microspectrophotometry. Histochem J. 1996;28:577–590. doi: 10.1007/BF02331378. [DOI] [PubMed] [Google Scholar]
- Kiviranta I, Jurvelin J, Tammi M. Microspectrophotometric quantitation of glycosaminoglycans in articular cartilage sections stained with Safranin O. Histochemistry. 1985;82:249–255. doi: 10.1007/BF00501401. [DOI] [PubMed] [Google Scholar]
- Lammi M, Tammi M. Densitometric assay of nanogram quantities of proteoglycans precipitated on nitrocellulose membrane with Safranin O. Anal Biochem. 1988;168:352–357. doi: 10.1016/0003-2697(88)90329-6. [DOI] [PubMed] [Google Scholar]
- Langsjo TK, Hyttinen M, Pelttari A, et al. Electron microscopic stereological study of collagen fibrils in bovine articular cartilage: volume and surface densities are best obtained indirectly (from length densities and diameters) using isotropic uniform random sampling. J Anat. 1999;195:281–293. doi: 10.1046/j.1469-7580.1999.19520281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little CB, Ghosh P. Variation in proteoglycan metabolism by articular chondrocytes in different joint regions is determined by post-natal mechanical loading. Osteoarth Cart. 1997;5:49–62. doi: 10.1016/s1063-4584(97)80031-3. [DOI] [PubMed] [Google Scholar]
- Maroudas A, Palla G, Gilav E. Racemization of aspartic acid in human articular cartilage. Connect Tissue Res. 1992;28:161–169. doi: 10.3109/03008209209015033. [DOI] [PubMed] [Google Scholar]
- Murray RC, Birch HL, Lakhani K, et al. Biochemical composition of equine carpal articular cartilage is influenced by short-term exercise in a site-specific manner. Osteoarth Cart. 2001;9:625–632. doi: 10.1053/joca.2001.0462. [DOI] [PubMed] [Google Scholar]
- Nieminen MT, Rieppo J, Toyras J, et al. T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med. 2001;46:487–493. doi: 10.1002/mrm.1218. [DOI] [PubMed] [Google Scholar]
- Rieppo J, Toyras J, Nieminen MT, et al. Structure-function relationships in enzymatically modified articular cartilage. Cells Tissues Organs. 2003;175:121–132. doi: 10.1159/000074628. [DOI] [PubMed] [Google Scholar]
- Rieppo J, Hallikainen J, Jurvelin JS, et al. Practical considerations in the use of polarized light microscopy in the analysis of the collagen network in articular cartilage. Microsc Res Tech. 2008;71:279–287. doi: 10.1002/jemt.20551. [DOI] [PubMed] [Google Scholar]
- Rieppo J, Hytinnen MM, Halmesmaki E, et al. Changes in spatial collagen content and collagen network architecture in porcine articular cartilage during growth and maturation. Osteoarth Cart. 2009;17:448–455. doi: 10.1016/j.joca.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Rogers CW, Firth EC, McIlwraith CW, et al. Evaluation of a new strategy to modulate skeletal development in Thoroughbred performance horses by imposing track-based exercise during growth. Equine Vet J. 2008;40:111–118. doi: 10.2746/042516408X268923. [DOI] [PubMed] [Google Scholar]
- Van Turnhout MC, Haazelager MB, Gijsen MAL, et al. Quantitative description of collagen structure in the articular cartilage of the young and adult equine distal metacarpus. Anim Biol. 2008;58:353–370. [Google Scholar]
- Verzijl N, DeGroot J, Bank RA, et al. Age-related accumulation of the advanced glycation endproduct pentosidine in human articular cartilage aggrecan: the use of pentosidine levels as a quantitative measure of protein turnover. Matrix Biol. 2001;20:409–417. doi: 10.1016/s0945-053x(01)00158-5. [DOI] [PubMed] [Google Scholar]
- van Weeren PR, Barneveld A. Study design to evaluate the influence of exercise on the development of the musculoskeletal system of foals up to age 11 months. Equine Vet J Suppl. 1999;31:4–8. doi: 10.1111/j.2042-3306.1999.tb05307.x. [DOI] [PubMed] [Google Scholar]
- Xia Y, Moody JB, Alhadlaq H, et al. Imaging the physical and morphological properties of a multi-zone young articular cartilage at microscopic resolution. J Magn Reson Imaging. 2003;17:365–374. doi: 10.1002/jmri.10269. [DOI] [PubMed] [Google Scholar]
- Yarker YE, Aspden RM, Hukins DWL. Birefringence of articular cartilage and the distribution of collagen fibril orientations. Connect Tissue Res. 1983;11:207–213. doi: 10.3109/03008208309004857. [DOI] [PubMed] [Google Scholar]